Resistance of Alternaria spp. Causing Strawberry Black Spot to Boscalid in China
Abstract
1. Introduction
2. Results
2.1. Sensitivity to Boscalid
2.2. Point Mutations in Target Genes Conferring Boscalid Resistance
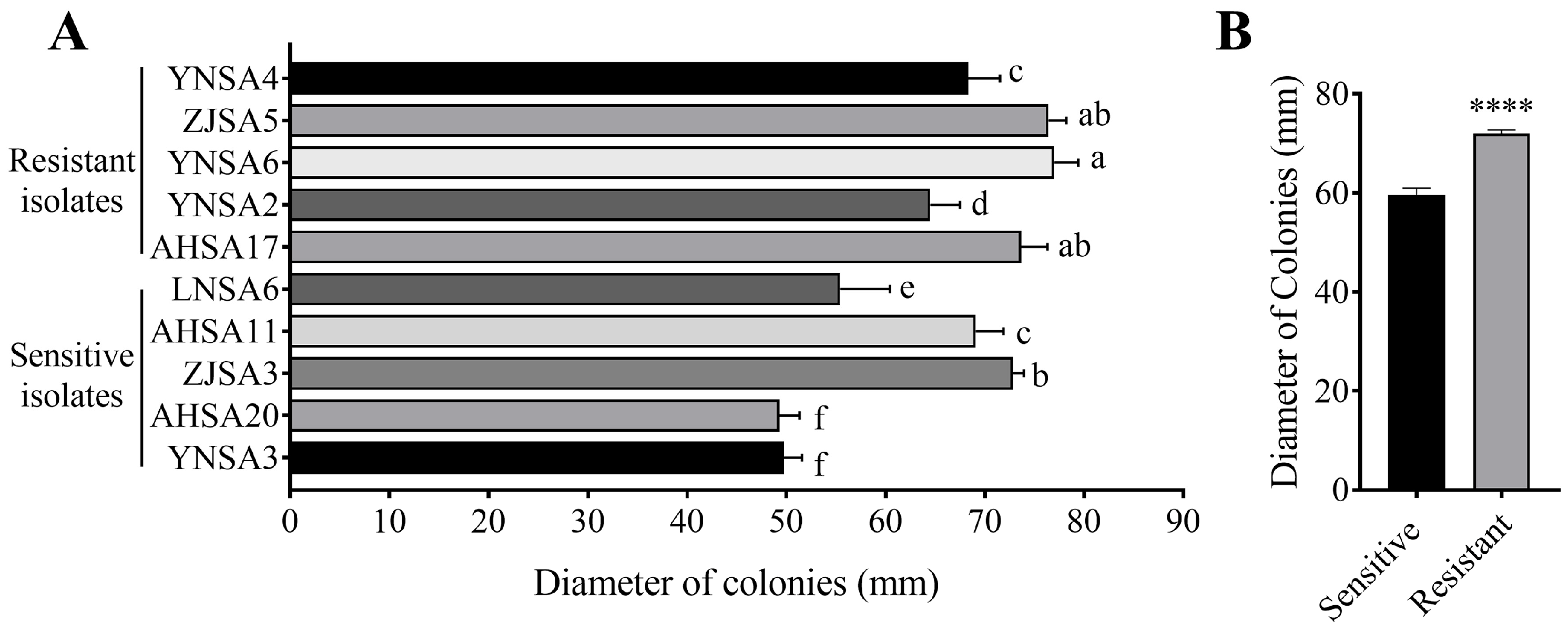
2.3. Increased Mycelial Growth in Resistant Isolates
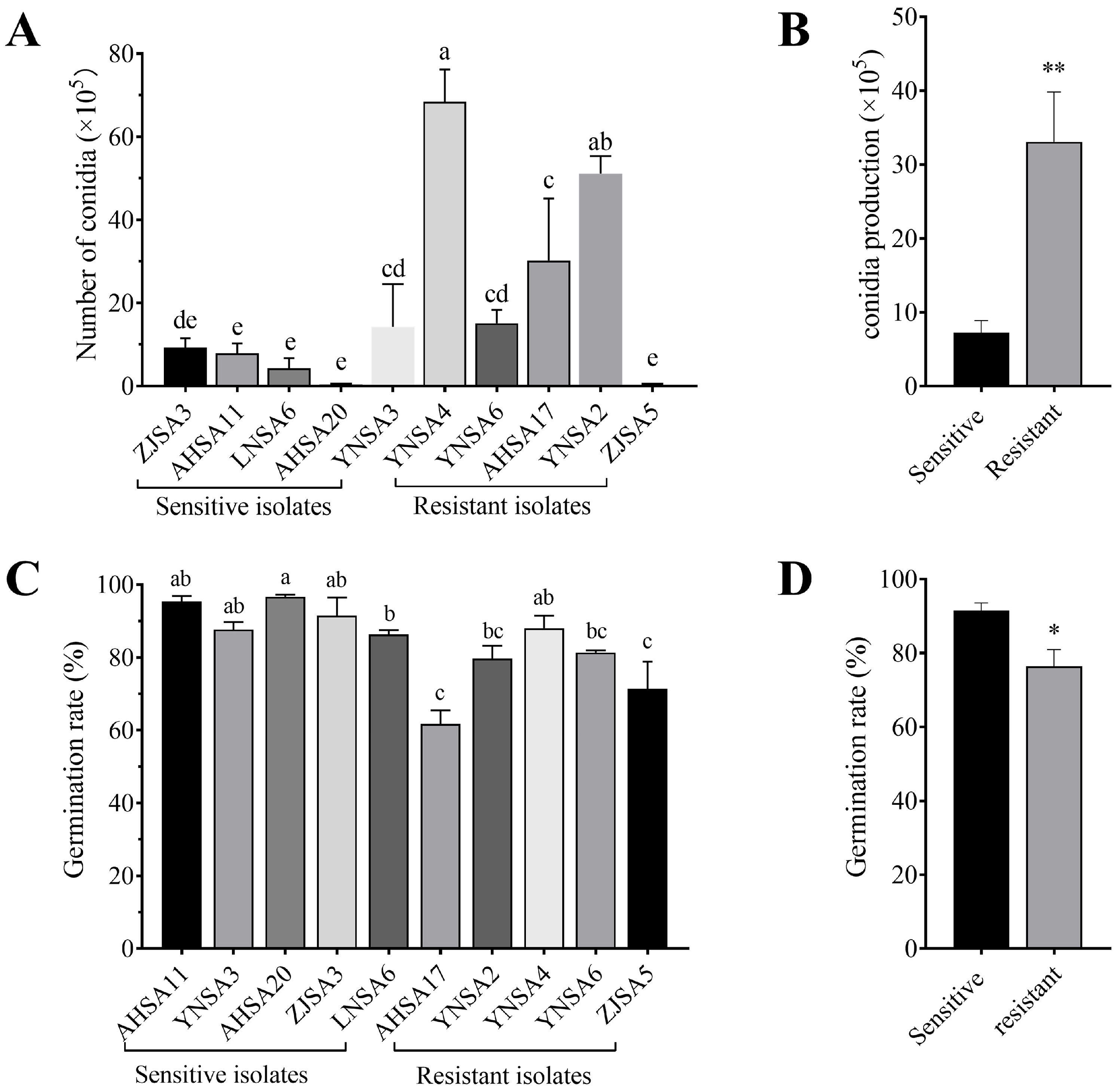
2.4. Conidia Production and Germination
2.5. Virulence
2.6. Responses to Stress Agents
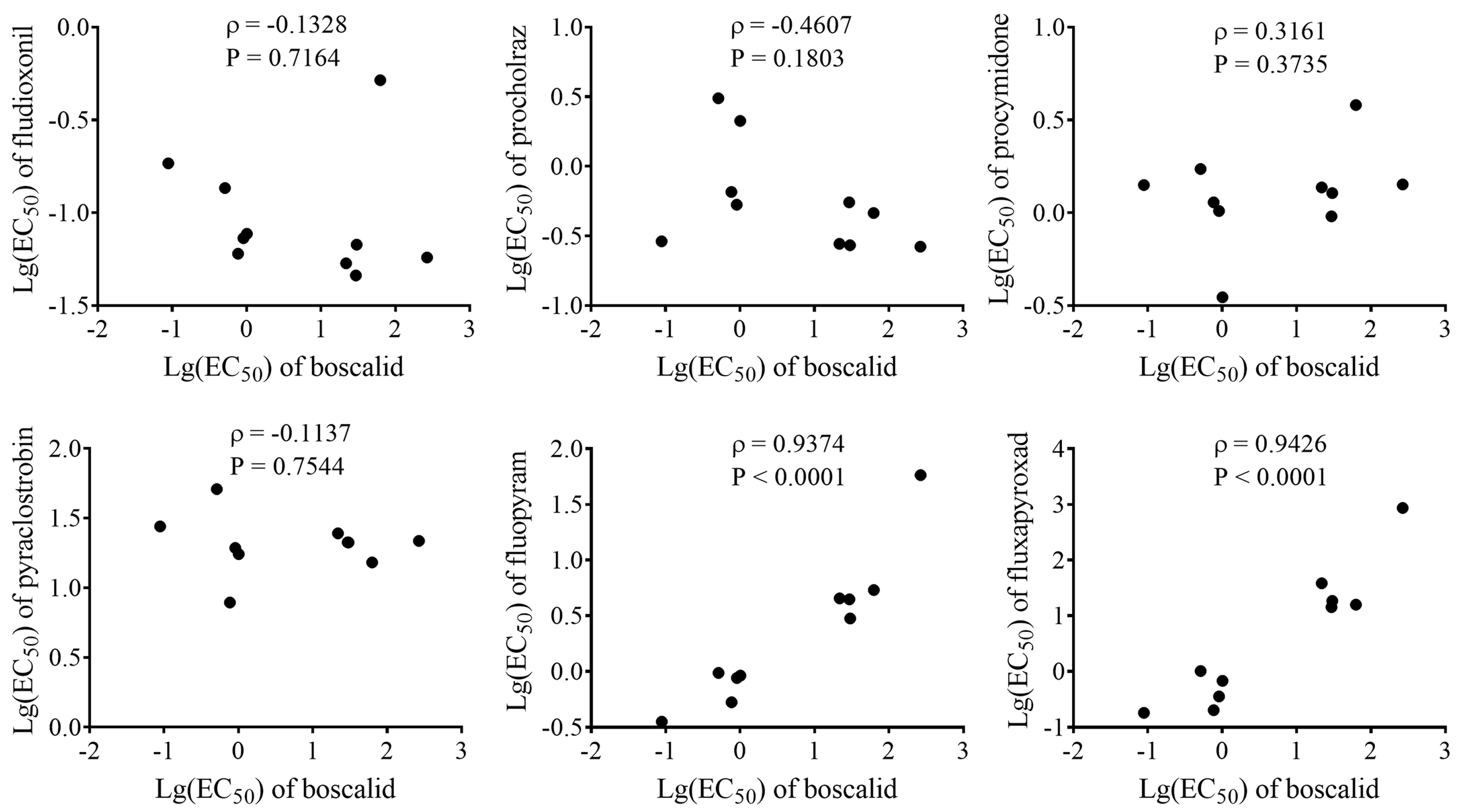
2.7. Cross-Resistance Between Boscalid and Other Fungicides
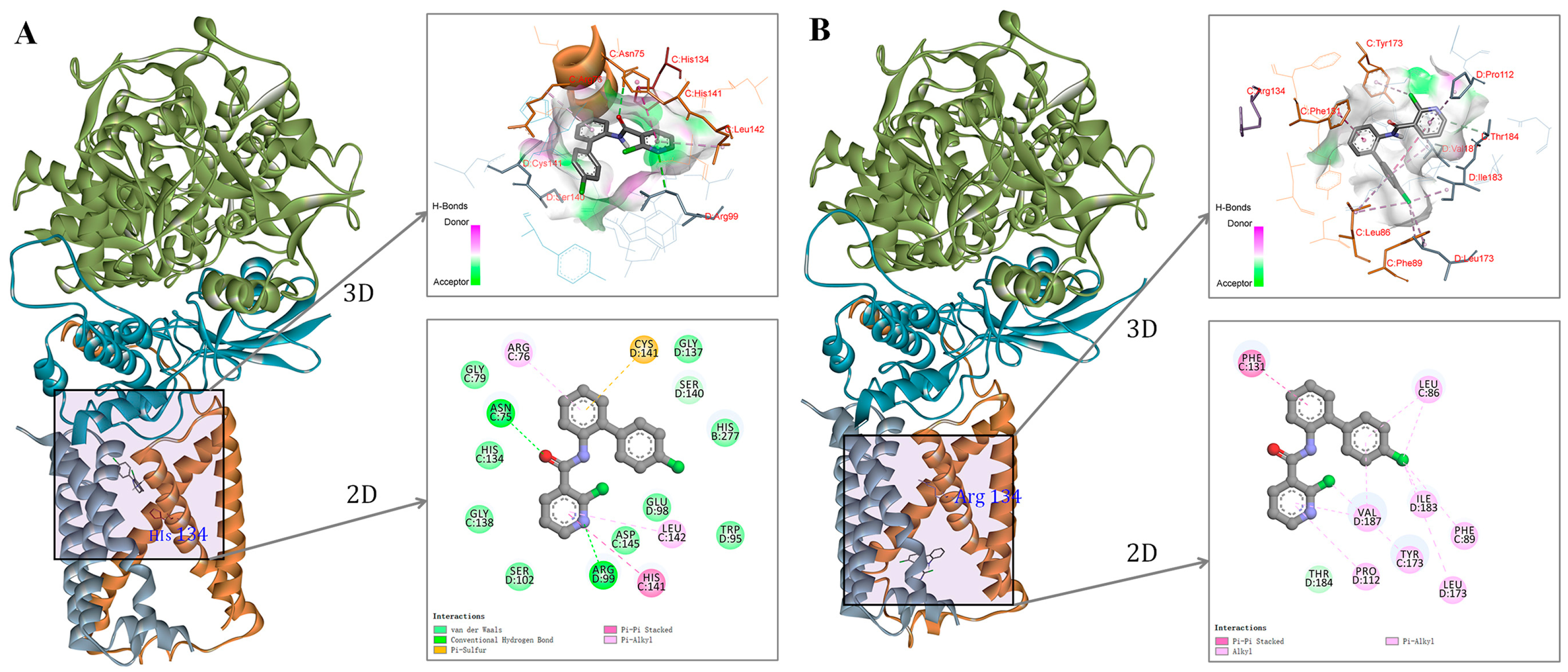
2.8. SDHC-H134R Mutation Reduces the Binding Affinity of Boscalid to Mitochondrial Complex II
3. Discussion
4. Materials and Methods
4.1. Isolates, Fungicides and Medium
4.2. Sensitivity Assay for Boscalid
4.3. Amplification and Sequence Analysis of the Target Genes
4.4. Biological Characterization of Sensitivity and Resistant Isolates
4.5. Sensitivity to Stress Agents
4.6. Cross-Resistance Analysis
4.7. Model Building and Molecular Docking
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDA | Potato dextrose agar |
| AEA | Acetic acid ester agar |
| SDHI | Succinate dehydrogenase inhibitor |
References
- Voća, S.; Šic Žlabur, J.; Dobričević, N.; Jakobek, L.; Šeruga, M.; Galić, A.; Pliestić, S. Variation in the bioactive compound content at three ripening stages of strawberry fruit. Molecules 2014, 19, 10370–10385. [Google Scholar] [CrossRef] [PubMed]
- Sallato, B.V.; Torres, R.; Zoffoli, J.P.; Latorre, B.A. Effect of boscalid on postharvest decay of strawberry caused by Botrytis cinerea and Rhizopus stolonifera. Span. J. Agric. Res. 2007, 5, 67–78. [Google Scholar] [CrossRef]
- Lattanzio, V.; Di Venere, D.; Linsalata, V.; Lima, G.; Ippolito, A.; Salerno, M. Antifungal activity of 2.5-dimethoxybenzoic acid on postharvest pathogens of strawberry fruits. Postharvest Biol. Technol. 1996, 9, 325–334. [Google Scholar] [CrossRef]
- Henry, P.M.; Haugland, M.; Lopez, L.; Munji, M.; Watson, D.C.; Gordon, T.R. The potential for Fusarium oxysporum f. sp. fragariae, cause of fusarium wilt of strawberry, to colonize organic matter in soil and persist through anaerobic soil disinfestation. Plant Pathol. 2020, 69, 12181226. [Google Scholar] [CrossRef]
- Hu, S.D.; Zhang, Y.T.; Yu, H.; Zhou, J.Y.; Hu, M.H.; Liu, A.C.; Wu, J.Y.; Wang, H.C.; Zhang, C.Q. Colletotrichum spp. diversity between leaf anthracnose and crown rot from the same strawberry plant. Front. Microbiol. 2022, 13, 860694. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Yu, H.; Hu, M.H.; Wu, J.Y.; Zhang, C.Q. Fungal pathogens associated with strawberry crown rot disease in China. J. Fungi 2022, 8, 1161. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Zhang, S.K.; Xu, J.J.; Ke, X.Y.; Peng, C.; Li, Z.; Chen, B.; Pan, S.; Gu, T.T. Monitoring the infection of powdery mildew pathogen on strawberry leaves by ATR-IR technique. J. Pathol. 2022, 170, 579–587. [Google Scholar] [CrossRef]
- Yuan, S.Z.; Wang, B.G.; Wang, M.; Sun, M.M.; Wang, X.Q.; Li, X.F.; Yang, N.; Xu, X.D.; Zheng, S.F.; Wang, Q. Antifungal mechanism of protocatechuic acid methyl ester against Botrytis cinerea in postharvest strawberry fruit. Postharvest Biol. Technol. 2024, 211, 112787. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, X.F.; Liu, S.J.H.; Hu, K.L.; Wu, X.H. Characterization of Alternaria species associated with black spot of strawberry in Beijing municipality of China. Can. J. Plant Pathol. 2020, 42, 235–242. [Google Scholar] [CrossRef]
- Patil, J.S.; Suryawanshi, N.S. Fruit rot of strawberry caused by Alternaria alternata control using homoeopathic medicines. Int. J. Pharm. Sci. Invent. 2014, 3, 57–58. [Google Scholar]
- Sun, X.Z.; Wang, C.Y.; Gao, X.; Wu, X.H.; Fu, Y. Characterization of Alternaria species associated with black spot of strawberry in Dandong, China. Agronomy 2023, 13, 1014. [Google Scholar] [CrossRef]
- Watanabe, Y.; Umekawa, M. Black spot of strawberry caused by Alternaria spp. Jpn. J. Phytopathol. 1977, 42, 82. [Google Scholar] [CrossRef]
- Wada, H.; Cavanni, P.; Bugiani, R.; Kodama, M.; Otani, H.; Kohmoto, K. Occurrence of the strawberry pathotypes of Alternaria alternata in Italy. Plant Dis. 1996, 104, 715–716. [Google Scholar] [CrossRef]
- Cho, J.T.; Moon, B.J. The occurrence of strawberry black leaf spot caused by Alternaria alternata (Fr.) Keissler in Korea. Korean J. Appl. Entomol. 1980, 19, 221–227. [Google Scholar]
- Mehmood, N.; Riaz, A.; Naz, F.; Hassan, I.; Jaabeen, N.; Anwaar, S.; Rosli, H.; Gleason, M.L. First report of strawberry leaf spot caused by Alternaria alternata in Pakistan. Plant Dis. 2018, 102, 820. [Google Scholar] [CrossRef]
- Bagherabadi, S.; Zafari, D.; Soleimani, M.J. First report of leaf spot of strawberry caused by Alternaria tenuissima in Iran. J. Plant Pathol. Microbiol. 2015, 6, 258. [Google Scholar] [CrossRef]
- Li, G.X.; Li, X.H.; Zeng, Y.; Liao, S.L.; Chen, Y.; Miao, J.Q.; Peng, Q.; Liu, X.L. Three point mutations in AaCYP51 combined with induced overexpression of AaCYP51 conferred low-level resistance to mefentrifluconazole in Alternaria alternata. Pestic. Biochem. Phys. 2023, 197, 105677. [Google Scholar] [CrossRef]
- Liu, Y.H.; Dai, D.J.; Shen, Y.; Zhang, C.Q. Detection of resistance of Alternaria Kikuchiana causing pear black spot to fungicides and baseline sensitivity of A. kikuchiana to boscalid. Chin. J. Pestic. Sci. 2015, 17, 274–278. [Google Scholar] [CrossRef]
- Vegas, B.; Dewdney, M.M. Sensitivity of Alternaria alternata from citrus to boscalid and polymorphism in iron-sulfur and in anchored membrane subunits of succinate dehydrogenase. Plant Dis. 2015, 99, 231–239. [Google Scholar] [CrossRef]
- Sun, J.Z.; Ran, W.Q.; Feng, L.C.; Cui, J.; Fan, W.Z.; Pan, Y.X.; Zhao, L.N. Pathogen identification of strawberry black spot and its susceptibility to fungicides. Agrochemicals 2023, 62, 828–832. [Google Scholar] [CrossRef]
- Al-Rahbi, B.A.A.; Al-Sadi, A.M.; Al-Mahmooli, I.H.; Al-Maawali, S.S.; Al-Mahruqi, N.M.T.; Celazhahan, R. Meyerozyma guilliermondii SQUCC-33Y suppresses postharvest fruit rot of strawberry caused by Alternaria alternata. Australas. Plant Pathol. 2021, 50, 349–352. [Google Scholar] [CrossRef]
- Demir, S.; Durak, E.D.; GÜneş, H.; Boyno, G.; Mulet, J.M.; Danesh, Y.R.; Procel, R. Biological control of three fungal diseases in strawberry (Fragaria × ananassa) with arbuscular mycorrhizal fungi. Agronomy 2023, 13, 2439. [Google Scholar] [CrossRef]
- Lin, Z.S.; Zhao, S.F.; Huang, F.Y.H.; Wu, J.Y.; Wang, H.D.; Zhang, C.Q. Resistance to boscalid in Botrytis cinerea in Zhejiang Province. Chin. J. Pestic. Sci. 2018, 20, 729–734. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Cheng, W.; Zeng, R.; Xu, H.L.; Gao, S.G.; Dai, F.M. Sensitivity and resistance mechanism to boscalid of Botrytis cinerea from strawberry in Shanghai. Chin. J. Pestic. Sci. 2018, 20, 452–458. [Google Scholar] [CrossRef]
- Liu, S.Y.; Fan, H.Y. The occurrence and mechanism of field resistance to boscalid and pyraclostrobin in Stemphylium solani, the causal agent of tomato gray leaf spot in China. Pestic. Biochem. Phys. 2024, 204, 106028. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Anastasios, A.; Apostolidou, Z.A.; Louka, D.; Markoglou, A.; Flouri, F. Biological and molecular characterization of field isolates of Alternaria alternata with single or double resistance to respiratory complex II and III inhibitors. Eur. J. Plant Pathol. 2018, 152, 199–211. [Google Scholar] [CrossRef]
- Budde-Rodriguez, S.; Pasche, J.S.; Mllik, I.; Gudmestad, N.C. Sensitivity of Alternaria spp. from potato to pyrimethanil, cyprodinil and fludioxonil. Crop Prot. 2022, 152, 105855. [Google Scholar] [CrossRef]
- He, M.X.; Fu, Y.S.; Ruan, R.X.; Li, H.Y. Sensitivity assay of Alternaria alternata from citrus in China to four new fungicides. J. Zhejiang Univ. (Agric. Life Sci.) 2016, 42, 535–542. [Google Scholar] [CrossRef]
- Lei, F.B.; Wang, H.C.; Dai, Y.F.; Zhang, C.Q. Sensitivity to boscalid of Alternaria alternata causing tobacco brown spot in Guizhou province. Chin. J. Pestic. Sci. 2021, 23, 812–816. [Google Scholar] [CrossRef]
- Landschoot, S.; Carrette, J.; Vandecasteele, M.; De Baets, B.; Hofte, M.; Audenaert, K.; Haesaert, G. Boscalid-resistance in Alternaria alternata and Alternaria solani populations: An emerging problem in Europe. Crop Prot. 2017, 92, 49–59. [Google Scholar] [CrossRef]
- Miles, T.D.; Fairchild, K.L.; Merlington, A.; Kirk, W.W.; Rosenzweig, N.; Wharton, P.S. First Report of boscalid and penthiopyrad-resistant isolates of Alternaria solani causing early blight of potato in Michigan. Plant Dis. 2013, 97, 1655–1656. [Google Scholar] [CrossRef] [PubMed]
- Avenot, H.F.; Luna, M.; Michailides, T.J. Phenotypic and molecular characterization of resistance to the SDHI fungicide fluopyram in populations of Alternaria alternata from pistachio orchards in California. Crop Prot. 2019, 124, 104838. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Occurrence and extent of boscalid resistance in populations of Alternaria alternata from California pistachio orchards. Plant Dis. 2020, 104, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bauske, M.J.; Mallik, I.; Yellareddygari, S.K.R.; Gudmestad, N.C. Spatial and temporal distribution of mutations conferring QoI and SDHI resistance in Alternaria solani across the United States. Plant Dis. 2018, 102, 349–358. [Google Scholar] [CrossRef]
- Förster, H.; Luo, Y.; Hou, L.L.; Adaskaveg, J.E. Mutations in Sdh gene subunits confer different cross-resistance patterns to SDHI fungicides in Alternaria alternata causing Alternaria leaf spot of almond in California. Plant Dis. 2022, 106, 1911–1918. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, J.H.; Fan, F.; Luo, C.X.; Schanabel, G. Fitness and competitive ability of Alternaria alternata field isolates with resistance to SDHI, QoI and MBC fungicides. Plant Dis. 2015, 99, 1744–1750. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.K.; Gao, H.; Cao, Z.G.; Wang, J.X.; Cai, Q.Y.; Duan, Y.B.; Zhou, M.G. The G143A/S substitution of mitochondrially encoded cytochrome b (Cytb) in Magnaporthe oryzae confers resistance to quinone outside inhibitors. Pest. Manag. Sci. 2022, 78, 4850–4858. [Google Scholar] [CrossRef]
- Li, T.; Li, N.; Lei, Z.Y.; Zhang, C.Q. Sensitivity and resistance risk of Botryosphaeria dothidea causing Chinese hickory trunk canker to fludioxonil. Pest. Biochem. Phys. 2023, 194, 105500. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.C.; Gan, J.H.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acid. Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
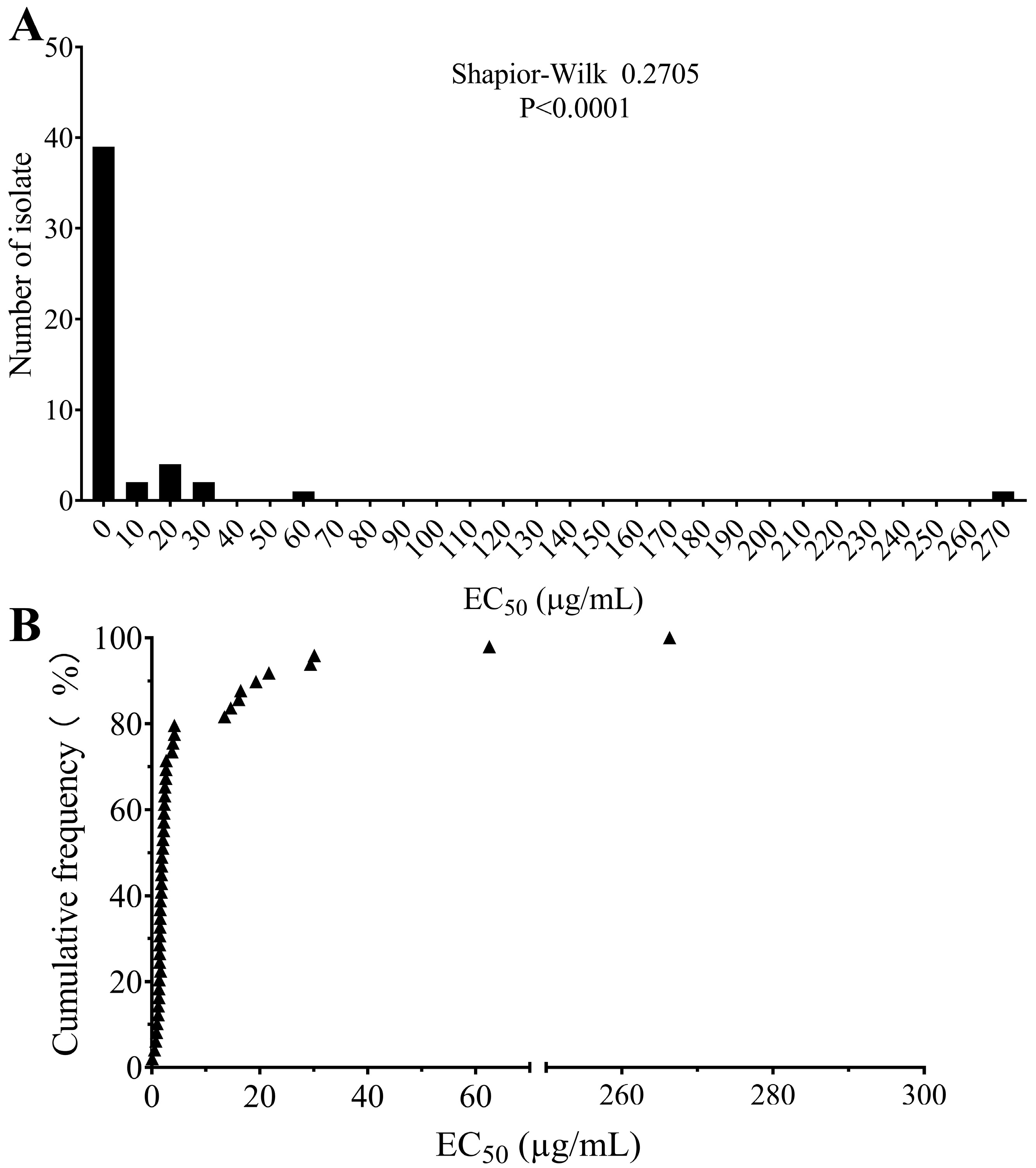



| Location | Number of Isolates | Range of EC50 (µg/mL) | EC50 (µg/mL) | Ratio of Resistant Isolates | Ratio of Highly Resistant Isolates |
|---|---|---|---|---|---|
| Anhui | 29 | 0.5129–266.3289 | 11.9290 ± 9.1095 | 28/29 | 3/29 |
| Liaoning | 8 | 1.0068–13.4895 | 3.6321 ± 1.4727 | 8/8 | 1/8 |
| Yunnan | 6 | 0.0884–62.4968 | 25.0278 ± 8.5018 | 5/6 | 5/6 |
| Zhejiang | 6 | 0.7673–29.4114 | 6.5811 ± 4.5846 | 5/6 | 1/6 |
| Primers | Sequence (5′-3′) | Purpose |
|---|---|---|
| B-SF | TGTCATAACCGAGGAAGC | Amplify a fragment of 1363 bp containing coding region of sdhB |
| B-SR | TAGATTGGCACAGGGAAC | |
| C-SF | CATAACGCCAGCAGACAA | Amplify a fragment of 1274 bp containing coding region of sdhC |
| C-SR | GATTCCCATAAACCACCC | |
| D-SF | GGGACGTCAGCAATCGACAT | Amplify a fragment of 937 bp containing coding region of sdhD |
| D-SR | CAGCAGGAAAGTGCTCCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yu, W.; Feng, J.; Mao, C.; Yu, H.; Liu, A.; Zhang, C. Resistance of Alternaria spp. Causing Strawberry Black Spot to Boscalid in China. Plants 2025, 14, 1941. https://doi.org/10.3390/plants14131941
Li T, Yu W, Feng J, Mao C, Yu H, Liu A, Zhang C. Resistance of Alternaria spp. Causing Strawberry Black Spot to Boscalid in China. Plants. 2025; 14(13):1941. https://doi.org/10.3390/plants14131941
Chicago/Turabian StyleLi, Tao, Wenbin Yu, Ji Feng, Chengxin Mao, Hong Yu, Aichun Liu, and Chuanqing Zhang. 2025. "Resistance of Alternaria spp. Causing Strawberry Black Spot to Boscalid in China" Plants 14, no. 13: 1941. https://doi.org/10.3390/plants14131941
APA StyleLi, T., Yu, W., Feng, J., Mao, C., Yu, H., Liu, A., & Zhang, C. (2025). Resistance of Alternaria spp. Causing Strawberry Black Spot to Boscalid in China. Plants, 14(13), 1941. https://doi.org/10.3390/plants14131941






