Potassium Fulvate Alleviates Salt–Alkali Stress and Promotes Comprehensive Growth of Oats in Saline–Alkali Soils of the Qaidam Basin
Abstract
1. Introduction
2. Materials and Methods
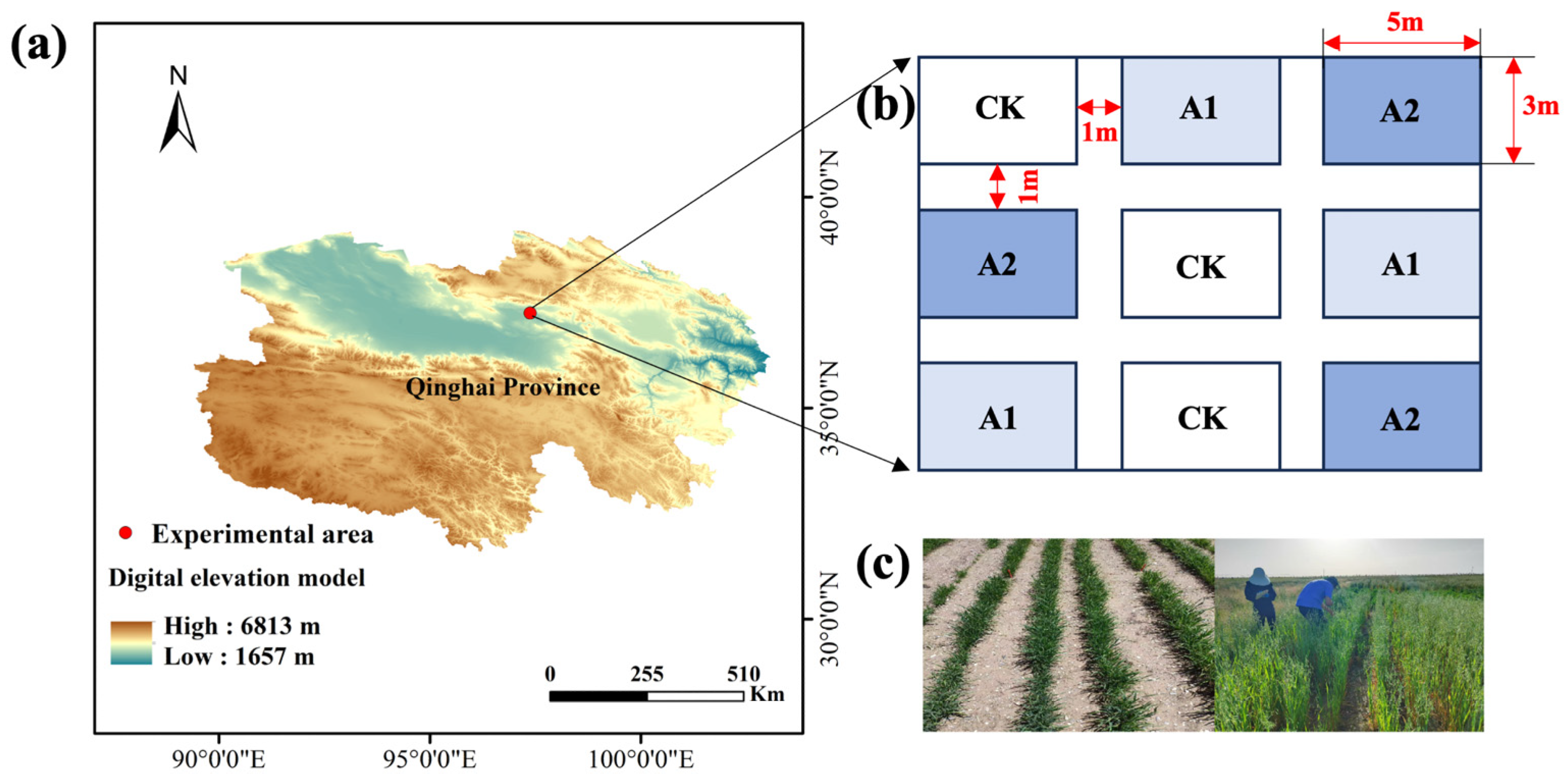
2.1. Experimental Site Description
2.2. Experimental Materials and Design
2.3. Soil and Microbial Sample Collection and Measurement
2.4. Plant and Soil Measurement Indicators and Methods
2.5. Extraction, Amplification, and Sequence Data Processing of Rhizosphere Microorganisms
2.6. Statistical Analysis
3. Results
3.1. Effects of Potassium Fulvate Application on Oat Growth
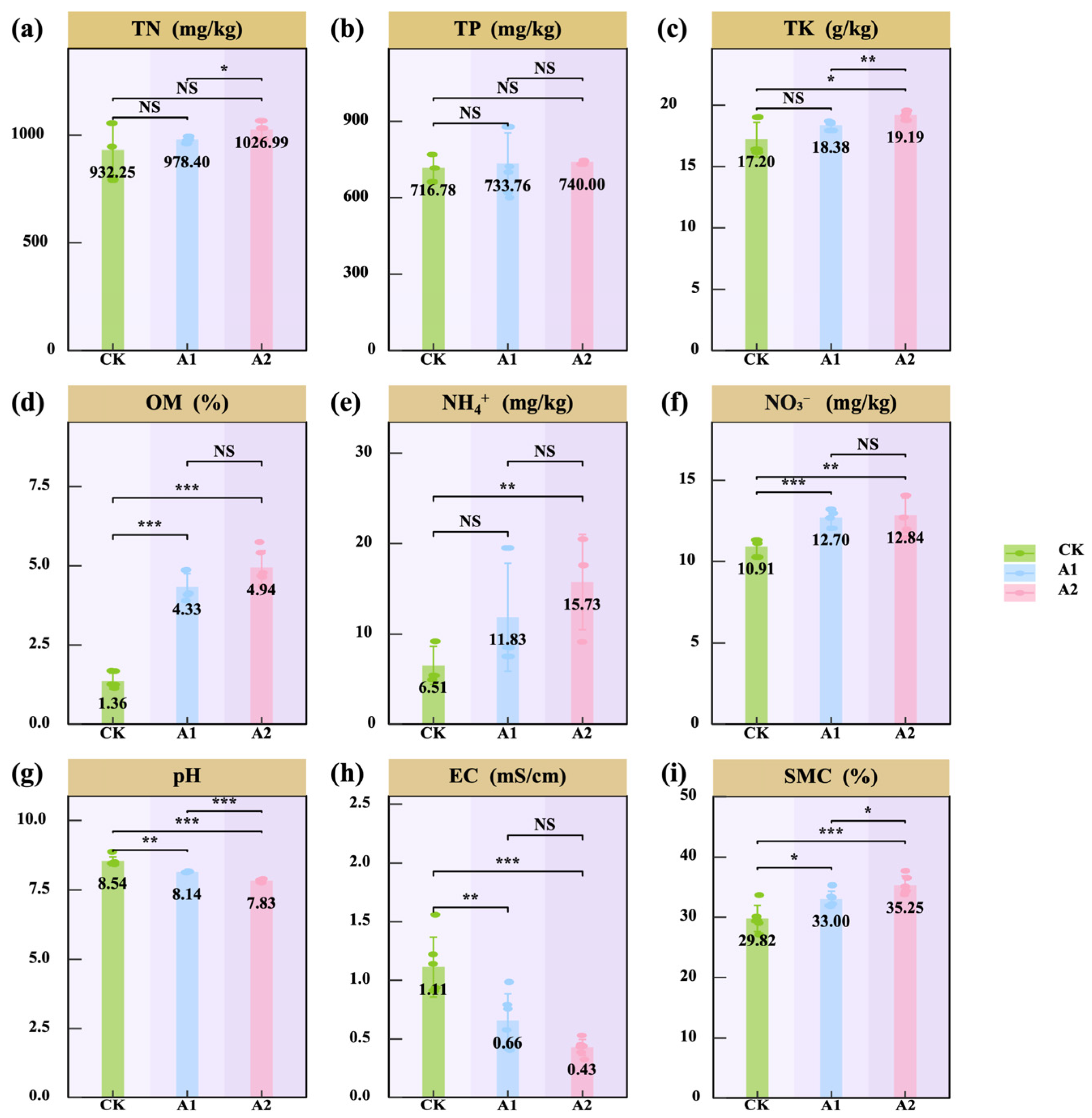
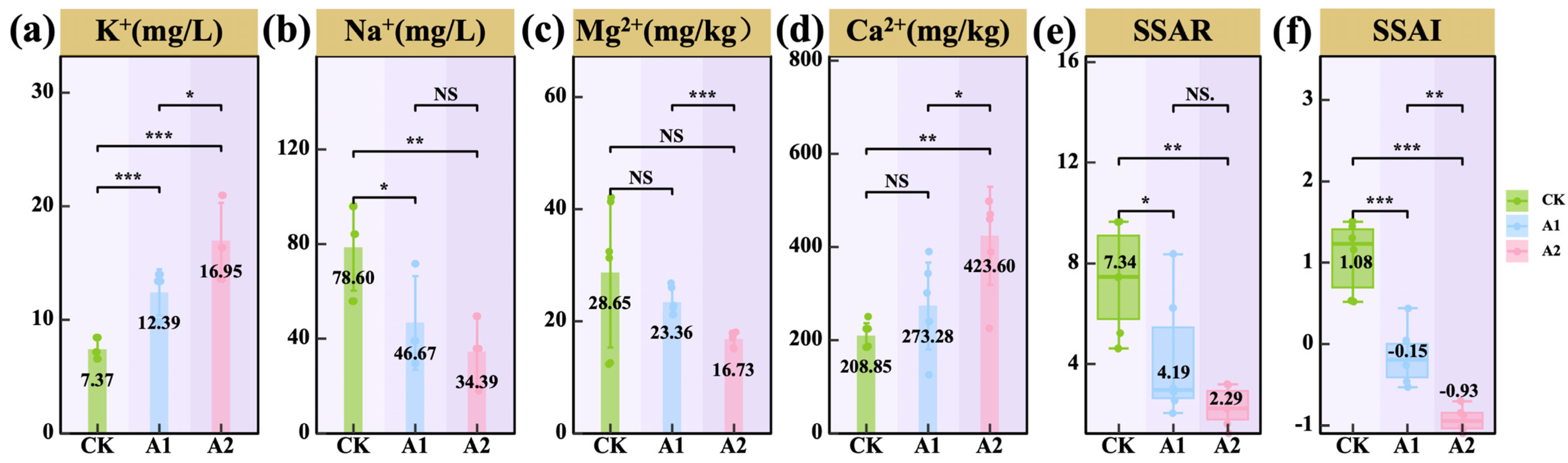
3.2. Effects of Potassium Fulvate Application on Rhizosphere Soil of Oats
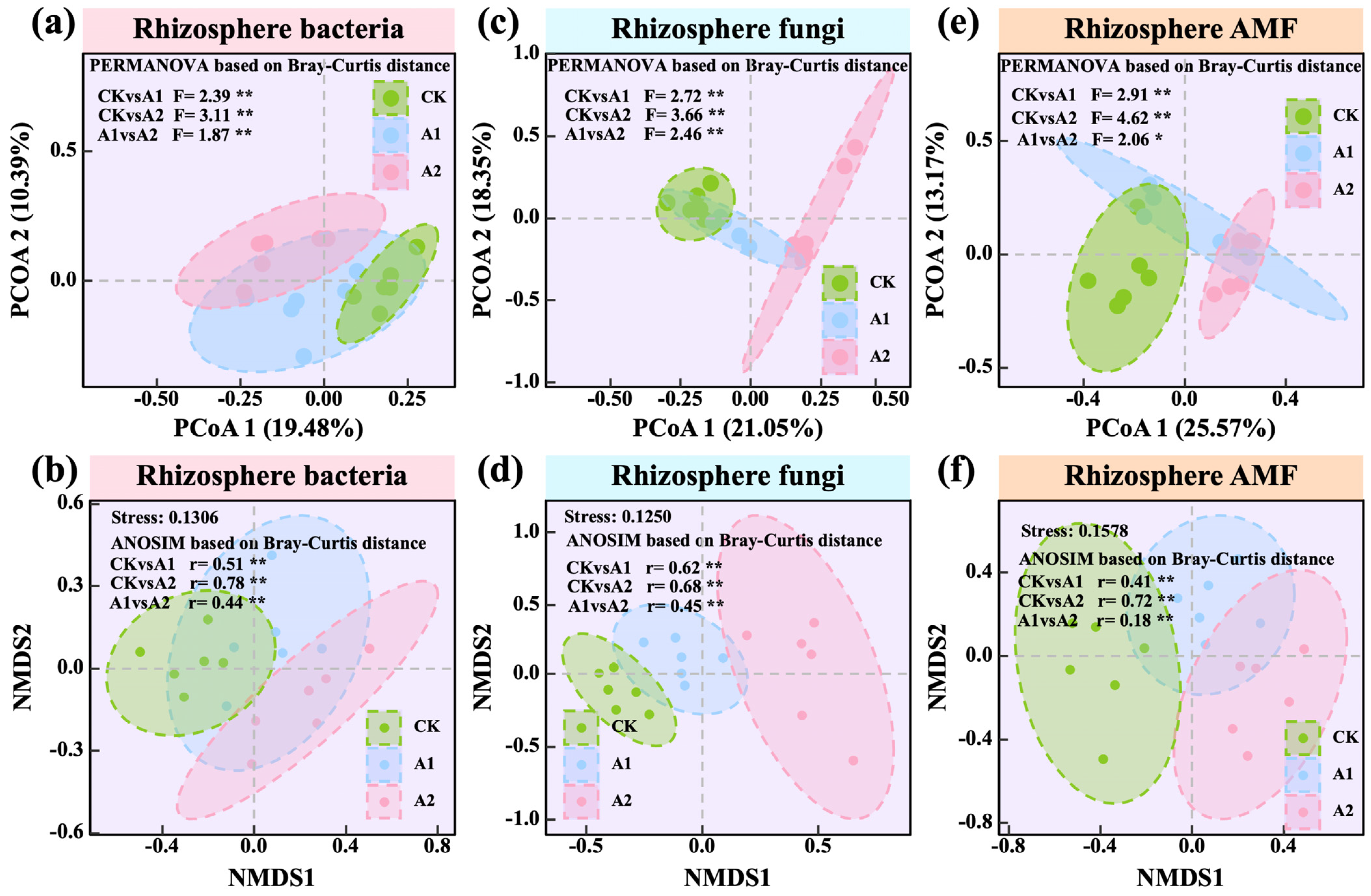
3.3. Effects of Potassium Fulvate on the Composition of Rhizosphere Soil Microbial Communities
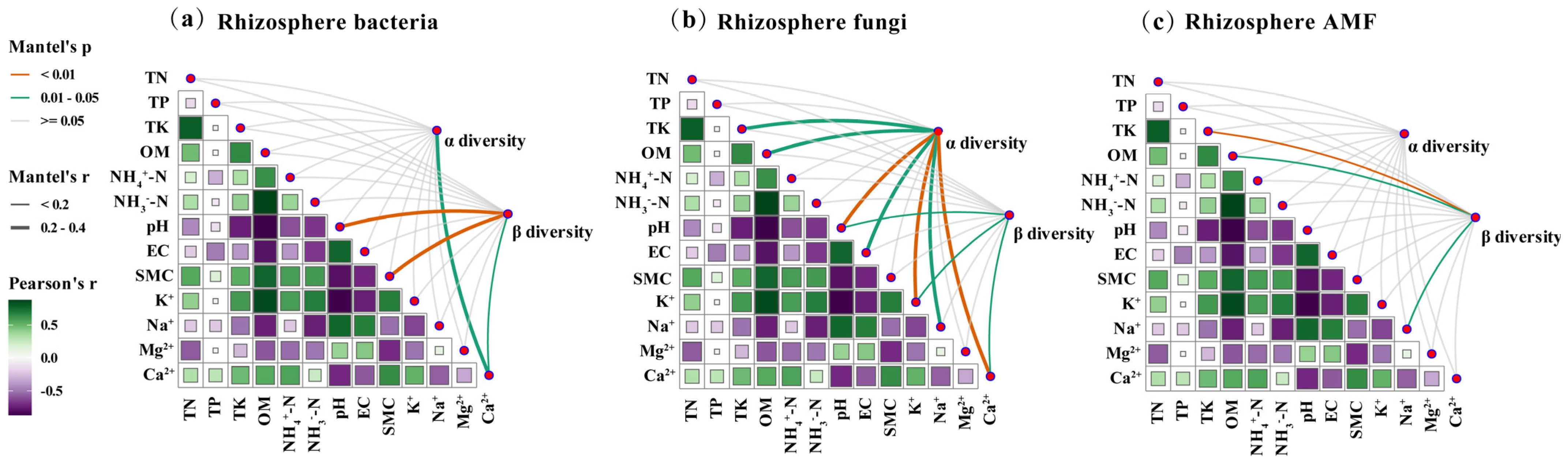
3.4. Effects of Potassium Fulvate on Rhizosphere Microbial Diversity and Community
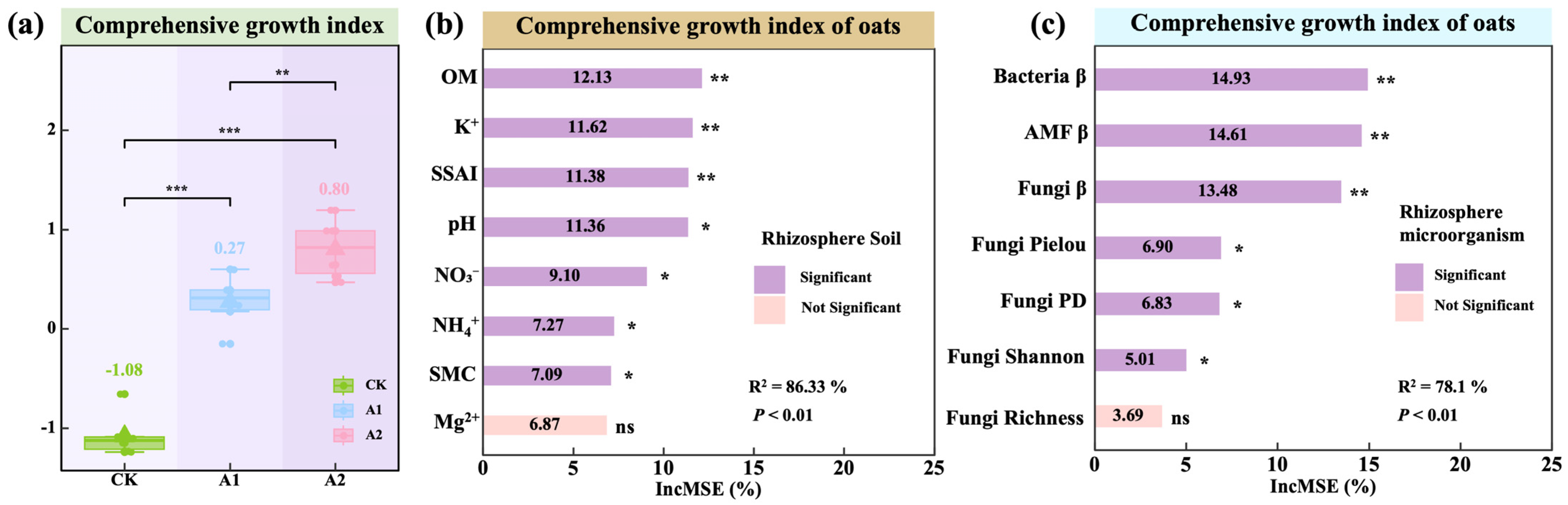
3.5. Effects and Regulatory Pathways of Potassium Fulvate on the Comprehensive Growth of Oats
4. Discussion
4.1. Potassium Fulvate Application Promotes Aboveground and Belowground Growth of Oat
4.2. Potassium Fulvate Enhances Nutrient Availability, Balances Ions, and Alleviates Saline–Alkali Stress in the Rhizosphere
4.3. Potassium Fulvate Alters Rhizosphere Microbial Community Composition
4.4. Potassium Fulvate Reshapes Rhizosphere Microbial Diversity and Underlying Environmental Drivers
4.5. Effects of Potassium Fulvate on Comprehensive Growth Index and Regulatory Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinity: A Global Threat to Sustainable Development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; He, X.; Lin, E.; Yang, G. Winter Irrigation Effects on Soil Moisture, Temperature and Salinity, and on Cotton Growth in Salinized Fields in Northern Xinjiang, China. Sustainability 2020, 12, 7573. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, K.; Qian, H.; Gao, Y.; Fang, Y.; Xiao, S.; Tang, S.; Zhang, Q.; Qu, W.; Ren, W. Characterization of Soil Salinization and Its Driving Factors in a Typical Irrigation Area of Northwest China. Sci. Total Environ. 2022, 837, 155808. [Google Scholar] [CrossRef]
- Zhang, Z.; He, K.; Zhang, T.; Tang, D.; Li, R.; Jia, S. Physiological Responses of Goji Berry (Lycium barbarum L.) to Saline-Alkaline Soil from Qinghai Region, China. Sci. Rep. 2019, 9, 12057. [Google Scholar] [CrossRef]
- She, X.; Jing, C.; Liu, W.; Zhang, L.; Zhao, Z.; Wang, Z.; Li, W.; Zhang, Z. Dynamics and Drivers of Soil Salinization in Arid and Semiarid Regions from 2002 to 2021: A Case Study in the Qaidam Basin. Ecol. Front. 2025. [Google Scholar] [CrossRef]
- Hui, R.; Tan, H.; Li, X.; Wang, B. Variation of Soil Physical-Chemical Characteristics in Salt-Affected Soil in the Qarhan Salt Lake, Qaidam Basin. J. Arid. Land 2022, 14, 341–355. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Sun, H.; Yang, F. In the Qaidam Basin, Soil Nutrients Directly or Indirectly Affect Desert Ecosystem Stability under Drought Stress through Plant Nutrients. Plants 2024, 13, 1849. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Sadhukhan, S. Bioremediation of Salt-Affected Soil through Plant-Based Strategies. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management: Principles, Monitoring and Remediation; Malik, J.A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 81–100. ISBN 978-3-030-89984-4. [Google Scholar]
- Zhu, G.; Liu, J.; Wu, H.; Zhu, Y.; Nimir, N.E.A.; Zhou, G. The Optimum Mixed Cropping Ratio of Oat and Alfalfa Enhanced Plant Growth, Forage Yield, and Forage Quality in Saline Soil. Plants 2024, 13, 3103. [Google Scholar] [CrossRef]
- Guo, C.; Xu, C.; Pu, X.; Zhao, Y.; Wang, J.; Fu, Y.; Wang, W. Oat and Forage Pea Mixed Sowing Improves Soil Chemical Fertility and Fresh and Dry Mass Yield in Light Saline–Alkali Land: Preliminary Results. Agronomy 2025, 15, 297. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.-G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil Salinity and Its Associated Effects on Soil Microorganisms, Greenhouse Gas Emissions, Crop Yield, Biodiversity and Desertification: A Review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, M.; Chen, H.; Chen, Y.; Wang, L.; Wang, R. Organic Amendments Promote Saline-Alkali Soil Desalinization and Enhance Maize Growth. Front. Plant Sci. 2023, 14, 1177209. [Google Scholar] [CrossRef]
- Aboelsoud, H. Effect of Gypsum, K-Humate and Plant Growth Promoting Bacteria on Improvement of Soil Properties and Productivity of Wheat and Maize Irrigated by Saline Water. J. Soil Sci. Agric. Eng. 2020, 11, 129–139. [Google Scholar] [CrossRef]
- Awwad, M.; El-Hedek, K.; Bayoumi, M.; Eid, T. Effect of Potassium Humate Appliction and Irrigation Water Levels on Maize Yield, Crop Water Productivity and Some Soil Properties. J. Soil Sci. Agric. Eng. 2015, 6, 461–482. [Google Scholar] [CrossRef][Green Version]
- Abd Elghany, S.H.; Saad, S.A.; Arafat, A.A.; Shaban, K. Effect of Different Irrigation Period and Potassium Humate on Some Soil Properties and Carrot Productivity under Saline Soil Conditions. Middle East J. Appl. Sci. 2019, 9, 1117–1127. [Google Scholar] [CrossRef]
- Lumactud, R.A.; Gorim, L.Y.; Thilakarathna, M.S. Impacts of Humic-Based Products on the Microbial Community Structure and Functions toward Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 977121. [Google Scholar] [CrossRef]
- Whitton, M.M.; Ren, X.; Yu, S.J.; Irving, A.D.; Trotter, T.; Bajagai, Y.S.; Stanley, D. Humate Application Alters Microbiota–Mineral Interactions and Assists in Pasture Dieback Recovery. Heliyon 2023, 9, e13327. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhu, X.; Ntoukakis, V.; Cernava, T.; Jin, D. Exploration of Phyllosphere Microbiomes in Wheat Varieties with Differing Aphid Resistance. Environ. Microbiome 2023, 18, 78. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, N.; Zhou, G.; Dang, P.; Yang, X.; Qiu, L.; Huang, M.; Gong, Y.; Zhao, S.; Chen, J. Response of Soil Microbial Community to Plant Composition Changes in Broad-Leaved Forests of the Karst Area in Mid-Subtropical China. PeerJ 2022, 10, e12739. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Zhu, X.; Chai, J.; Ji, X. Ecological Effects of Heavy Metal Pollution on Soil Microbial Community Structure and Diversity on Both Sides of a River around a Mining Area. Int. J. Environ. Res. Public Health 2020, 17, 5680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Schmidt, O.; Luan, L.; Xue, J.; Fan, J.; Geisen, S.; Sun, B.; Jiang, Y. Bacterial Keystone Taxa Regulate Carbon Metabolism in the Earthworm Gut. Microbiol. Spectr. 2022, 10, e01081-22. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Li, Y.; Yao, Y.; Zhang, Y.; Jiang, Y.; Lei, X.; Liu, H.; Wu, N.; Fohrer, N. Succession and Driving Factors of Periphytic Community in the Middle Route Project of South-to-North Water Division (Henan, China). Int. J. Environ. Res. Public Health 2022, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Sun, S.; Liu, W.; Zhu, L.; Yan, X. Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity. Plants 2022, 11, 1057. [Google Scholar] [CrossRef]
- Bai, Y.; Qin, Y.; Lu, X.; Zhang, J.; Chen, G.; Li, X. Fractal Dimension of Particle-Size Distribution and Their Relationships with Alkalinity Properties of Soils in the Western Songnen Plain, China. Sci. Rep. 2020, 10, 20603. [Google Scholar] [CrossRef]
- García-Palacios, P.; Gross, N.; Gaitán, J.; Maestre, F.T. Climate Mediates the Biodiversity-Ecosystem Stability Relationship Globally. Proc. Natl. Acad. Sci. USA 2018, 115, 8400–8405. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Günal, E. Delineating Reclamation Zones for Site-Specific Reclamation of Saline-Sodic Soils in Dushak, Turkmenistan. PLoS ONE 2021, 16, e0256355. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Jin, L.; Ogrinc, N.; Kaye, J.; Krajnc, B.; Ma, L. Dryland Irrigation Increases Accumulation Rates of Pedogenic Carbonate and Releases Soil Abiotic CO2. Sci. Rep. 2022, 12, 464. [Google Scholar] [CrossRef]
- Kreyling, J.; Jentsch, A.; Beier, C. Beyond Realism in Climate Change Experiments: Gradient Approaches Identify Thresholds and Tipping Points. Ecol. Lett. 2014, 17, 125-e1. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, X.; Yu, W.; Ye, X.; Erdenebileg, E.; Wang, C.; Ma, L.; Wang, R.; Huang, Z.; Indree, T.; et al. Aridity and Decreasing Soil Heterogeneity Reduce Microbial Network Complexity and Stability in the Semi-Arid Grasslands. Ecol. Indic. 2023, 151, 110342. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing Aridity Reduces Soil Microbial Diversity and Abundance in Global Drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- Sgarbossa, J.; Lúcio, A.D.; Silva, J.A.G.D.; Diel, M.I.; Lambrecht, D.M.; Peripolli, M.; Engers, L.B.D.O.; Nardini, C.; Alessi, O.; Peter, C.L.; et al. Implications of Removing Model Parameters on the Linear Relationships of Trials with Oat. Ciênc. Rural 2024, 54, e20230379. [Google Scholar] [CrossRef]
- Jin, X.; Deng, A.; Fan, Y.; Ma, K.; Zhao, Y.; Wang, Y.; Zheng, K.; Zhou, X.; Lu, G. Diversity, Functionality, and Stability: Shaping Ecosystem Multifunctionality in the Successional Sequences of Alpine Meadows and Alpine Steppes on the Qinghai-Tibet Plateau. Front. Plant Sci. 2025, 16, 1436439. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil Microbiomes with Distinct Assemblies through Vertical Soil Profiles Drive the Cycling of Multiple Nutrients in Reforested Ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise Structural Equation Modelling in r for Ecology, Evolution, and Systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of Humic and Fulvic Acid under Saline Soil Conditions to Improve Growth and Yield in Barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.N. Effect of Salicylic, Humic and Fulvic Acids Application on the Growth, Productivity and Elements Contents of Two Wheat Varieties Grown under Salt Stress. J. Soil Sci. Agric. Eng. 2021, 12, 657–671. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in Root Growth and Development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fu, H.; Zhu, B.; Hussain, H.A.; Zhang, K.; Tian, X.; Duan, M.; Xie, X.; Wang, L. Potassium Improves Drought Stress Tolerance in Plants by Affecting Root Morphology, Root Exudates, and Microbial Diversity. Metabolites 2021, 11, 131. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, K.; Guo, Y.; Li, T.; Kuang, N.; Liu, Z.; Yang, H. Investigating the Mechanism of Auxin-Mediated Fulvic Acid-Regulated Root Growth in Oryza Sativa through Physiological and Transcriptomic Analyses. Planta 2024, 261, 9. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Dar, A.A.; Wang, Z. The Influence of Humic and Fulvic Acids on Cd Bioavailability to Wheat Cultivars Grown on Sewage Irrigated Cd-Contaminated Soils. Ecotoxicol. Environ. Saf. 2020, 205, 111347. [Google Scholar] [CrossRef]
- Abbas, G.; Rehman, S.; Siddiqui, M.H.; Ali, H.M.; Farooq, M.A.; Chen, Y. Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes. Plants 2022, 11, 263. [Google Scholar] [CrossRef]
- Wang, C.; Feng, G.; Zhang, Z.; Huang, M.; Qi, W.; Ma, L. Geometrical and Statistical Analysis of Dynamic Crack Morphology in Shrink-Swell Soils with Addition of Maize Roots or Salinity (NaCl). Soil Tillage Res. 2021, 212, 105057. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Liu, X.; Gu, L.; Zhu, B.; Wang, H.; Du, X. The SbWRKY54–SbHKT2b Transcriptional Cascade Confers Cadmium Stress Tolerance in Sorghum. Environ. Exp. Bot. 2023, 214, 105478. [Google Scholar] [CrossRef]
- Yu, B.; Chen, L.; Baoyin, T. Effects of Restoration Strategies on the Ion Distribution and Transport Characteristics of Medicago Sativa in Saline–Alkali Soil. Agronomy 2023, 13, 3028. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- El-Shaboury, H.; Baddour, A.G.A. Effects of Various Organic Fertilizer Sources and External Applications of Potassium Fulvate and Potassium Citrate on the Yield and Quality of Two Barley Varieties Grown under Salt Affected Soil. J. Soil Sci. Agric. Eng. 2024, 15, 117–123. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Gan, Q.; Li, Y.; Huang, T.; Chen, S.; Su, J.; Lv, H.; Jia, H.; Zhao, G. Effects of Bio-Based Compound Amendments on Na+, Ca2+, Mg2+, and K+ Distribution in Salt-Tolerant Alfalfa (Medicago sativa L.) Grown in Saline Soils from Two Regions. Soil Sci. Plant Nutr. 2025, 71, 145–154. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L.; Li, M. Combined Application of Humic Acid and Arbuscular Mycorrhizal Fungi Regulates Microbial Community Dynamics and Enhances Mercury-Resistant Genes in Mercury-Polluted Paddy Soil. J. Clean. Prod. 2022, 369, 133317. [Google Scholar] [CrossRef]
- Yao, R.; Yang, J.; Zhu, W.; Li, H.; Yin, C.; Jing, Y.; Wang, X.; Xie, W.; Zhang, X. Impact of Crop Cultivation, Nitrogen and Fulvic Acid on Soil Fungal Community Structure in Salt-Affected Alluvial Fluvo-Aquic Soil. Plant Soil 2021, 464, 539–558. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. Interactions between Arbuscular Mycorrhizal Fungi and Potassium-Solubilizing Microorganisms on Agricultural Productivity. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 111–125. ISBN 978-81-322-2776-2. [Google Scholar]
- Jin, Q.; Zhang, Y.; Wang, Q.; Li, M.; Sun, H.; Liu, N.; Zhang, L.; Zhang, Y.; Liu, Z. Effects of Potassium Fulvic Acid and Potassium Humate on Microbial Biodiversity in Bulk Soil and Rhizosphere Soil of Panax Ginseng. Microbiol. Res. 2022, 254, 126914. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Q.; Guo, X.; Li, H.; Chen, X.; Men, K.; Liu, X.; Shang, X.; Gao, Y.; Zhang, L.; et al. Effect of Potassium Fulvate on Continuous Tobacco Cropping Soils and Crop Growth. Front. Plant Sci. 2024, 15, 1457793. [Google Scholar] [CrossRef]
- Yang, B.; Feng, W.; Zhou, W.; He, K.; Yang, Z. Association between Soil Physicochemical Properties and Bacterial Community Structure in Diverse Forest Ecosystems. Microorganisms 2024, 12, 728. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, R.; Qi, D.; Xu, T.; Chang, W.; Li, K.; Fang, X.; Song, F. The Effect of AMF Combined with Biochar on Plant Growth and Soil Quality under Saline-Alkali Stress: Insights from Microbial Community Analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef]
- Cai, R.; Guo, Y.; Li, Y.; Li, L.; Xu, S.; Gong, P.; Li, P.; Liu, H. Effect of Fulvic Acid on Aggregate Characteristics and Humus Composition in Saline-Alkali Soil. Plant Soil 2025. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective Strategies for Reclamation of Saline-Alkali Soil and Response Mechanisms of the Soil-Plant System. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef] [PubMed]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D.; et al. Plant Diversity Predicts Beta but Not Alpha Diversity of Soil Microbes across Grasslands Worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, L.; Wang, C.; Huang, H.; Zhuang, M. Synergistic Improvement of Carbon Sequestration and Crop Yield by Organic Material Addition in Saline Soil: A Global Meta-Analysis. Sci. Total Environ. 2023, 891, 164530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Xu, Y.; Yu, J.; Zuo, W.; Shan, Y.; Bai, Y. Environmental Constraints Mitigation Directly Drove the Diversifications of Fungal Community and Functional Profile in Amended Coastal Salt-Affected Soils. Agronomy 2024, 14, 2772. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Li, Y.; Yuan, Z.; Wen, Z.; Shi, L.; Chen, Y. Ectomycorrhizal Plant-Mediated Carbon Fixation and Microbial Mechanisms of Organic Carbon Stabilization in Coastal Saline Soils. J. Soils Sediments 2025, 25, 811–824. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Wang, J.; Liu, X.; Chang, J.; Li, C.; Lu, G. Potassium Fulvate Alleviates Salt–Alkali Stress and Promotes Comprehensive Growth of Oats in Saline–Alkali Soils of the Qaidam Basin. Plants 2025, 14, 1982. https://doi.org/10.3390/plants14131982
Jin X, Wang J, Liu X, Chang J, Li C, Lu G. Potassium Fulvate Alleviates Salt–Alkali Stress and Promotes Comprehensive Growth of Oats in Saline–Alkali Soils of the Qaidam Basin. Plants. 2025; 14(13):1982. https://doi.org/10.3390/plants14131982
Chicago/Turabian StyleJin, Xin, Jie Wang, Xinyue Liu, Jianping Chang, Caixia Li, and Guangxin Lu. 2025. "Potassium Fulvate Alleviates Salt–Alkali Stress and Promotes Comprehensive Growth of Oats in Saline–Alkali Soils of the Qaidam Basin" Plants 14, no. 13: 1982. https://doi.org/10.3390/plants14131982
APA StyleJin, X., Wang, J., Liu, X., Chang, J., Li, C., & Lu, G. (2025). Potassium Fulvate Alleviates Salt–Alkali Stress and Promotes Comprehensive Growth of Oats in Saline–Alkali Soils of the Qaidam Basin. Plants, 14(13), 1982. https://doi.org/10.3390/plants14131982






