In Vitro Polyploidy Induction of Longshan Lilium lancifolium from Regenerated Shoots and Morphological and Molecular Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Induction of Longshan L. lancifolium Shoot Regeneration
2.3. Colchicine-Induced Variations in Regenerated Shoots
2.4. Stable Cultivation of Variant Organs
2.5. Identification of Chromosome Ploidy in Mutant Plantlets
2.6. Morphological Observation of Polyploid Plantlets
2.7. ISSR Molecular Marker Analysis
2.8. Statistical Analysis
3. Results
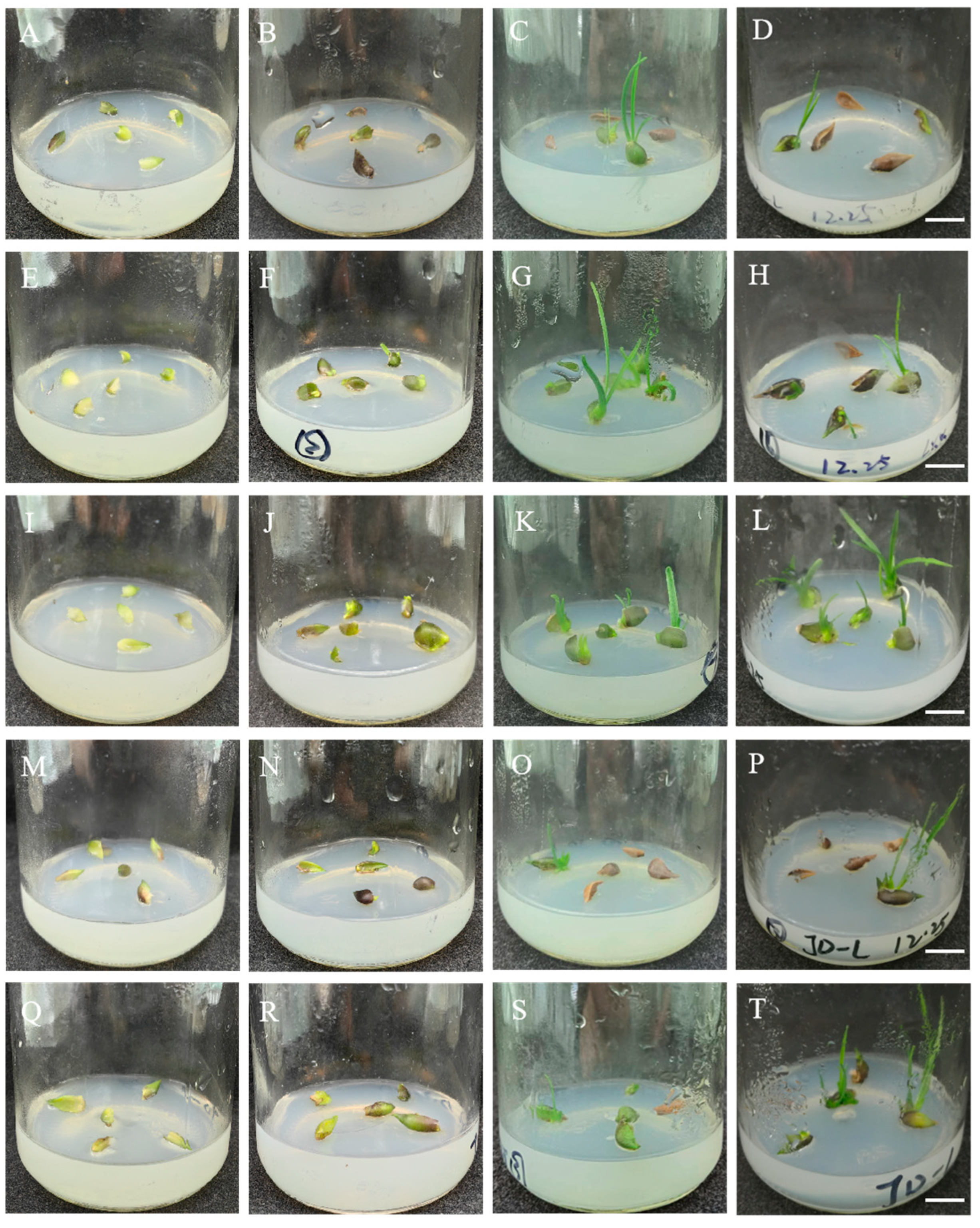
3.1. Induction of Longshan L. lancifolium Regenerated Shoots
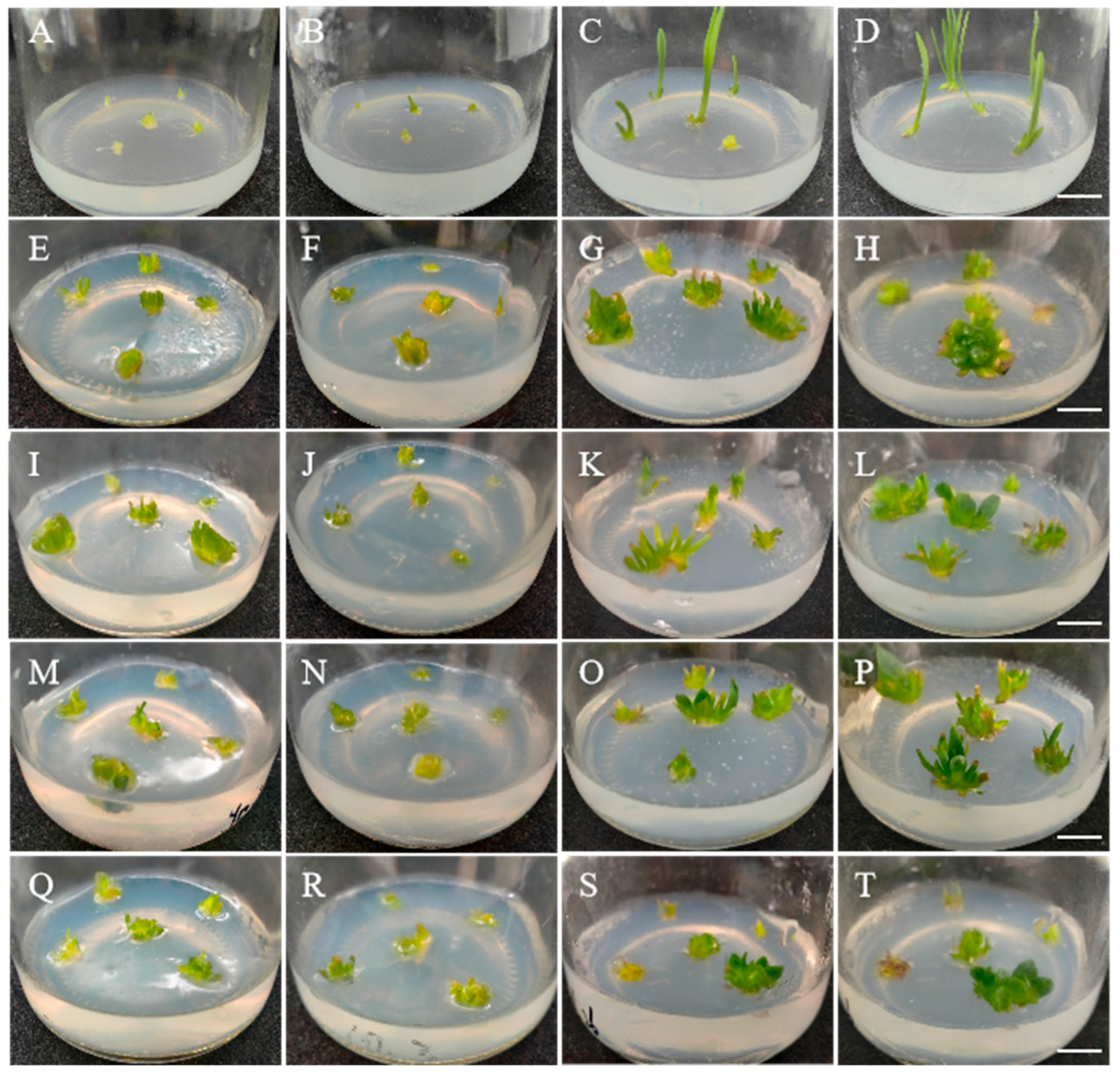
3.2. Colchicine-Induced Variations in Longshan L. lancifolium Shoots
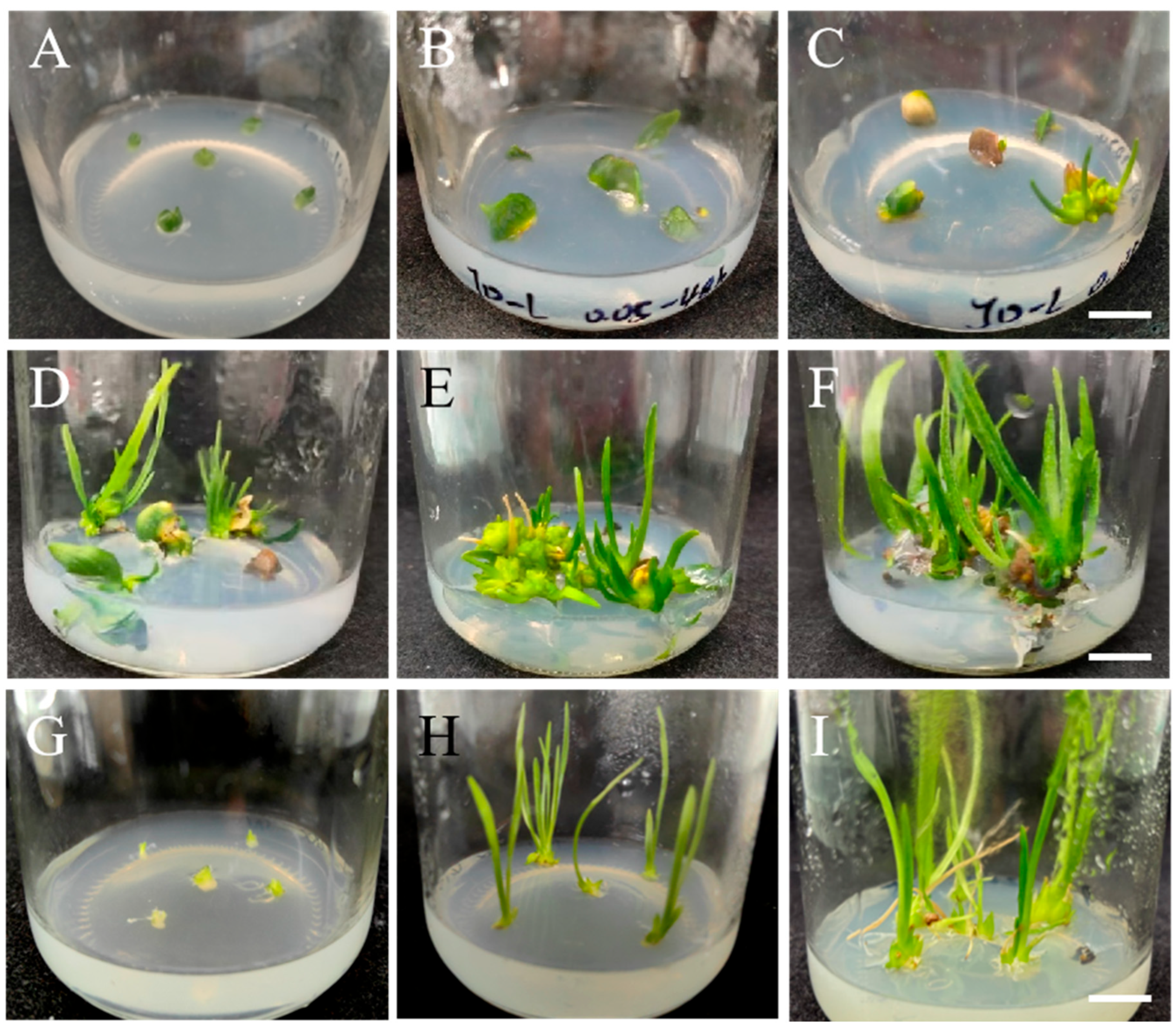
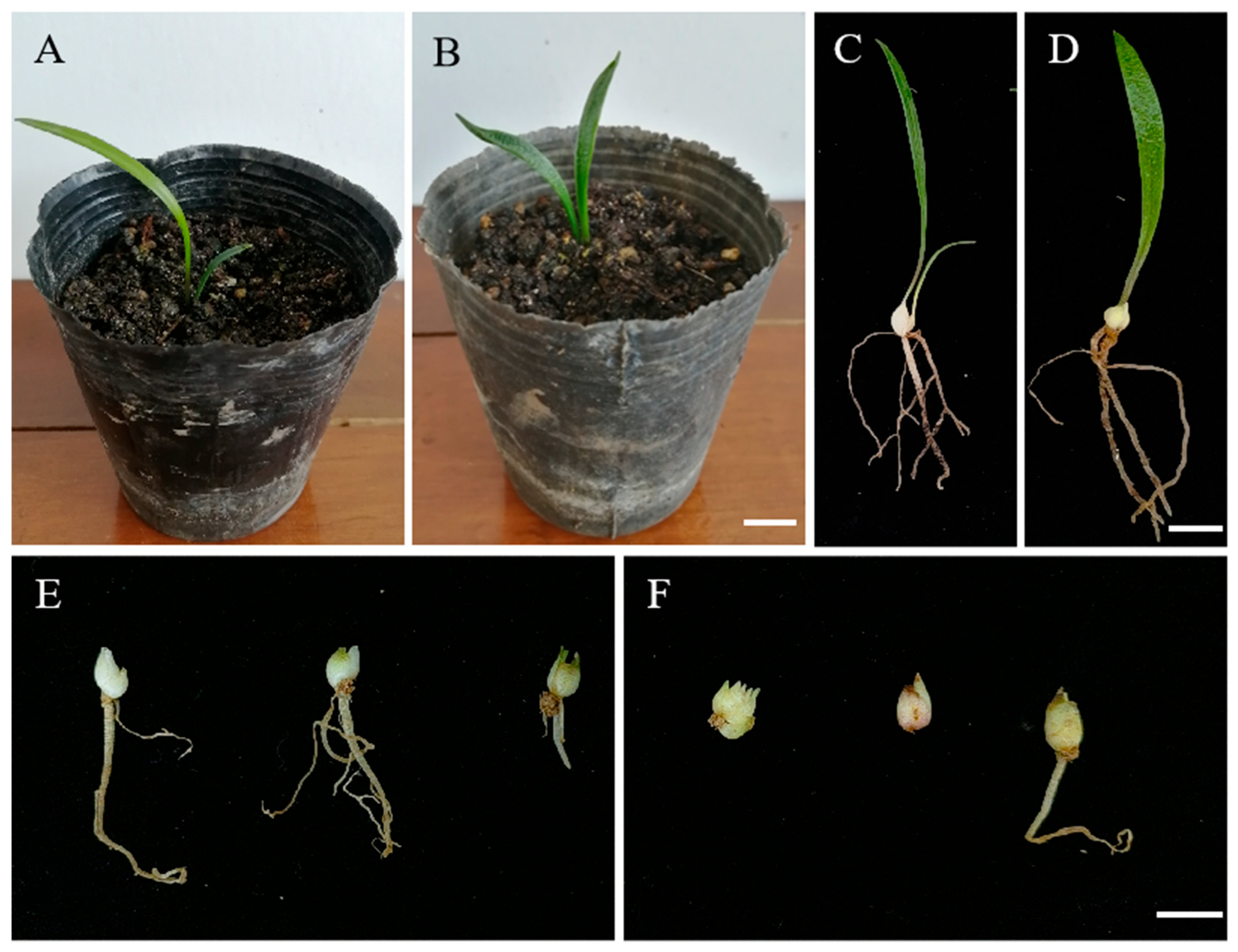
3.3. Stable Cultivation and Generation of Mutant Plantlets
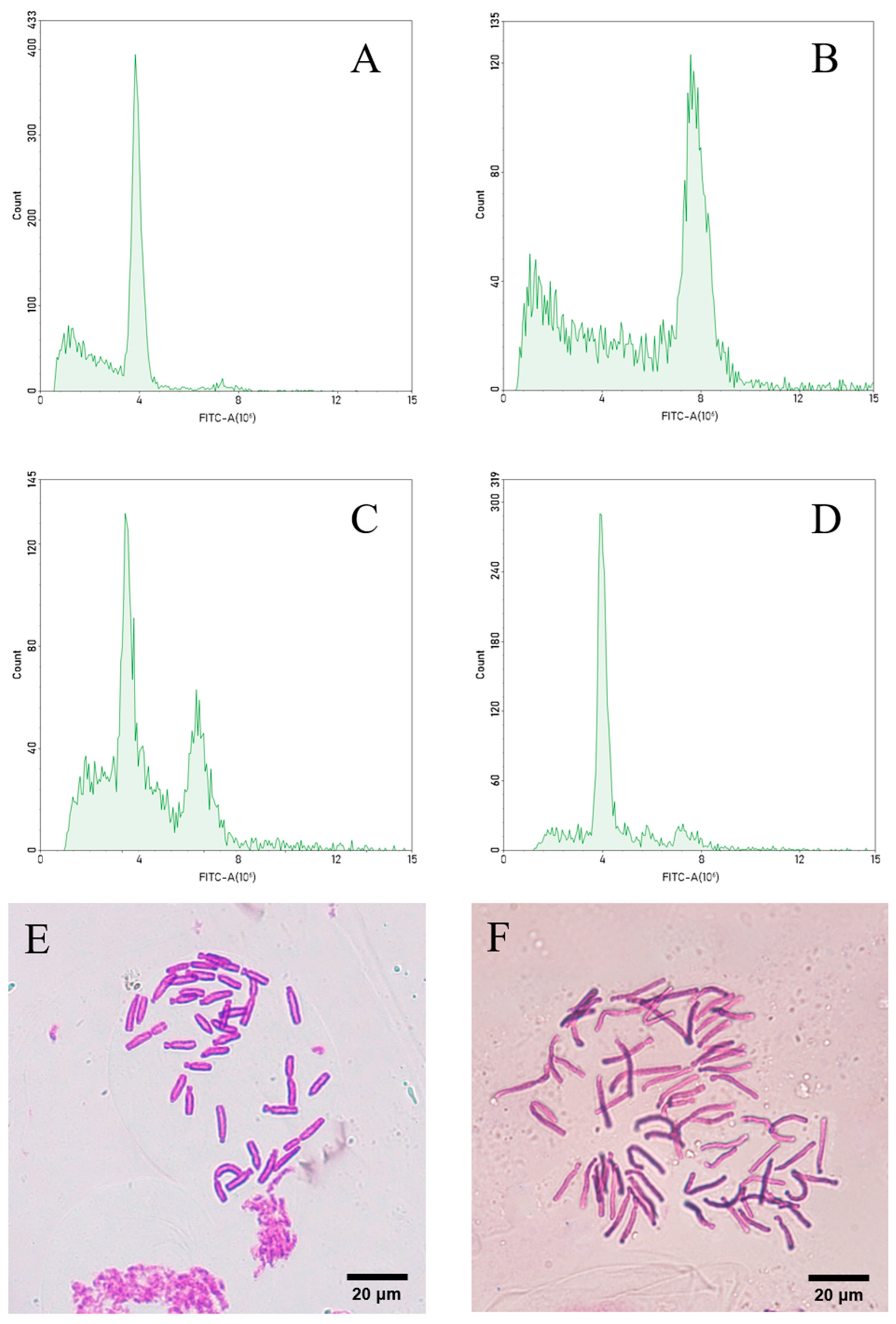
3.4. Identification of Longshan L. lancifolium Polyploids
3.5. Morphological Observation of Tissue-Cultured ‘JD-12’ Plantlets
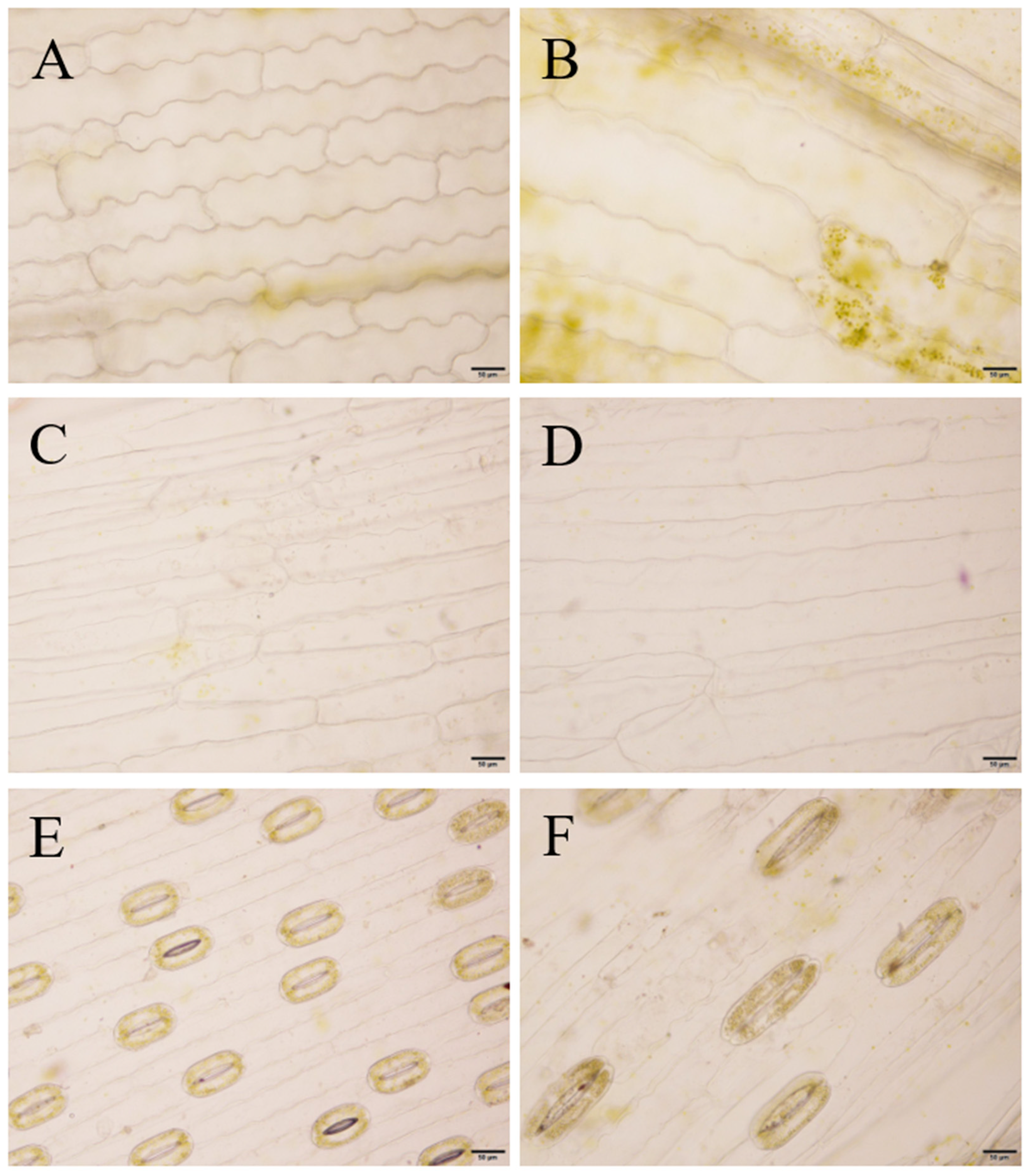
3.6. Phenotypic and Cellular Observations of ‘JD-12’ Plants Grown in a Greenhouse
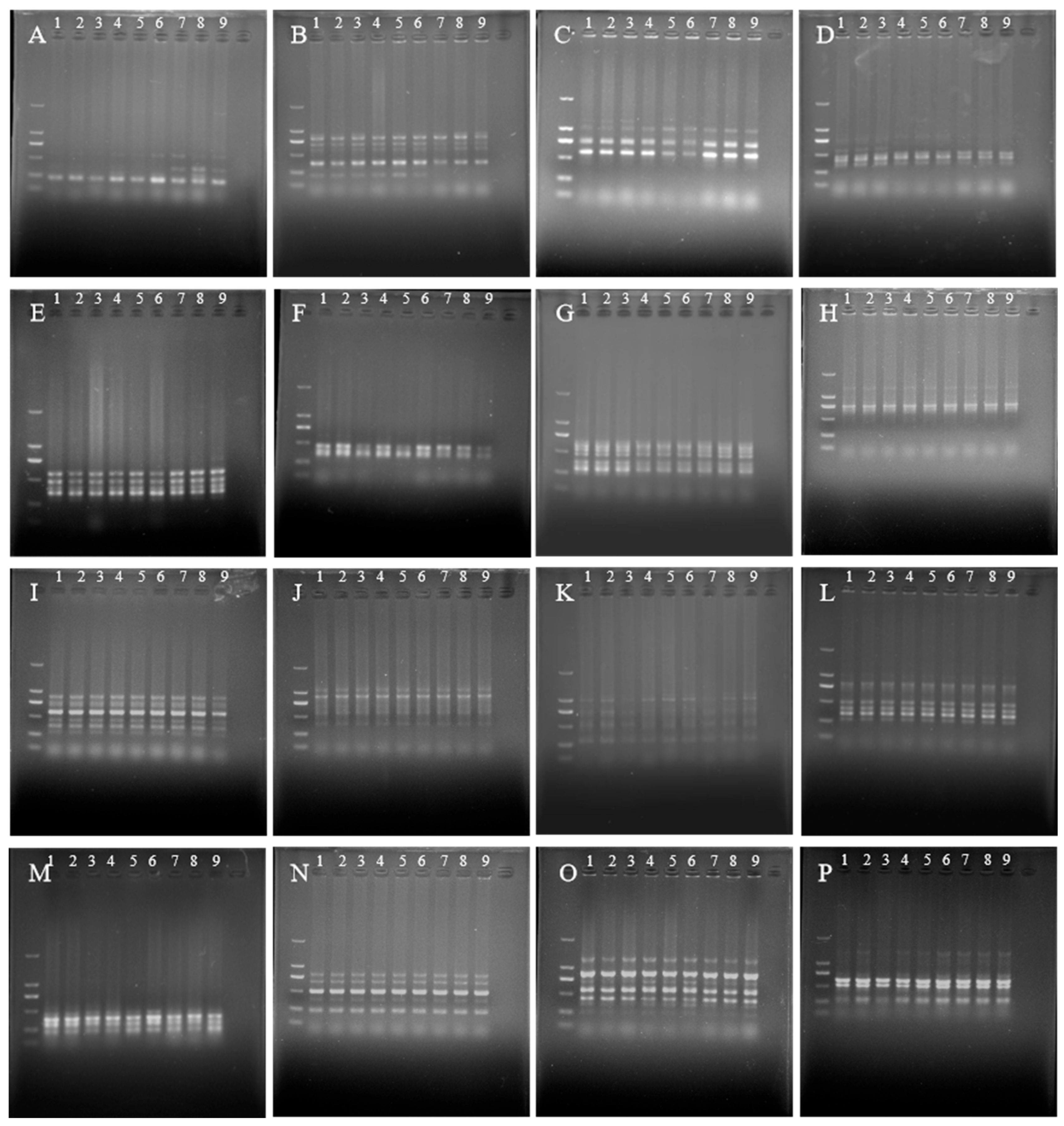
3.7. ISSR Marker Detection in ‘JD-12’ Plantlets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, F.Z.; Tang, J. Flora of China; Science Press: Beijing, China, 1980. [Google Scholar]
- Zhao, X.Y.; Wang, W.H. Status and Development Proposals of Lily Industry in China[M]//Ming J, Yuan SX. Papers (Abstracts) of the 12th Symposium of Chinese Society of Flower Bulbs; Beijing Zhonglv Garden Science Research Institute: Beijing, China, 2017; pp. 61–69. [Google Scholar]
- Luo, C.Y.; Yuan, Z.T.; Liu, H.J.; Yuan, Y.; Zheng, S.X. Research progress in the utilization of medicinal lily germplasm resources. Hunan Agric. Sci. 2024, 98–102. [Google Scholar] [CrossRef]
- Yang, L.P.; Fu, Y.Y. Research on the Utilization of Lily Resources in China; Northeast Forestry University Press: Harbin, China, 2018. [Google Scholar]
- Yang, L.P.; Song, X.H. Tissue culture system construction of Lilium lancifolium Thunb. J. Agric. Univ. Hebei 2013, 36, 17–21, 30. [Google Scholar]
- Wang, Y.L.; He, W.R.; Wang, S.H.; Hu, L.Q.; Yu, X.L.; Li, J.G. Industry status and development direction of Longshan medicinal lily. Hunan Agric. Sci. 2023, 12, 65–68. [Google Scholar] [CrossRef]
- Pan, Q.P.; Zhou, R.B.; He, Y.S.; Luo, Y.L. Investigation of lily bulb implantation garden in Longshan County. Hunan Guid. J. TCM 2003, 56–57. [Google Scholar]
- Yang, Q. Lily Cultivation, Yield and Quality Improving Policy and Technology in Longshan County. Master’s Thesis, Hunan Agriculture University, Changsha, China, 2014. [Google Scholar]
- Lei, L.H. Antioxidant Capacity and Lung Cancer Cell Rejection Characterization of Lilium lancifolium Bulbs from Different Populations. Master’s Thesis, Northwest A&F University, Xianyang, China, 2015. [Google Scholar]
- Wang, C.L.; Yuan, C.Z.; Chen, H.X.; Jiang, H. Detection of CMV infecting on Longshan Lilium tigrinum. Tianjin Agric. Sci. 2015, 21, 15–18. [Google Scholar]
- Jiang, H.; Yuan, C.Z.; Chen, H.X. Detection of LSV infecting Longshan Lilium tigrinum. Tianjin Agric. Sci. 2014, 20, 27–30. [Google Scholar]
- Fu, Y.Y.; Yang, L.P.; Gao, H.H.; Xu, W.J.; Lei, M.Y.; Wang, A.X. Virus detection of Lilium lancifolium Thunb. in Chongqing and study on its detoxification technology. Acta Agric. Boreali-Occident. Sin. 2020, 29, 1068–1077. [Google Scholar]
- Hu, Y.; Li, H.G.; Lei, X.Y.; Zhou, Y.J.; Zhang, Y.; Li, L.H. Study on mutagenic effects of 60Co-γray and chemical mutagen on Lilium lancifolium. Acta Agric. Jiangxi 2022, 34, 58–63. [Google Scholar]
- Han, X.; Yang, L.Y.; Chen, M.M.; Li, X.; Yang, Y.Y.; Zhang, Y.C. Research progress of the techniques applicable in lily breeding. J. Plant Genet. Resour. 2024, 25, 763–776. [Google Scholar]
- Yamagishi, M.; Jitsuyama, Y.; Hoshino, Y. Agronomic performance in tetraploid Lilium leichtlinii: Larger flowers and earlier flowering. Euphytica 2023, 219, 126. [Google Scholar] [CrossRef]
- Wu, X.J.; Yang, L.P.; Chen, M. In vitro polyploid induction of Lilium callosum. Guizhou Agric. Sci. 2016, 44, 84–86. [Google Scholar]
- Sun, H.M.; Fu, L.L.; Wang, Z.P.; Gai, M.Z.; Wang, C.X. Polyploidy induction and identification of Lilium pumilum and Lilium davidii var. unicolor based on somatic embryogenesis. Acta Hortic. Sin. 2018, 45, 1136–1146. [Google Scholar]
- Wang, Y.T.; Zhang, Y.Q.; Yang, Q.J. Effect of colchicine on polyploid induction of Lilium concolor seeds. Guizhou Agric. Sci. 2019, 47, 5–9. [Google Scholar]
- Fu, Y.Y.; Cai Li Li, F.Y.; Yang, W.J.; Xu, W.J.; Jiang, S.J.; Yang, L.P. Induction and molecular and cellular identification of polyploid Lilium davidii var. unicolor. Acta Pratacult. Sin. 2024, 33, 172–181. [Google Scholar]
- Wang, Z.G.; Yin, D.S.; Zhang, H.H.; Wu, T.T.; Pan, B.S.; Yuan, X.F. Research progress on genetic breeding of Lilium lancifolium Thumb. Hortic. Seed 2014, 1, 1–3, 10. [Google Scholar]
- Shu, K.; Li, J.H.; Chen, L.; Fu, H.Y.; Cai, W.; Liu, L.C.; Li, Y.F. Distant hybridization of Lilium lancifolium Thunb. and identification of its hybrid progeny. J. Hunan Agric. Univ. (Nat. Sci.) 2022, 48, 556–562. [Google Scholar]
- Tang, Y.C.; Yang, P.P.; He, G.R.; Cao, Y.W.; Liu, Y.J.; Xu, L.F.; Ming, J. ITS sequence analysis used for parent selection in Lilium lancifolium Thunb. cross-breeding. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100362. [Google Scholar] [CrossRef]
- Chen, A.; Yang, L.P.; Tan, Y.; Peng, C.T.; Tang, B. Study on polyploid induction of Lilium lancifolium in vitro with colchicine treatment. J. Plant Genet. Resour. 2014, 15, 1385–1389. [Google Scholar]
- Lei, M.Y.; Yang, L.P.; Yang, T.J.; Fu, Y.Y.; Han, L.; Quan, J.; Pu, S.C. Cultivation of a new polyploid Lilium lancifolium variety ‘Yu Baihe 1’. Mol. Plant Breed. 2020, 18, 4714–4724. [Google Scholar]
- Fu, Y.Y.; Yi, D.Y.; Yang, X.M.; Cai, L.; Liang, Y.H.; Lei, M.Y.; Yang, L.P. Analysis of morphological characteristics and genetic variation in a new germplasm Lilium lancifolium JD-h-15. Biotechnol. Bull. 2022, 38, 140–150. [Google Scholar]
- Fu, Y.Y.; Liang, X.M.; Zhang, H.; Cheng, S.Y.; Li, A.Q.; Liao, M.J.; Tan, L.; Yang, L.P.; Qi, X.Y. Establishment of an efficient regeneration system and in vitro polyploid induction based on the bulblet centre in Lilium rosthornii Diels. In Vitro Cell. Dev. Biol.-Plant 2024, 60, 508–522. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhou, Y.; Sun, Y.J.; Li, X.; Sun, J.J. Polyploidy induction of Lilium spp. by colchicine and ploidy identification by flow cytometry. Acta Agric. Shanghai 2018, 34, 81–87. [Google Scholar]
- Fu, Y.Y.; Ren, J.J.; Guo, R.Y.; Lu, X.X.; Lei, Y.X.; Xu, W.J. In vitro induction and molecular and cellular identification of mutant plants of Lilium leichtlinii var. Maximowiczii (Regel) Baker by colchicine. Ornam. Plant Res. 2025, 5, e014. [Google Scholar] [CrossRef]
- Wu, Q.Q.; Hu, X.J.; Cui, W.; Yang, L.; Shi, L.J.; Xu, H.J.; Ban, T.T.; Xia, J.F. Study on ployploid induction of lily Yelloween by colchicine. Seeds 2019, 38, 96–100. [Google Scholar]
- Zhang, J.F.; Liu, Q.H.; Wang, K.L.; Liu, Q.C.; Sun, Y. Tetraploid induction of Lilium tsingtauense by colchicine. J. Nucl. Agric. Sci. 2009, 23, 454–457. [Google Scholar]
- Liu, J.; Zhao, Q.F.; Ding, L. Polyploid induction and indentification of Lilium davidii var. unicolor (Hoog) Cotton. North. Hortic. 2011, 18, 138–141. [Google Scholar]
- Yang, Y.J.; Ge, B.B.; Wei, Q.; Gao, J.P.; Hong, B. Colchicines-induced polyploid plants and identification in Lilium pumilum DC. J. China Agric. Univ. 2013, 18, 128–133. [Google Scholar]
- Zhang, X.Q.; Wang, L.J.; Cao, Q.Z.; Jia, G.X. Polyploidy induction and identification in Lilium concolor var. pulchellum. J. Beijing For. Univ. 2017, 39, 96–102. [Google Scholar]
- Wu, H.Z.; Zhang, X.Q.; Zheng, S.X.; Shi, J.F.; Bi, Y.F. Polyploid induction of coloured Zantedeschia aethiopica. Acta Hortic. Sin. 2008, 35, 443–446. [Google Scholar]
- Yang, H.; Gou, H.; Zhou, D.; Du, T.; Wu, H.Z. Studies on polyploid induction of different varieties of the colored Zantedeschia hybrid. J. Yunnan Agric. Univ. 2014, 29, 229–234. [Google Scholar]
- Fu, L.L.; Zhu, Y.Y.; Li, M.; Wang, C.X.; Sun, H.M. Autopolyploid induction via somatic embryogenesis in Lilium distichum Nakai and Lilium cernuum Komar. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 139, 237–248. [Google Scholar] [CrossRef]
- Okazaki, K.; Hane, Y. Comparison of diploid and chimeric forms (4×/2×) of Asiatic hybrid lilies (Lilium spp.) under natural and early forcing culture. N. Z. J. Exp. Agric. 2005, 33, 261–267. [Google Scholar]
- Wang, L.Y.; Jing, R.Y. Tetraploid induction of Lilium davidii var. unicolor through colchicine treatment. J. Nucl. Agric. Sci. 2008, 22, 581–584. [Google Scholar]
- Wang, M.L.; Li, X.T.; Wang, W.H.; Du, F. Ploidy identification of 100 lily accessions based on stomatal characteristics. Acta Agric. Jiangxi 2021, 33, 27–33. [Google Scholar]
- Mallick, P.; Chattaraj, S.; Sikdar, S.R. Molecular characterizations of somatic hybrids developed between Pleurotus florida and Lentinus squarrosulus through inter-simple sequence repeat markers and sequencing of ribosomal RNA-ITS gene. Biotechnology 2017, 7, 298. [Google Scholar] [CrossRef]
- Mcgregor, C.E.; Lambert, C.A.; Greyling, M.M.; Louw, J.H.; Warnich, L. A comparative assessment of DNA fingerprinting techniques (RAPD, ISSR, AFLP and SSR) in tetraploid potato (Solanum tuberosum L.) germplasm. Euphytica 2000, 113, 135–144. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zheng, Z.W.; Zheng, S.X.; Liao, X.S.; Song, Z.W.; Song, R.; Lin, Q.D. Cultivation and identification of allotriploid lily. Mol. Plant Breed. 2022, 20, 6424–6432. [Google Scholar]



| Colchicine Concentration (w/v) | Treatment Duration (h) | Treated Number | Survived Number | Survival Frequency (%) | Number of Morphological Variants | Frequency of Morphological Variants (%) |
|---|---|---|---|---|---|---|
| 0.05% | 24 | 30 | 29 | 96.67 | 16 | 53.33 |
| 48 | 30 | 28 | 93.33 | 20 | 66.67 | |
| 72 | 30 | 24 | 80.00 | 15 | 50.00 | |
| 0.10% | 24 | 30 | 27 | 90.00 | 20 | 66.67 |
| 48 | 30 | 26 | 86.67 | 24 | 80.00 | |
| 72 | 30 | 26 | 86.67 | 23 | 76.67 | |
| 0.15% | 24 | 30 | 28 | 93.33 | 21 | 70.00 |
| 48 | 30 | 25 | 83.33 | 23 | 76.67 | |
| 72 | 30 | 23 | 76.67 | 20 | 66.67 | |
| 0.20% | 24 | 30 | 26 | 86.67 | 22 | 73.33 |
| 48 | 30 | 27 | 90.00 | 17 | 56.67 | |
| 72 | 30 | 21 | 70.00 | 17 | 56.67 |
| Samples | Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) | Leaf Thickness (mm) | Bulblet Diameter (cm) | Scale Length (cm) | Scale Width (cm) | Scale Thickness (mm) |
|---|---|---|---|---|---|---|---|---|
| Control | 7.53 ± 0.12 * | 6.45 ± 0.13 ** | 0.23 ± 0.01 ** | 0.37 ± 0.00 | 0.48 ± 0.02 | 0.52 ± 0.02 | 0.28 ± 0.01 | 1.76 ± 0.07 |
| ‘JD-12’ | 7.01 ± 0.26 | 5.97 ± 0.11 | 0.17 ± 0.01 | 0.76 ± 0.00 ** | 0.60 ± 0.03 ** | 0.51 ± 0.01 | 0.30 ± 0.01 | 2.12 ± 0.10 ** |
| Samples | Upper Epidermis Cell Length (μm) | Upper Epidermis Cell Width (μm) | Cell Length–Width Ratio | Guard Cell Length (μm) | Guard Cell Width (μm) | Stomatal Frequency (No.·mm−2) |
|---|---|---|---|---|---|---|
| Control | 418.07 ± 7.87 | 81.75 ± 1.45 | 5.46 ± 0.18 | 90.73 ± 0.55 | 57.03 ± 0.25 | 29.75 ± 1.49 ** |
| ‘JD-12’ | 569.62 ± 20.33 ** | 91.12 ± 2.40 ** | 7.08 ± 0.37 ** | 146.53 ± 1.97 ** | 70.67 ± 0.76 ** | 9.66 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-Q.; Zhang, H.; Qian, Q.; Cheng, S.-Y.; Lu, X.-X.; Liu, X.-Y.; Han, G.-Q.; Fu, Y.-Y. In Vitro Polyploidy Induction of Longshan Lilium lancifolium from Regenerated Shoots and Morphological and Molecular Characterization. Plants 2025, 14, 1987. https://doi.org/10.3390/plants14131987
Tang Y-Q, Zhang H, Qian Q, Cheng S-Y, Lu X-X, Liu X-Y, Han G-Q, Fu Y-Y. In Vitro Polyploidy Induction of Longshan Lilium lancifolium from Regenerated Shoots and Morphological and Molecular Characterization. Plants. 2025; 14(13):1987. https://doi.org/10.3390/plants14131987
Chicago/Turabian StyleTang, Yu-Qin, Hong Zhang, Qin Qian, Shi-Yuan Cheng, Xiu-Xian Lu, Xiao-Yu Liu, Guo-Qiang Han, and Yong-Yao Fu. 2025. "In Vitro Polyploidy Induction of Longshan Lilium lancifolium from Regenerated Shoots and Morphological and Molecular Characterization" Plants 14, no. 13: 1987. https://doi.org/10.3390/plants14131987
APA StyleTang, Y.-Q., Zhang, H., Qian, Q., Cheng, S.-Y., Lu, X.-X., Liu, X.-Y., Han, G.-Q., & Fu, Y.-Y. (2025). In Vitro Polyploidy Induction of Longshan Lilium lancifolium from Regenerated Shoots and Morphological and Molecular Characterization. Plants, 14(13), 1987. https://doi.org/10.3390/plants14131987





