Insight into the Functional Role of SiMPK6 in Stress Response and Photosynthetic Efficiency in Setaria italica
Abstract
1. Introduction
2. Results
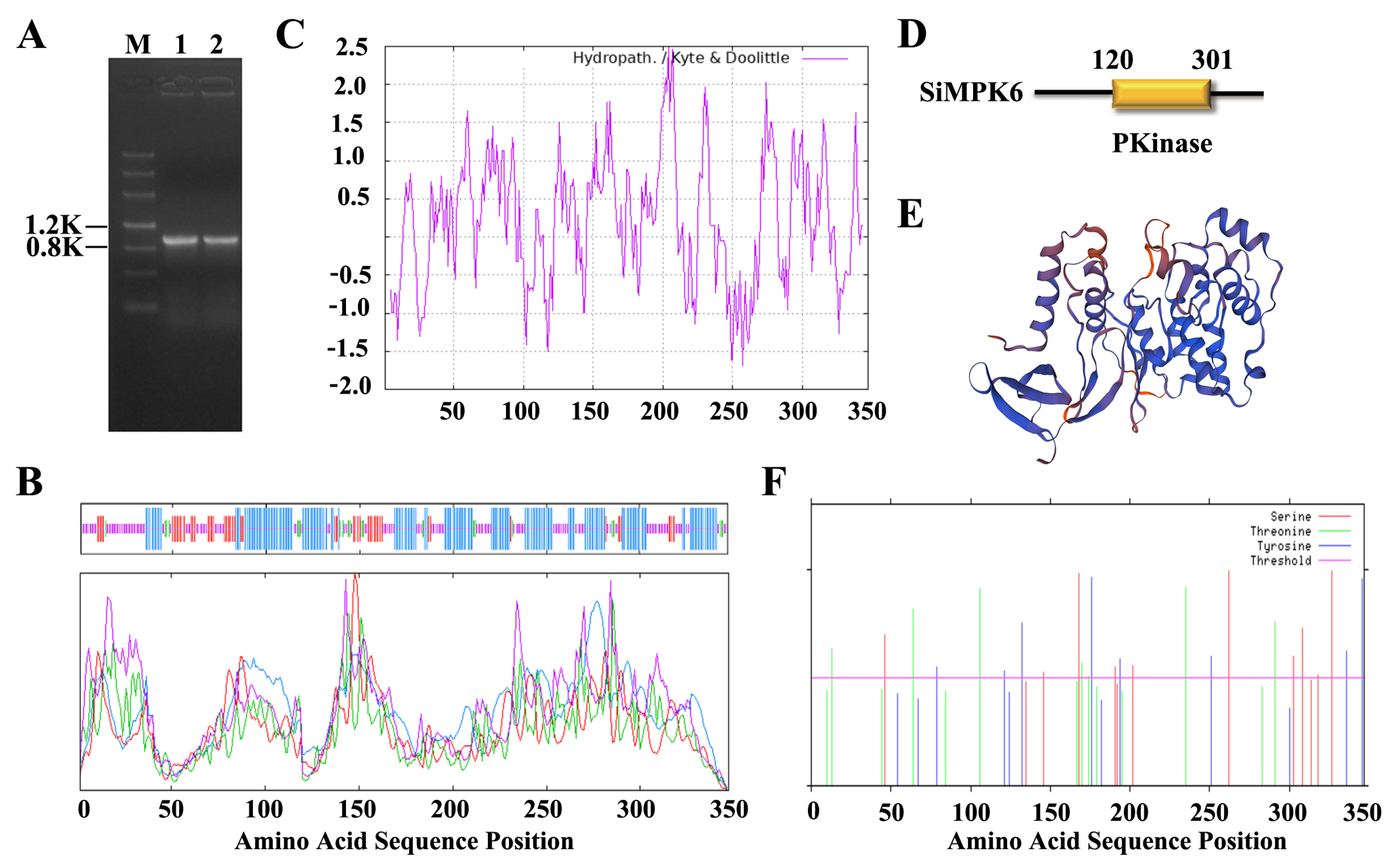
2.1. Cloning and Bioinformatics Analysis of the SiMPK6 in S. italica
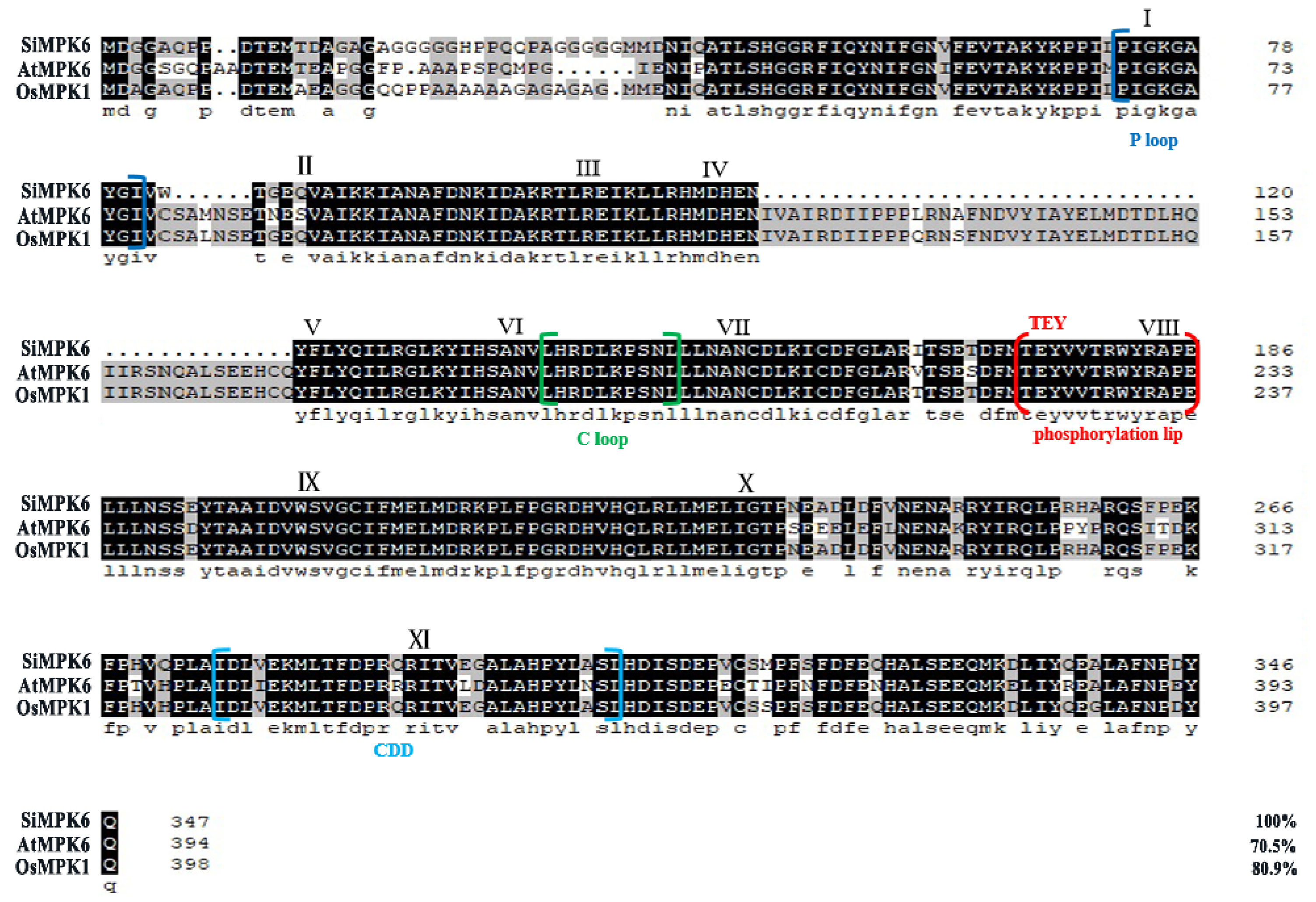
2.2. Molecular Evolutionary Analysis of SiMPK6
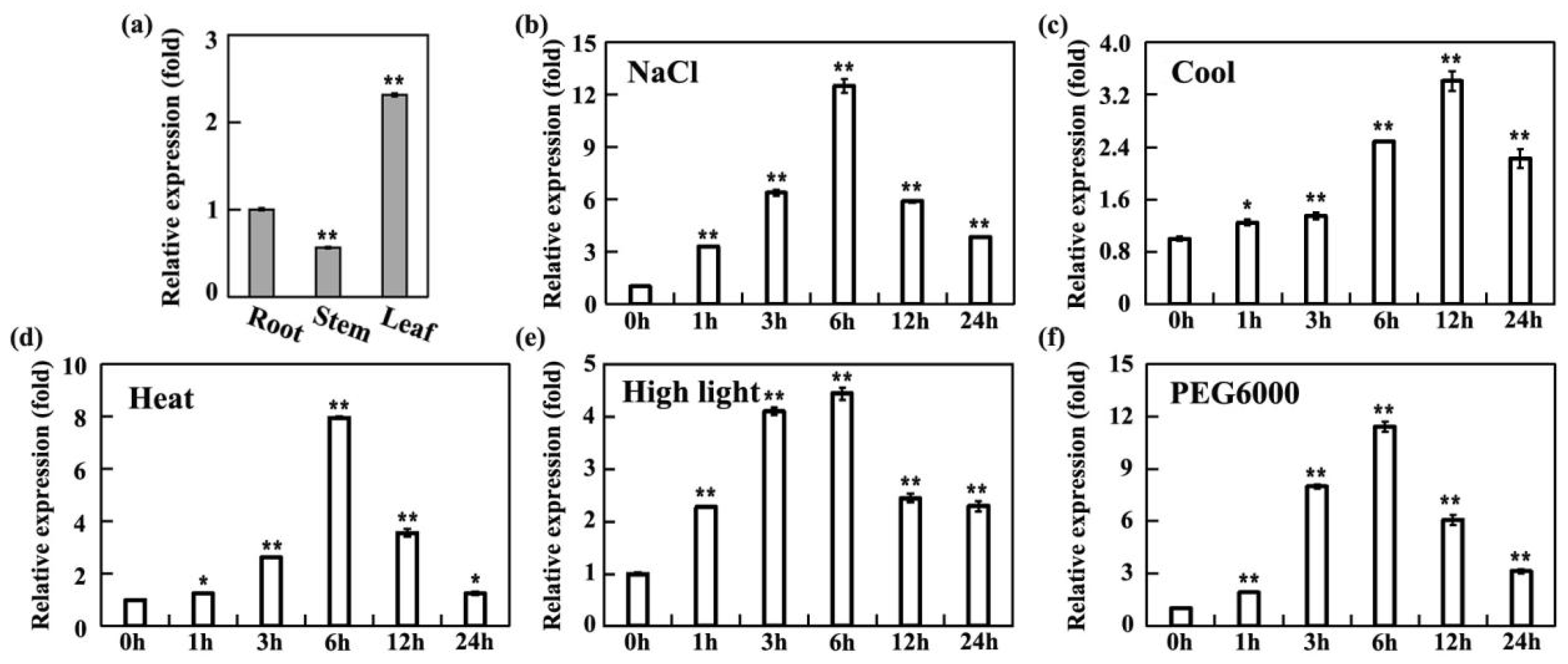
2.3. Specific Expression Analysis of the SiMPK6
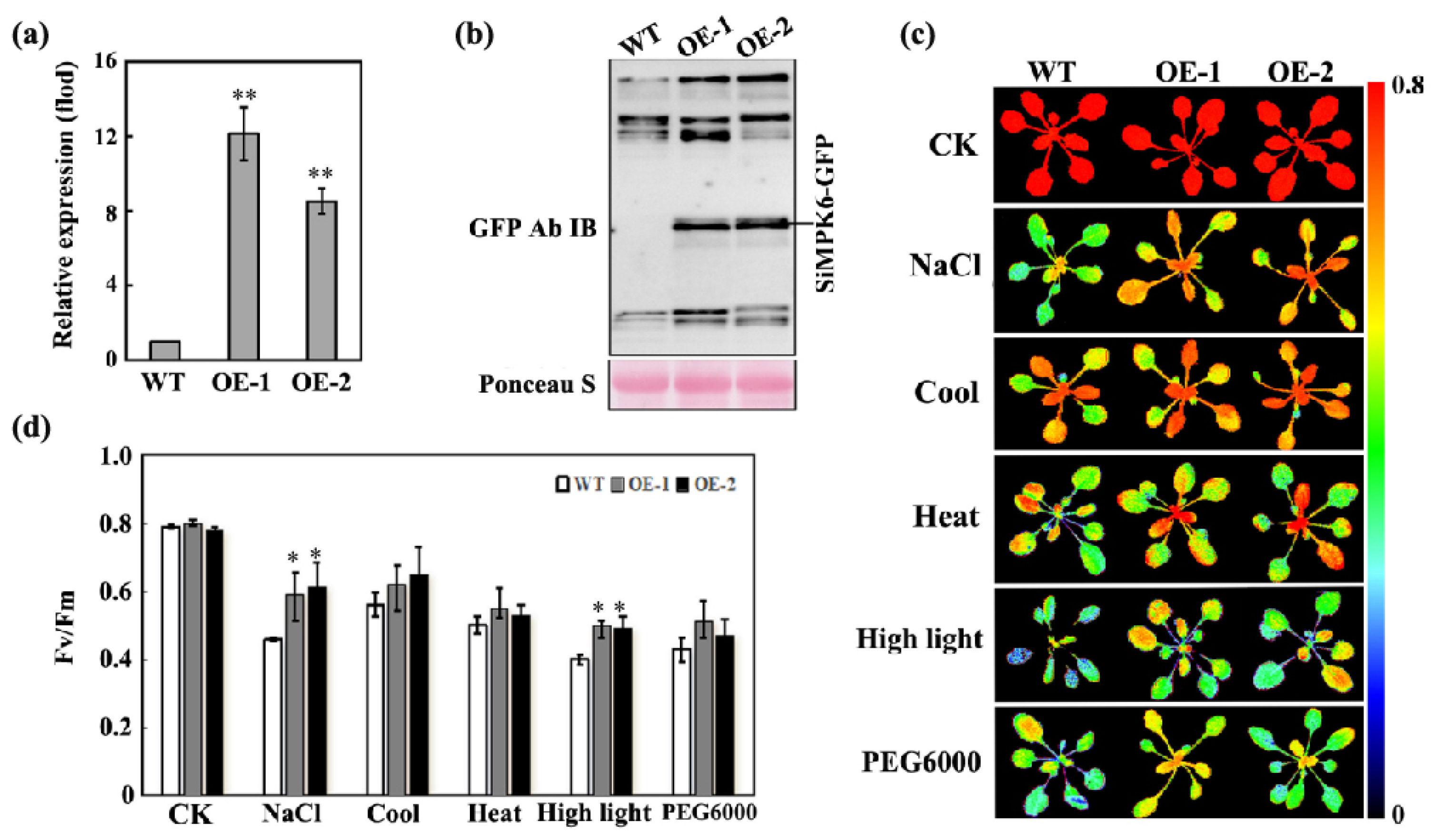
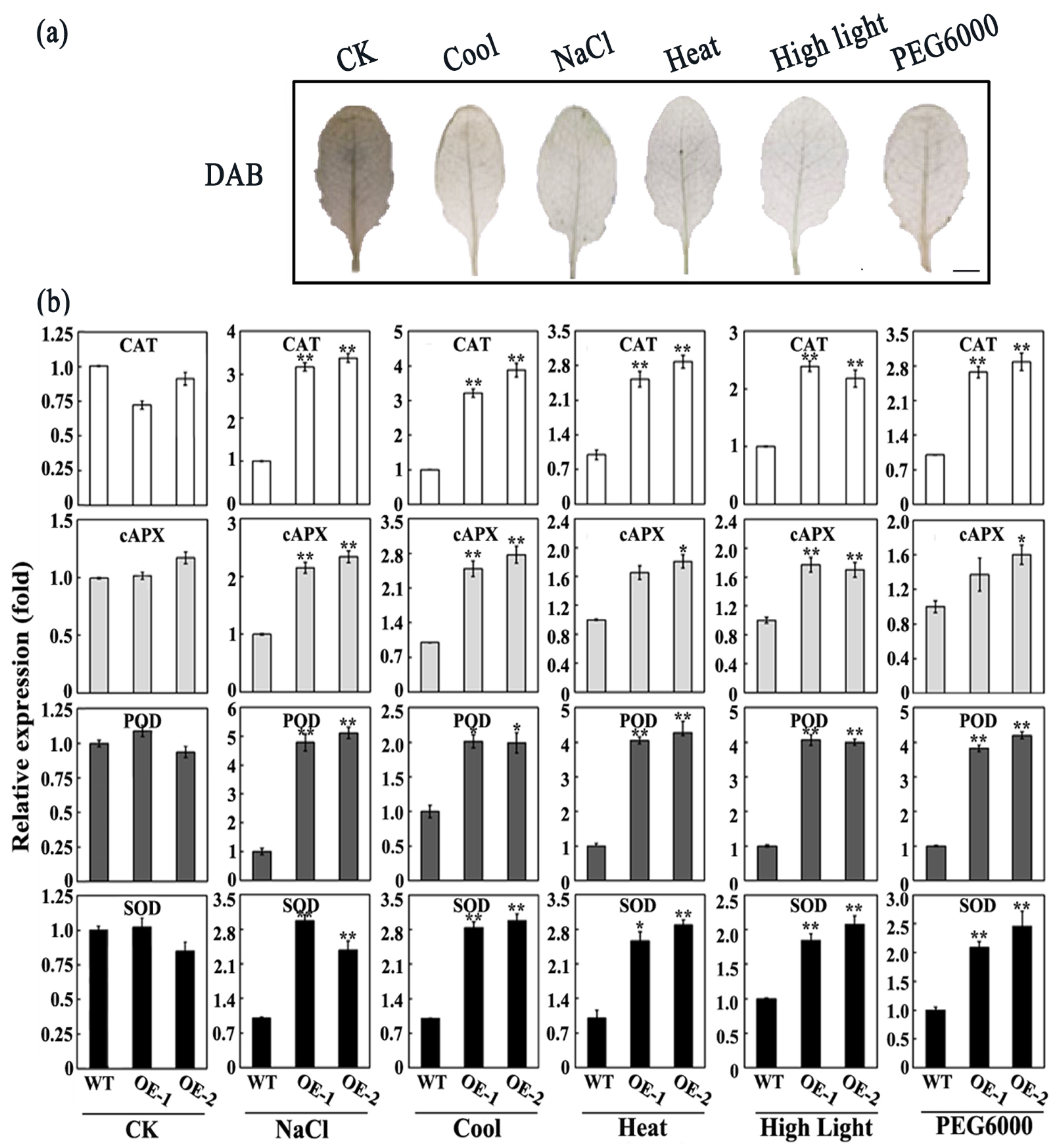
2.4. The SiMPK6 Enhances Plant Stress Resistance
2.5. Overexpression of the SiMPK6 Can Enhance Transcription of Antioxidant Enzyme
3. Materials and Methods
3.1. Plant Material
3.2. Molecular Cloning and Plant Transformation
3.3. Protein Extraction and Immunoblot Analysis
3.4. Bioinformatics Analysis of SiMPK6
3.5. qRT-PCR Expression Analysis
3.6. Chlorophyll Fluorescence Analysis
3.7. Analysis of H2O2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Tang, S.; Zhi, H.; Chen, J.; Zhang, J.; Liang, H.; Alam, O.; Li, H.; Zhang, H.; Xing, L.; et al. A graph-based genome and pan-genome variation of the model plant Setaria. Nat. Genet. 2023, 55, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, F.; Xie, K.; Zeng, X.; Cao, Y.; Zeng, J.; He, Z.; Ren, Y.; Li, W.; Deng, Q.; et al. The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol. J. 2016, 14, 2134–2146. [Google Scholar] [CrossRef]
- Kasinathan, R.; Subramani, P.; Manikandan, R. Physiological and Biochemical Response of Finger Millet Plants Exposed to Arsenic and Nickel Stress. Plant Stress 2024, 11, 100389. [Google Scholar]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. In Methods in Enzymology; Haussinger, D., Sies, H., Eds.; Academic Press: New York, NY, USA, 2007; pp. 419–438. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. 2009, 23, 185–212. [Google Scholar] [CrossRef]
- Zhu, J. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Paszkowski, U.; Scott, D. Editorial overview: Biotic interactions: The diverse and dynamic nature of perception and response in plant interactions: From cells to communities. Curr. Opin. Plant Biol. 2015, 26, v–viii. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015, 20, 56–64. [Google Scholar] [CrossRef]
- Ren, W.; Shi, Z.; Zhao, Y.; Zhang, Q.; Zhou, M.; Cheng, C.; Liu, M.; Zhao, B.; Guo, Y.; Du, H.; et al. Transcriptional analysis of maize elite inbred line Jing24 and the function of ZmMAPKKK21 in the response to drought stress. Agric. Commun. 2024, 2, 100063. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Rodriguez, M.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Ann. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar]
- Ichimura, K.; Mizoguchi, T.; Yoshida, R.; Yuasa, T.; Shinozaki, K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000, 24, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Ökrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAPkinase signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Xie, K.; Chen, J.; Wang, Q.; Yang, Y. Direct phosphorylation and activation of a mitogen-activated protein kinase by a calciumdependent protein kinase in rice. Plant Cell 2014, 26, 3077–3089. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Wu, Y.; Tian, Y.; Han, Y.; Liu, C.; Hao, J.; Fan, S. Genome-wide identification and expression analysis of MAPK gene family in lettuce (Lactuca sativa L.) and functional analysis of LsMAPK4 in high-temperature-induced bolting. Int. J. Mol. Sci. 2022, 23, 11129. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, S.; Pan, J.; Kong, X.; Zhou, Y.; Liu, Y.; Li, D. The overexpression of a maize mitogen-activated protein kinase gene (ZmMPK5) confers salt stress tolerance and induces defence responses in tobacco. Plant Biol. 2014, 16, 558–570. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Kong, X.; Xing, X.; Liu, Y.; Zhou, Y.; Liu, Y.; Sun, L.; Li, D. ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 2012, 235, 661–676. [Google Scholar] [CrossRef]
- Zhu, D.; Chang, Y.; Pei, T.; Zhang, X.; Liu, L.; Li, Y.; Zhuang, J.; Yang, H.; Qin, F.; Song, C.; et al. The MAPK-like protein ZmMPKL1 positively regulates maize seedling drought sensitivity by suppressing ABA biosynthesis. Plant J. 2020, 102, 747–760. [Google Scholar] [CrossRef]
- Long, L.; Gao, W.; Xu, L.; Liu, M.; Luo, X.; He, X.; Yang, X.; Zhang, X.; Zhu, L. GbMPK3, a mitogen-activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell Tiss. Organ. Cult. 2014, 116, 153–162. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, M.; Gu, C.; Jiang, H.; Sun, J.; Li, J. Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensi). Open Life Sci. 2022, 17, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Osuna, A.; Calatrava, V.; Galvan, A.; Fernandez, E.; Llamas, A. Identification of the MAPK Cascade and its Relationship with Nitrogen Metabolism in the Green Alga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2020, 12, 3417. [Google Scholar] [CrossRef]
- Verma, D.; Jalmi, S.K.; Bhagat, P.K.; Verma, N.; Sinha, A.K. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2020, 287, 2560–2576. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, J.; Wang, F.; Xie, C.; Lv, B.; Yu, Z.; Dai, S.; Liu, X.; Xia, G.; Tian, H.; et al. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis. EMBO Rep. 2021, 22, e52457. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, F.; Zheng, Y.; Liu, G.; Zhuang, Y.; Wang, Z.; Zhang, Y.; He, J.; Fu, C.; Lin, H. Pamp-induced secreted peptide 3 modulates salt tolerance through receptor-like kinase 7 in plants. Plant Cell 2022, 34, 927–944. [Google Scholar] [CrossRef]
- Abulfaraj, A. Stepwise signal transduction cascades under salt stress in leaves of wild barley (Hordeum spontaneum). Biotechnol. Biotechnol. Equip. 2020, 34, 860–872. [Google Scholar] [CrossRef]
- Shan, D.; Wang, C.; Song, H.; Bai, Y.; Zhang, H.; Hu, Z.; Wang, L.; Shi, K.; Zheng, X.; Yan, T.; et al. The MdMEK2-MdMPK6-MdWRKY17 pathway stabilizes chlorophyll levels by directly regulating MdSUFB in apple under drought stress. Plant J. 2021, 108, 814–828. [Google Scholar] [CrossRef]
- Kumar, K.; Raina, S.; Sultan, S. Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. J. Plant Biochem. Biotech. 2020, 29, 700–714. [Google Scholar] [CrossRef]
- Ding, Y.; Lv, J.; Shi, Y.; Gao, J.; Hua, J.; Song, C.; Gong, Z.; Yang, S. EGR 2 phosphatase regulates OST1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 2019, 38, e99819. [Google Scholar] [CrossRef]
- Ponce-Pineda, I.G.; Carmona-Salazar, L.; Saucedo-García, M.; Cano-Ramírez, D.; Morales-Cedillo, F.; Peña-Moral, A.; Guevara-García, Á.A.; Sánchez-Nieto, S.; Gavilanes-Ruíz, M. MPK6 kinase regulates plasma membrane H+-ATPase activity in cold acclimation. Int. J. Mol. Sci. 2021, 22, 6338. [Google Scholar] [CrossRef]
- Song, A.; Hu, Y.; Ding, L.; Zhang, X.; Li, P.; Liu, Y.; Chen, F. Comprehensive analysis of mitogen-activated protein kinase cascades in Chrysanthemum. Peer J. 2018, 6, e5037. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Li, Y.; Ren, D.; Song, C.-P. Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 2010, 22, 2981–2998. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Z.; Xing, D. Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death: NO and MPK6 regulate Cd2+-induced PCD. Plant Cell Environ. 2013, 36, 1–15. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, J.; Liu, P.; Ming, D.; Sun, J. Overexpression of TaJAZ1 increases powdery mildew resistance through promoting reactive oxygen species accumulation in bread wheat. Sci. Rep. 2019, 9, 5691. [Google Scholar] [CrossRef]
- Jalmi, S.; Sinha, A. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, N.; Liu, X.; Li, S.; Yang, J.; Hong, X.; Wang, F.; Si, H. Mitogen-activated protein kinase 11 (MAPK11) maintains growth and photosynthesis of potato plant under drought condition. Plant Cell Rep. 2021, 40, 491–506. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Qi, J.; Dang, P.; Xia, T. Cadmium activates ZmMPK3-1 and ZmMPK6-1 via induction of reactive oxygen species in maize roots. Biochem. Biophys. Res. Commun. 2019, 516, 747–752. [Google Scholar] [CrossRef]
- Moon, H.; Lee, B.; Choi, G.; Shin, D.; Prasad, D.T.; Lee, O.; Kwak, S.-S.; Kim, D.H.; Nam, J.; Bahk, J.; et al. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc. Natl. Acad. Sci. USA 2003, 100, 358–363. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2014, 65, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Guan, R.; Li, S.; Xu, X.; Zhang, S.; Xu, J. Co-regulation of indole glucosinolates and camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6 signaling pathways. J. Integr. Plant Biol. 2020, 62, 1780–1796. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.G.; Zhang, Y.; Sun, O.W.; Yang, Q.Q. Comprehensive evaluation and construction of drought resistance index system in Hydrangea macrophylla. Yingyong Shengtai Xuebao 2018, 29, 3175–3182. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Zhao, C.; Yang, H.; Ren, D. Expression of the inactive ZmMEK1 induces salicylic acid accumulation and salicylic acid-dependent leaf senescence. J. Integr. Plant Biol. 2016, 58, 724–736. [Google Scholar] [CrossRef]
- Kumar, M.; Kesawat, M.S.; Du, X.; Siddique, K.H.; Kant, S.; Chung, S.M. In silico analysis and expression profiling reveal the presence of abiotic stress and developmental stage specific Aconitase genes in rice (Oryza sativa L.). Plant Stress 2024, 11, 100416. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Duan, H.; Ma, Y.; Liu, R.; Li, Q.; Yang, Y.; Song, J. Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol. Biochem. 2018, 127, 231–237. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Differential response of photosystem II photochemistry in young and mature leaves of Arabidopsis thaliana to the onset of drought stress. Acta Physiol. Plant 2012, 34, 1267–1276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Hu, X.; Wang, H.; Zhang, Y.; Li, X.; Song, W.; Wen, R.; Feng, F.; Chai, R.; Wei, J.; et al. Insight into the Functional Role of SiMPK6 in Stress Response and Photosynthetic Efficiency in Setaria italica. Plants 2025, 14, 1960. https://doi.org/10.3390/plants14131960
Zhu D, Hu X, Wang H, Zhang Y, Li X, Song W, Wen R, Feng F, Chai R, Wei J, et al. Insight into the Functional Role of SiMPK6 in Stress Response and Photosynthetic Efficiency in Setaria italica. Plants. 2025; 14(13):1960. https://doi.org/10.3390/plants14131960
Chicago/Turabian StyleZhu, Dan, Xiaobing Hu, Hailong Wang, Yonghu Zhang, Xianglong Li, Wenqing Song, Rui Wen, Feng Feng, Ran Chai, Jianhua Wei, and et al. 2025. "Insight into the Functional Role of SiMPK6 in Stress Response and Photosynthetic Efficiency in Setaria italica" Plants 14, no. 13: 1960. https://doi.org/10.3390/plants14131960
APA StyleZhu, D., Hu, X., Wang, H., Zhang, Y., Li, X., Song, W., Wen, R., Feng, F., Chai, R., Wei, J., & Zhang, J. (2025). Insight into the Functional Role of SiMPK6 in Stress Response and Photosynthetic Efficiency in Setaria italica. Plants, 14(13), 1960. https://doi.org/10.3390/plants14131960





