Valorizing Brazilian Propolis Residue: Comprehensive Characterization for Sustainable Reutilization Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Crude Propolis Samples and Industrial Residues
2.3. Preparation of Extracts
2.4. HPLC-ESI-MS/MS Analysis
2.5. HPLC-DAD Analysis
2.6. Spectrophotometric Assays
2.6.1. Antioxidant Activity (DPPH)
2.6.2. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.7. GC-MS Analysis
2.8. Nutrients
2.9. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Compound Analysis in Propolis Residue
3.2. Spectrophotometric Assays in Propolis Residue
3.2.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.2.2. Antioxidant Activity (DPPH Assay)
3.3. Analysis of Volatile Compounds
3.4. Nutrient Composition of Propolis Residue
3.4.1. Macronutrient Profile
3.4.2. Micronutrient Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.Y.; Ambrosio, C.M.; Miano, A.C.; Rosalen, P.L.; Gloria, E.M.; Alencar, S.M. Essential oils extracted from organic propolis residues: An exploratory analysis of their antibacterial and antioxidant properties and volatile profile. Molecules 2021, 26, 4694. [Google Scholar] [CrossRef] [PubMed]
- El-Deen, N.; AI M, Z.S.; Shalaby, S.I.; Nasr, S. Propolis, with reference of chemical composition, antiparasitic, antimycotic, antibacterial and antiviral activities: A review. Life Sci. J. 2013, 10, 1778–1782. [Google Scholar]
- Aygun, A. Effects of propolis on eggshell. In Egg Innovations and Strategies for Improvements; Academic Press: London, UK, 2017; pp. 145–156. [Google Scholar]
- Belmehdi, O.; El Menyiy, N.; Bouyahya, A.; El Baaboua, A.; El Omari, N.; Gallo, M.; Abrini, J. Recent advances in the chemical composition and biological activities of propolis. Food Rev. Int. 2023, 39, 6078–6128. [Google Scholar] [CrossRef]
- Meccatti, V.M.; Martins, K.M.C.; Ramos, L.D.P.; Pereira, T.C.; de Menezes, R.T.; Marcucci, M.C.; de Oliveira, L.D. Synergistic antibiofilm action of Cinnamomum verum and Brazilian green propolis hydroethanolic extracts against multidrug-resistant strains of Acinetobacter baumannii and Pseudomonas aeruginosa and their biocompatibility on human keratinocytes. Molecules 2023, 28, 6904. [Google Scholar] [CrossRef]
- Daffalla, K.A.; Mahmoud, A.S. Propolis as a natural remedy. J. Int. Oral Health 2016, 8, 646–649. [Google Scholar] [CrossRef]
- De Carvalho, C.; Fernandes, W.H.C.; Mouttinho, T.B.F.; de Souza, D.M.; Marcucci, M.C.; D’Alpino, P.H.P. Evidence-based studies and perspectives of the use of Brazilian green and red propolis in dentistry. Eur. J. Dent. 2019, 13, 459–465. [Google Scholar] [CrossRef]
- Pedrinha, V.F.; Santos, L.M.; Gonçalves, C.P.; Garcia, M.T.; Lameira, O.A.; Queiroga, C.L.; Marcucci, M.C.; Shahbazi, M.-A.; Sharma, P.K.; Junqueira, J.C.; et al. Effects of natural antimicrobial compounds propolis and copaiba on periodontal ligament fibroblasts, molecular docking, and in vivo study in Galleria mellonella. Biomed. Pharmacother. 2024, 171, 116139. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality–A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Segueni, N.; Khadraoui, F.; Rhouati, S. Volatile compounds as propolis characterization markers. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions; Springer: Cham, Switzerland, 2023; pp. 1271–1273. [Google Scholar]
- Pu, Y.; Jiang, H.; Zhang, Y.; Cao, J.; Jiang, W. Advances in propolis and propolis functionalized coatings and films for fruits and vegetables preservation. Food Chem. 2023, 414, 135662. [Google Scholar] [CrossRef]
- Pant, K.; Chopra, H.K.; Nanda, V. Characterization and anti-oxidant potential of polyphenolic biomarker compounds of Indian propolis: A multivariate and ANN-based approach. Eur. Food Res. Technol. 2024, 250, 253–271. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Propolis: A potential preservation agent for the food industry. Interciencia 2013, 38, 705–711. [Google Scholar]
- Nalbantsoy, A.; Sarıkahya, N.B.; Özverel, C.S.; Barlas, A.B.; Kırcı, D.; Akgün, İ.H.; Goren, A.C. Chemical composition and biological activities of Cypriot propolis. J. Apic. Res. 2022, 61, 233–245. [Google Scholar] [CrossRef]
- Pant, K.; Sharma, A.; Chopra, H.K.; Nanda, V. Impact of biodiversification on propolis composition, functionality, and application in foods as natural preservative: A review. Food Control 2023, 155, 110097. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Katekhaye, S.; Fearnley, H.; Fearnley, J.; Paradkar, A. Gaps in propolis research: Challenges posed to commercialization and the need for an holistic approach. J. Apic. Res. 2019, 58, 604–616. [Google Scholar] [CrossRef]
- Soós, Á.; Bódi, É.; Várallyay, S.; Molnár, S.; Kovács, B. Mineral content of propolis tinctures in relation to the extraction time and the ethanol content of the extraction solvent. LWT 2019, 111, 719–726. [Google Scholar] [CrossRef]
- Shabani, S.; Halbertal, D.; Wu, W.; Chen, M.; Liu, S.; Hone, J.; Pasupathy, A.N. Deep moiré potentials in twisted transition metal dichalcogenide bilayers. Nat. Phys. 2021, 17, 720–725. [Google Scholar] [CrossRef]
- Matochek, M.H.M.; Tomaz, P.L.S.; de Sá Oliveira, T.; Polassi, M.R.; Alonso, R.C.B.; Scremin, F.M.; D’Alpino, P.H.P. Influence of a propolis-based irrigant solution on gap formation and bond strength of posts bonded to root canal dentin using different resin cements. Dent. Mater. J. 2020, 39, 490–499. [Google Scholar] [CrossRef]
- Mokhtarian, M.; Shabani, M.; Kazempoor, R. Extending oxidative stability of fish burgers using propolis ethanolic extract during storage. Iran. J. Nutr. Sci. Food Technol. 2021, 16, 109–122. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef] [PubMed]
- Santanatoglia, A.; Caprioli, G.; Cespi, M.; Ciarlantini, D.; Cognigni, L.; Fioretti, L.; Vittori, S. A comprehensive comparative study among the newly developed Pure Brew method and classical ones for filter coffee production. LWT 2023, 175, 114471. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Cespi, M.; Perinelli, D.R.; Fioretti, L.; Sagratini, G.; Vittori, S.; Caprioli, G. Impact of the human factor on the reproducibility of different coffee brewing methods. J. Food Compos. Anal. 2023, 124, 105698. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). NIST/EPA/NIH Mass Spectral Library with Search Program (Data Version: NIST 14); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2014. Available online: https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf (accessed on 1 May 2025).
- Santanatoglia, A.; Nzekoue, F.K.; Sagratini, G.; Ricciutelli, M.; Vittori, S.; Caprioli, G. Development and application of a novel analytical method for the determination of 8 plant sterols/stanols in 22 legumes samples. J. Food Compos. Anal. 2023, 118, 105195. [Google Scholar] [CrossRef]
- ISO 22184|IDF 244:2021; Milk and Milk Products—Determination of the Sugar Contents—High Performance Anion Exchange Chromatography with Pulsed Amperometric Detection Method (HPAEC-PAD). International Organization for Standardization: Geneva, Switzerland, 2021.
- Pacquette, L.H.; Thompson, J.J.; Malaviole, I.; Zywicki, R.; Woltjes, F.; Ding, Y.; Mittal, A.; Ikeuchi, Y.; Sadipiralla, B.; Kimura, S.; et al. Minerals and Trace Elements in Milk, Milk Products, Infant Formula, and Adult/Pediatric Nutritional Formula—ICP-MS Method: Collaborative Study, ISO/DIS 21424:2018|IDF 243. J. AOAC Int. 2018, 101, 536–561. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Caleja, C.; Oliveira, M.B.P.; Pereira, E.; Barros, L. Reuse of fruits and vegetables biowaste for sustainable development of natural ingredients. Food Biosci. 2023, 101, 102711. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, J.; Cong, J.; Zhang, T.; Lei, H.; Xu, H.; Li, M. The road to reuse of walnut by-products: A comprehensive review of bioactive compounds, extraction and identification methods, biomedical and industrial applications. Trends Food Sci. Technol. 2023, 143, 104264. [Google Scholar] [CrossRef]
- de Francisco, L.; Pinto, D.; Rosseto, H.; Toledo, L.; Santos, R.; Tobaldini-Valério, F.; Rodrigues, F. Evaluation of radical scavenging activity, intestinal cell viability and antifungal activity of Brazilian propolis by-product. Food Res. Int. 2018, 105, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.S.; Finger, D.; Felsner, M.L.; Torres, Y.R. In-house validation of an HPLC-DAD method for the simultaneous determination of specific bioactive compounds in southern Brazilian propolis. J. Apic. Res. 2019, 58, 104–113. [Google Scholar] [CrossRef]
- Hernández-Martínez, J.A.; Zepeda-Bastida, A.; Morales-Rodríguez, I.; Fernández-Luqueño, F.; Campos-Montiel, R.; Hereira-Pacheco, S.E.; Medina-Pérez, G. Potential Antidiabetic Activity of Apis mellifera Propolis Extraction Obtained with Ultrasound. Foods 2024, 13, 348. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, C.; Yang, Z.; Jia, G.; Liu, X.; Li, X.; Li, A. Simultaneous determination of 20 phenolic compounds in propolis by HPLC-UV and HPLC-MS/MS. J. Food Compos. Anal. 2023, 115, 104877. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid.-Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; Mohamed, S.A. Propolis applications in food industries and packaging. Biomass Convers. Biorefinery 2023, 14, 13731–13746. [Google Scholar] [CrossRef]
- Greene, C.A.; Thirumalai, K.; Kearney, K.A.; Delgado, J.M.; Schwanghart, W.; Wolfenbarger, N.S.; Blankenship, D.D. The climate data toolbox for MATLAB. Geochem. Geophys. Geosystems 2019, 20, 3774–3781. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Custódio, A.R.; Ferreira, M.M.; Bankova, V.S.; García-Viguera, C.; Bretz, W.A. Evaluation of phenolic compounds in Brazilian propolis from different geographic regions. Z. Naturforschung C 2000, 55, 76–81. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Sawaya, A.C.H.F.; Custodio, A.R.; Paulino, N.; Eberlin, M.N. HPLC and ESI-MS typification: New approaches for natural therapy with Brazilian propolis. In Scientific Evidence of the Use of Propolis in Ethnomedicine; 2008; Transworld Research Network: Kerala, India; pp. 33–54. [Google Scholar]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Sung, J.; Lee, J. Antioxidant and antiproliferative activities of grape seeds from different cultivars. Food Sci. Biotechnol. 2010, 19, 321–326. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Hejtmánková, K.; Horníčková, Š.; Pivec, V.; Skala, O.; Přibyl, J. Towards complex utilisation of winemaking residues: Characterisation of grape seeds by total phenols, tocols and essential elements content as a by-product of winemaking. Ind. Crops Prod. 2013, 49, 445–453. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Cedeño-Pinos, C.; Marcucci, M.C.; Bañón, S. Contribution of green propolis to the antioxidant, physical, and sensory properties of fruity jelly candies made with sugars or fructans. Foods 2021, 10, 2586. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Olegário, L.S.; Andrade, J.K.S.; Andrade, G.R.S.; Denadai, M.; Cavalcanti, R.L.; da Silva, M.A.A.P.; Narain, N. Chemical characterization of four Brazilian brown propolis: An insight in tracking of its geographical location of production and quality control. Food Res. Int. 2019, 123, 481–502. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.S.; Cruz, J.N.; Ferreira, O.O.; Pereira, D.S.; Pereira, N.S.; Oliveira, M.E.C.; Andrade, E.H.D.A. Chemical composition of volatile compounds in Apis mellifera propolis from the northeast region of Pará state, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef]
- Da Silva Mendonça, L.; Frota, V.M.; Pinto, B.J.F.; dos Santos Moita, E.C.; da Hora, J.P.; Costa, M.F.; Rodrigues, T.H.S. Seasonality in the volatile oil composition of Green Propolis from the Caatinga Biome. Rev. Bras. Farmacogn. 2021, 31, 497–501. [Google Scholar] [CrossRef]
- Sales, A.; Felipe, L.D.O.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Weimer, P.; Kreutz, T.; Limberger, R.P.; Rossi, R.C.; de Lima, Á.A.; Veiga, V.F., Jr.; de Araújo, B.V.; Koester, L.S. Correlation between the skin permeation profile of the synthetic sesquiterpene compounds, beta-caryophyllene and caryophyllene oxide, and the antiedematogenic activity by topical application of nanoemulgels. Biomolecules 2022, 12, 1102. [Google Scholar] [CrossRef]
- Melliou, E.; Stratis, E.; Chinou, I. Volatile constituents of propolis from various regions of Greece–Antimicrobial activity. Food Chem. 2007, 103, 375–380. [Google Scholar] [CrossRef]
- Kamatou, G.; Sandasi, M.; Tankeu, S.; van Vuuren, S.; Viljoen, A. Headspace analysis and characterisation of South African propolis volatile compounds using GCxGC–ToF–MS. Rev. Bras. De Farmacogn. 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Benvenuti, S. Headspace solid-phase microextraction-gas chromatography–mass spectrometry characterization of propolis volatile compounds. J. Pharm. Biomed. Anal. 2013, 84, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Quintino, R.L.; Reis, A.C.; Fernandes, C.C.; Martins, C.H.G.; Colli, A.C.; Crotti, A.E.M.; Squarisi, I.S.; Ribeiro, A.B.; Tavares, D.C.; Miranda, M.L.D. Brazilian Green Propolis: Chemical Composition of Essential Oil and Their In Vitro Antioxidant, Antibacterial and Antiproliferative Activities. Braz. Arch. Biol. Technol. 2020, 63, e20190408. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, Z.H.; Guo, X.F.; Hu, X.S.; Wu, J.H. Geographical origin identification of propolis using GC–MS and electronic nose combined with principal component analysis. Food Res. Int. 2013, 51, 813–822. [Google Scholar] [CrossRef]
- Miguel, M.G.; Figueiredo, A.C. Propolis and Geopropolis Volatiles. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 113–136. [Google Scholar]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Day, L.; Swanson, B.G. Functionality of protein-fortified extrudates. Compr. Rev. Food Sci. Food Saf. 2013, 12, 546–564. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Binkoski, A.E.; Zhao, G.; Coval, S.M.; Clemmer, K.F.; Hecker, H.D.; Etherton, T.D. Dietary fat: Assessing the evidence in support of a moderate-fat diet; the benchmark based on lipoprotein metabolism. Proc. Nutr. Soc. 2002, 61, 287–298. [Google Scholar] [CrossRef]
- Pant, K.; Thakur, M.; Chopra, H.K.; Dar, B.N.; Nanda, V. Assessment of fatty acids, amino acids, minerals, and thermal properties of bee propolis from Northern India using a multivariate approach. J. Food Compos. Anal. 2022, 111, 104624. [Google Scholar] [CrossRef]
- Welch, R.M. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 2004, 22, 491–502. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Propolis Residue 2,3 |
|---|---|
| Gallic acid | 7.49 ± 0.89 a |
| Neochlorogenic acid | 207.67 ± 19.32 b |
| Delphinidin 3-galactoside | n.d. 1 |
| Catechin | n.d. |
| Procyanidin B2 | n.d. |
| Chlorogenic acid | 497.93 ± 28.33 c |
| 4-Hydroxybenzoic acid | 14.37 ± 1.05 a |
| Epicatechin | n.d. |
| Cyanidin-3-glucoside | 0.14 ± 0.01a |
| Petunidin-3-glucoside | n.d. |
| 3-Hydroxy benzoic acid | n.d. |
| Caffeic acid | 115.11 ± 6.51 a |
| Vanillic acid | 2.69 ± 0.08 a |
| Pelargonidin-3-glucoside | n.d. |
| Pelargonidin-3-rutinoside | n.d. |
| Malvidin-3-galactoside | n.d. |
| Syringic acid | n.d. |
| Procyanidin A2 | n.d. |
| P-Coumaric acid | 637.80 ± 27.35 d |
| Ferulic acid | n.d. |
| Rutin | 5.59 ± 0.47 a |
| Hyperoside | 104.16 ± 5.61 a |
| Isoquercitrin | 34.75 ± 2.30 a |
| Delphindin 3,5 diglucoside | 34.56 ± 3.17 a |
| Phloridzin | n.d. |
| Naringin | n.d. |
| Quercitrin | 0.82 ± 0.04 a |
| Myricetin | n.d. |
| Kaempferol-3-glucoside | 5.70 ± 0.32 a |
| Hesperidin | n.d. |
| Ellagic acid | 23.29 ± 2.40 a |
| Trans-cinnamic acid | 17.13 ± 0.80 a |
| Quercetin | 31.21 ± 29.94 a |
| Phloretin | n.d. |
| Kaempferol | 295.82 ± 11.67 e |
| Isorhamnetin | 5.91 ± 0.39 a |
| TOTAL | 2041.34 |
| Artepillin-C | 56.56 ± 1.75 mg/kg |
| Total Phenolic Content (TPC) mg GAE/kg DW | Total Flavonoid Content (TFC) mg RE/kg DW | Antioxidant Activity (DPPH) mg TE/kg DW | |
|---|---|---|---|
| Propolis Residue | 16,726.67 ± 36.07 a | 6802.96 ± 32.40 b | 2921.61 ± 24.04 c |
| RT 1,2 | Compounds | RI | RI lit | % |
|---|---|---|---|---|
| 7.286 | Ethyl acetate | 881.4193 | 884 | 1.21 |
| 7.458 | Diethyl acetal | 890.7367 | 898 | 5.03 |
| 9.355 | Ethyl 2-methylpropanoate | 964.9116 | 960 | 1.57 |
| 9.689 | Isobutanal diethyl acetal | 977.4727 | 976 | 0.24 |
| 10.853 | α-pinene | 1020.207 | 1020 | 20.09 |
| 11.273 | Ethyl butanoate | 1035.229 | 1040 | 2.28 |
| 11.787 | Ethyl 2-methylbutanoate | 1053.612 | 1057 | 0.59 |
| 12.232 | Ethyl 3-methylbutanoate | 1069.528 | 1072 | 0.34 |
| 13.346 | β-pinene | 1108.049 | 1108 | 6.79 |
| 14.647 | 3-carene | 1148.018 | 1146 | 6.20 |
| 15.08 | β-myrcene | 1161.321 | 1160 | 0.39 |
| 15.161 | α-phellandrene | 1163.81 | 1164 | 0.76 |
| 15.615 | α-Terpinene | 1177.757 | 1176 | 0.38 |
| 16.206 | D-limonene | 1195.914 | 1196 | 7.60 |
| 16.514 | β-phellandrene | 1205.686 | 1201 | 0.87 |
| 17.623 | γ-terpinene | 1241.715 | 1243 | 0.92 |
| 17.739 | cis-β-Ocimene | 1245.484 | 1245 | 1.19 |
| 17.88 | styrene | 1250.065 | 1250 | 0.16 |
| 18.356 | o-cymene | 1265.53 | 1268 | 6.34 |
| 23.147 | acetic acid | 1434.643 | 1433 | 0.28 |
| 23.747 | α-cubebene | 1457.535 | 1458 | 0.67 |
| 24.479 | ylangene | 1485.464 | 1485 | 1.38 |
| 24.727 | copaene | 1494.926 | 1493 | 6.93 |
| 25.301 | benzaldehyde | 1517.978 | 1520 | 0.38 |
| 25.438 | (-)-β-bourbonene | 1523.563 | 1517 | 1.60 |
| 27.442 | caryophyllene | 1605.558 | 1604 | 9.76 |
| 27.699 | aromandendrene | 1616.631 | 1610 | 4.64 |
| 28.628 | alloaromadendrene | 1656.657 | 1662 | 0.83 |
| 29.181 | humulene | 1680.483 | 1681 | 1.39 |
| 29.536 | γ-muurolene | 1695.778 | 1695 | 3.99 |
| 30.315 | α-muurolene | 1730.814 | 1727 | 2.51 |
| 31.189 | γ-cadinene | 1770.362 | 1767 | 2.10 |
| 34.567 | α-calacorene | 1929.507 | 1936 | 0.59 |
| Nutrients | ||
|---|---|---|
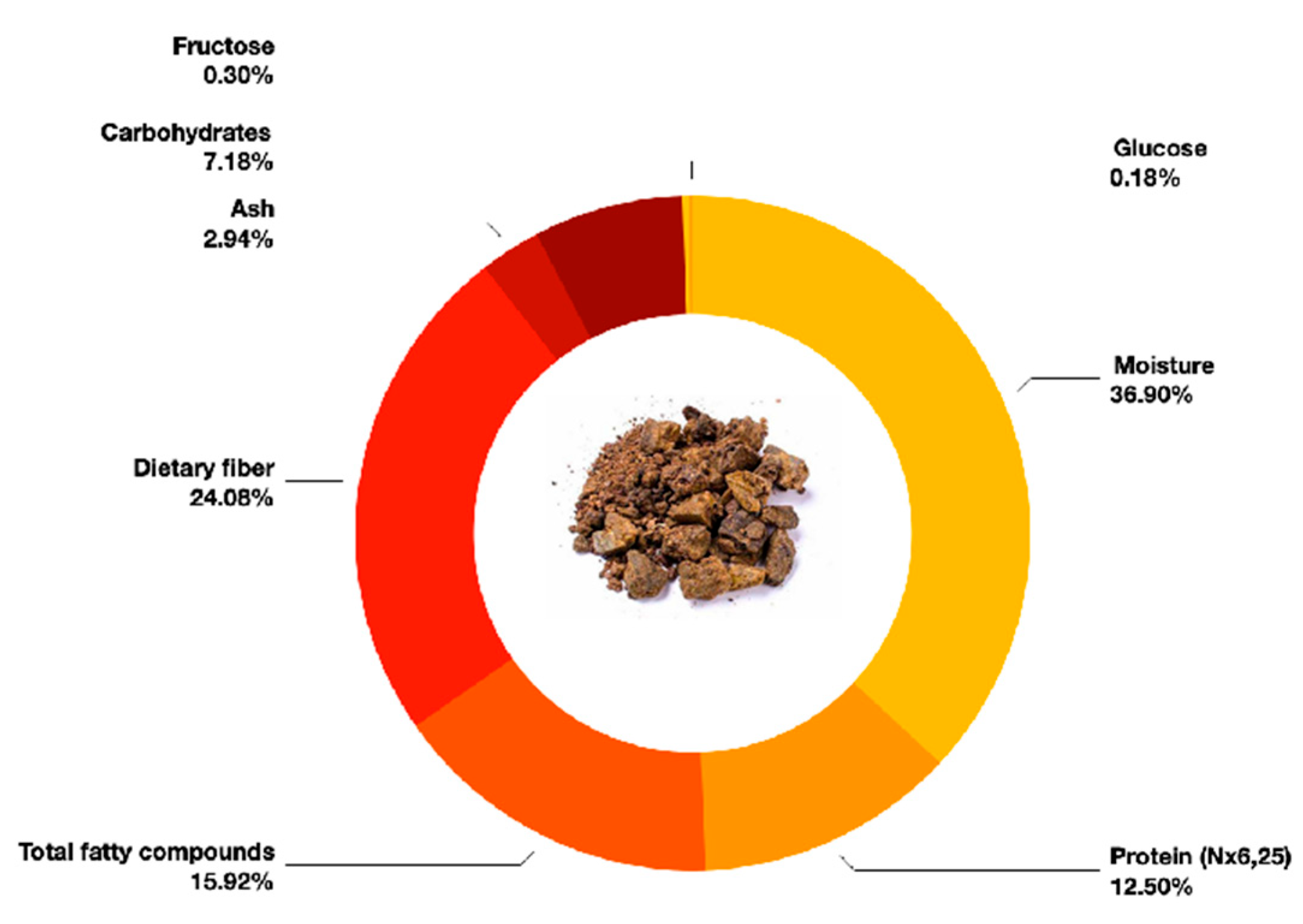
| Moisture | g/100 g | 37.08 ± 1.07 i |
| Protein (Nx6,25) | g/100 g | 12.56 ± 0.75 m |
| Total fatty compounds | g/100 g | 16.0 ± 1.3 l |
| Dietary fiber | g/100 g | 24.2 ± 3.9 k |
| Ash | g/100 g | 2.95 ± 0.20 p |
| Carbohydrates | g/100 g | 7.21 ± 4.32 n |
| Fructose | g/100 g | 0.299 ± 0.06 |
| Glucose | g/100 g | 0.183 ± 0.04 |
| Sucrose | g/100 g | n.d. 1 |
| Maltose | g/100 g | n.d. |
| Lactose | g/100 g | n.d. |
| Total Sugars | g/100 g | 0.482 ± 0.07 q |
| Energy value | kCal/100 g | 271 ± 12 f |
| kJ/100 g | 1122 ± 48 e | |
| Minerals | ||
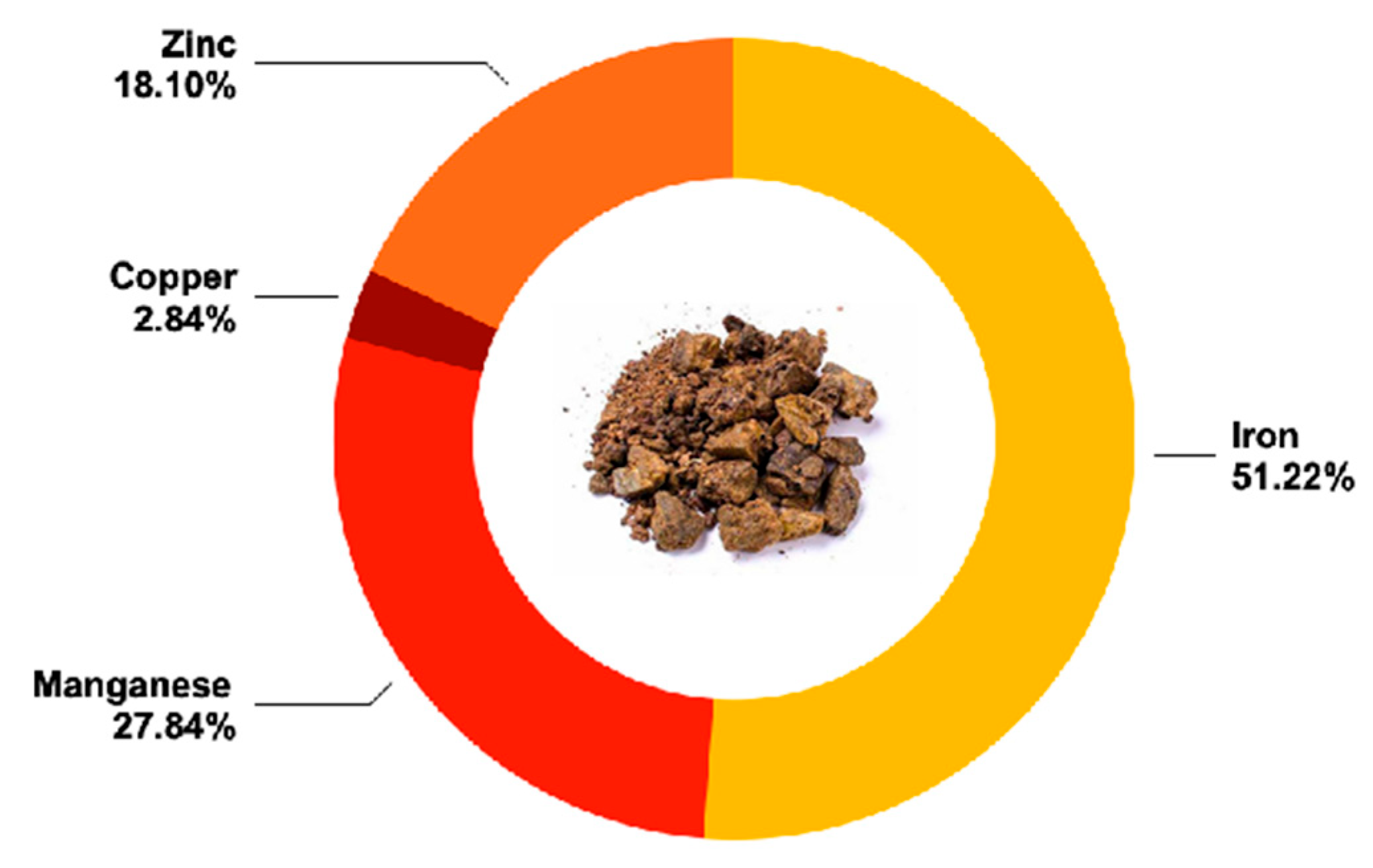
| Calcium | mg/kg | 2100 ± 290 c |
| Iron | mg/kg | 92 ± 18 g |
| Phosphorus | mg/kg | 2520 ± 470 b |
| Magnesium | mg/kg | 1210 ± 230 d |
| Manganese | mg/kg | 50 ± 11 h |
| Potassium | mg/kg | 9800 ± 1900 g a |
| Copper | mg/kg | 5.1 ± 1.1 |
| Selenium | mg/kg | n.d. |
| Sodium | mg/kg | n.d. |
| Zinc | mg/kg | 32.5 ± 6.3 j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santanatoglia, A.; Acquaticci, L.; Marcucci, M.C.; Maggi, F.; Oliveira, C.R.; Caprioli, G. Valorizing Brazilian Propolis Residue: Comprehensive Characterization for Sustainable Reutilization Strategies. Plants 2025, 14, 1989. https://doi.org/10.3390/plants14131989
Santanatoglia A, Acquaticci L, Marcucci MC, Maggi F, Oliveira CR, Caprioli G. Valorizing Brazilian Propolis Residue: Comprehensive Characterization for Sustainable Reutilization Strategies. Plants. 2025; 14(13):1989. https://doi.org/10.3390/plants14131989
Chicago/Turabian StyleSantanatoglia, Agnese, Laura Acquaticci, Maria Cristina Marcucci, Filippo Maggi, Carlos Rocha Oliveira, and Giovanni Caprioli. 2025. "Valorizing Brazilian Propolis Residue: Comprehensive Characterization for Sustainable Reutilization Strategies" Plants 14, no. 13: 1989. https://doi.org/10.3390/plants14131989
APA StyleSantanatoglia, A., Acquaticci, L., Marcucci, M. C., Maggi, F., Oliveira, C. R., & Caprioli, G. (2025). Valorizing Brazilian Propolis Residue: Comprehensive Characterization for Sustainable Reutilization Strategies. Plants, 14(13), 1989. https://doi.org/10.3390/plants14131989










