Chemotypic and Seasonal Variations in Essential Oils from Mespilodaphne cymbarum (Kunth) Trofimov and Their Antibacterial and Antibiofilm Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Yields of the Essential Oils (EOs) from Bark, Leaf and Fruit of Mespilodaphne cymbarum
2.2. Chemical Composition and Statistical Analysis of the Essential Oils (EOs) from Bark, Leaves and Fruits of Mespilodaphne cymbarum
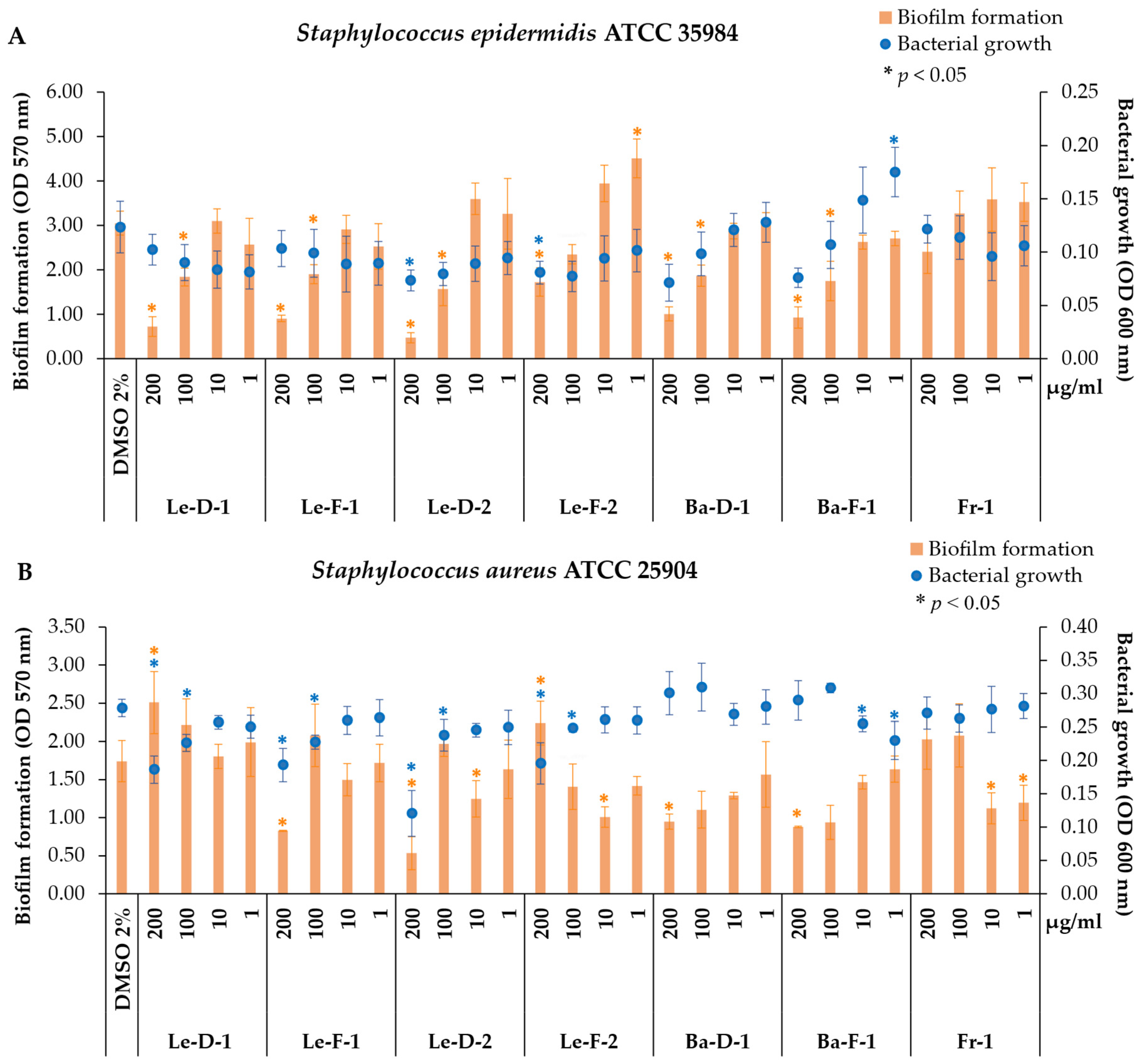
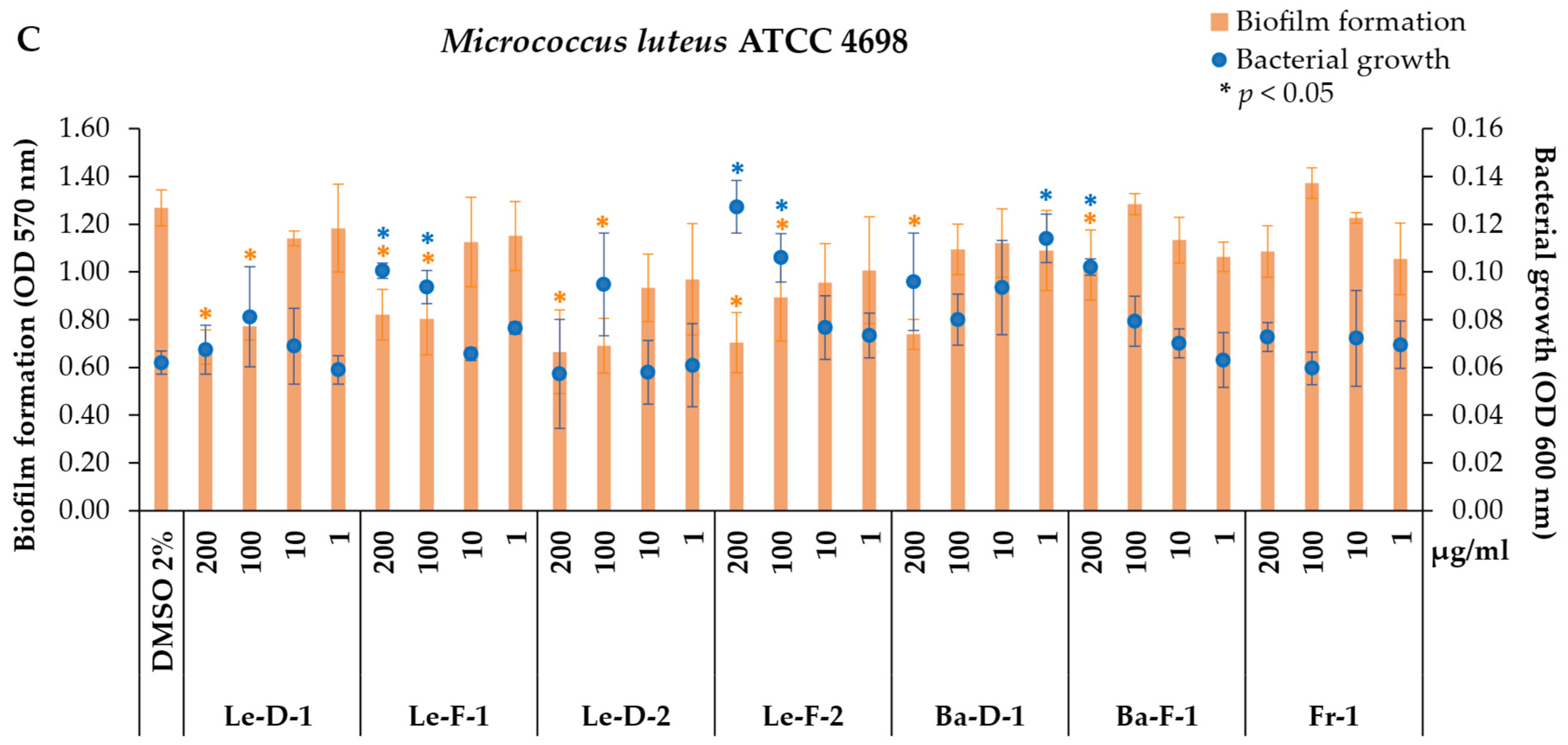
2.3. Antibiofilm and Antibacterial Activity of Essential Oils from Mespilodaphne cymbarum
3. Materials and Methods
3.1. Study Area
3.2. Processing the Vegetal Material
3.3. Extraction and Yield of Essential Oils (EOs) from Mespilodaphne cymbarum
3.4. Analysis of the Essential Oils (EOs) by Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
3.5. Data Processing
3.6. Molecular Networking
3.7. Bacterial Strains
3.8. Bacterial Growth and Biofilm Formation Assays
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO/EOs | Essential oil/Essential oils |
| GC-MS | Gas chromatography–mass spectrometry |
| DMSO | Dimethyl sulfoxide |
| Le-D-1 | Leaves from chemotype-1 collected in the dry season |
| Le-F-1 | Leaves from chemotype-1 collected in the flooding season |
| Le-D-2 | Leaves from chemotype-2 collected in the dry season |
| Le-F-2 | Leaves from chemotype-2 collected in the flooding season |
| Ba-D-1 | Bark from chemotype-1 collected in the dry season |
| Ba-F-1 | Bark from chemotype-1 collected in the flooding season |
| Fr-1 | Fruit from chemotype-1 collected in the flooding season |
| ATCC | American Type Culture Collection |
| PAO1 | Pseudomonas aeruginosa resistant to chloramphenicol |
References
- Damasceno, C.S.B.; Fabri Higaki, N.T.; Dias, J.d.F.G.; Miguel, M.D.; Miguel, O.G. Chemical Composition and Biological Activities of Essential Oils in the Family Lauraceae: A Systematic Review of the Literature. Planta Med. 2019, 85, 1054–1072. [Google Scholar] [CrossRef] [PubMed]
- Farias, K.S.; Alves, F.M.; Santos-Zanuncio, V.S.; de Sousa, P.T.J.; Silva, D.B.; Carollo, C.A. Global Distribution of the Chemical Constituents and Antibacterial Activity of Essential Oils in Lauraceae Family: A Review. S. Afr. J. Bot. 2023, 155, 214–222. [Google Scholar] [CrossRef]
- Trofimov, D.; Cadar, D.; Schmidt-Chanasit, J.; Rodrigues de Moraes, P.L.; Rohwer, J.G. A Comparative Analysis of Complete Chloroplast Genomes of Seven Ocotea Species (Lauraceae) Confirms Low Sequence Divergence within the Ocotea Complex. Sci. Rep. 2022, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, D.; de Moraes, P.L.R.; Rohwer, J.G. Towards a Phylogenetic Classification of the Ocotea Complex (Lauraceae): Classification Principles and Reinstatement of Mespilodaphne. Bot. J. Linn. Soc. 2019, 190, 25–50. [Google Scholar] [CrossRef]
- Souza, D.A.T.; de Lima, A.A.; Wrege, M.S.; de Aguiar, A.V.; Bezerra, C.d.S.; Meneses, C.H.S.G.; Lopes, R.; Ramos, S.L.F.; Paranatinga, I.L.D.; Lopes, M.T.G. Impacts of Climate Change on the Natural Distribution of Species of Lowland High and Low in the Amazon. Rev. Arvore 2024, 48, e4808. [Google Scholar] [CrossRef]
- Marinho, T.A.S.; Piedade, M.T.F.; Wittmann, F. Distribution and Population Structure of Four Central Amazonian High-Várzea Timber Species. Wetl. Ecol. Manag. 2010, 18, 665–677. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; López-Camacho, R.; González, M.C.; Castillo, O.J.; Cervantes-Díaz, M.; Celis, M. Prospecting for Non-Timber Forest Products by Chemical Analysis of Four Species of Lauraceae from the Amazon Region of Colombia. J. Wood Sci. 2024, 70, 33. [Google Scholar] [CrossRef]
- Diaz, A.M.P.; Gottlieb, H.E.; Gottlieb, O.R. Dehydrodieugenols from Ocotea cymbarum. Phytochemistry 1980, 19, 681–682. [Google Scholar] [CrossRef]
- Cabral, M.M.O.; Barbosa-Filho, J.M.; Maia, G.L.A.; Chaves, M.C.O.; Braga, M.V.; De Souza, W.; Soares, R.O.A. Neolignans from Plants in Northeastern Brazil (Lauraceae) with Activity against Trypanosoma cruzi. Exp. Parasitol. 2010, 124, 319–324. [Google Scholar] [CrossRef]
- Ferreira, B.A.; de Moura, F.B.R.; Gomes, K.S.; da Silva Souza, D.C.; Lago, J.H.G.; Araújo, F.d.A. Biseugenol from Ocotea cymbarum (Lauraceae) Attenuates Inflammation, Angiogenesis and Collagen Deposition of Sponge-Induced Fibrovascular Tissue in Mice. Inflammopharmacology 2023, 31, 1539–1549. [Google Scholar] [CrossRef]
- Ávila, W.A.D.; Suárez, L.E.C.; Fernando, J. Composición química del aceite esencial de Ocotea cymbarum Kunth (cascarillo y/o sasafrás) de la región Orinoquia. Rev. Cuba. De Plantas Med. 2016, 21, 248–260. [Google Scholar]
- Zoghbi, M.G.B.; Andrade, e.H.A.; Santos, A.S.; Silva, M.H.L.; Maia, J.G.S. Constituintes Voláteis de Espécies de Lauraceae com Ocorrência na Floresta Nacional de Caxiuanã-Melgaço-PA. In Estação Científica Ferreira Penna-Dez Anos de Pesquisa; Dez Anos de Pesquisa: Belém, Brazil, 2003; pp. 1–3. [Google Scholar]
- Passos, B.G.; De Albuquerque, R.D.D.G.; Muñoz-Acevedo, A.; Echeverria, J.; Llaure-Mora, A.M.; Ganoza-Yupanqui, M.L.; Rocha, L. Essential Oils from Ocotea Species: Chemical Variety, Biological Activities and Geographic Availability. Fitoterapia 2022, 156, 105065. [Google Scholar] [CrossRef] [PubMed]
- Shukis, A.J.; Wachs, H. Determination of Safrole in the Oil of Ocotea cymbarum. A Cryoscopic Method. Anal. Chem. 1948, 20, 248–249. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Cunha, R.M.; Dias, C.S.; Athayde-Filho, P.F.; Silva, M.S.; Da-Cunha, E.V.L.; Machado, M.I.L.; Craveiro, A.A.; Medeiros, I.A. GC-MS Analysis and Cardiovascular Activity of the Essential Oil of Ocotea Duckei. Rev. Bras. Farmacogn. 2008, 18, 37–41. [Google Scholar] [CrossRef]
- Chaverri, C.; Díaz, C.; Cicció, J.F. Chemical Analysis of Essential Oils from Ocotea gomezii W.C. Burger and Ocotea morae Gómez-Laur. (Lauraceae) Collected at “Reserva Biológica Alberto M. Brenes” in Costa Rica and Their Cytotoxic Activity on Tumor Cell Lines. J. Braz. Chem. Soc. 2011, 22, 741–745. [Google Scholar] [CrossRef]
- Silva, J.K.; Da Trindade, R.; Moreira, E.C.; Maia, J.G.S.; Dosoky, N.S.; Miller, R.S.; Cseke, L.J.; Setzer, W.N. Chemical Diversity, Biological Activity, and Genetic Aspects of Three Ocotea Species from the Amazon. Int. J. Mol. Sci. 2017, 18, 1081. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.L.; Pedrosa, T.D.N.; de Vasconcellos, M.C.; Lima, E.S.; Veiga-Junior, V.F. Ocotea (Lauraceae) Amazonian Essential Oils Chemical Composition and Their Tyrosinase Inhibition to Use in Cosmetics. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2020, 19, 519–526. [Google Scholar] [CrossRef]
- Gilardoni, G.; Montalván, M.; Vélez, M.; Malagón, O. Chemical and Enantioselective Analysis of the Essential Oils from Different Morphological Structures of Ocotea quixos (Lam.) Kosterm. Plants 2021, 10, 2171. [Google Scholar] [CrossRef]
- Takaku, S.; Haber, W.A.; Setzer, W.N. Leaf Essential Oil Composition of 10 Species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem. Syst. Ecol. 2007, 35, 525–532. [Google Scholar] [CrossRef]
- Rambo, M.A.; Soares, K.D.; Danielli, L.J.; Lana, D.F.D.; Bordignon, S.A.d.L.; Fuentefria, A.M.; Apel, M.A. Biological Activities of Essential Oils from Six Genotypes of Four Ocotea Species. Braz. J. Pharm. Sci. 2022, 58, e181097. [Google Scholar] [CrossRef]
- Xavier, J.K.A.M.; Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes. Biomolecules 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Guerrini, A.; Noriega, P.; Bianchi, A.; Bruni, R. Essential Oil of Wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) Leaves from Amazonian Ecuador. Flavour Fragr. J. 2006, 21, 674–676. [Google Scholar] [CrossRef]
- Radice, M.; Pietrantoni, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; Venturi, G.; Fortuna, C. Inhibitory Effect of Ocotea quixos (Lam.) Kosterm. and Piper aduncum L. Essential Oils from Ecuador on West Nile Virus Infection. Plant Biosyst. 2019, 153, 344–351. [Google Scholar] [CrossRef]
- Arteaga-Crespo, Y.; Ureta-Leones, D.; García-Quintana, Y.; Montalván, M.; Gilardoni, G.; Malagón, O. Preliminary Predictive Model of Termiticidal and Repellent Activities of Essential Oil Extracted from Ocotea quixos Leaves against Nasutitermes corniger (Isoptera: Termitidae) Using One-Factor Response Surface Methodology Design. Agronomy 2021, 11, 1249. [Google Scholar] [CrossRef]
- Valarezo, E.; Vullien, A.; Conde-Rojas, D. Variability of the Chemical Composition of the Essential Oil from the Amazonian Ishpingo Species (Ocotea quixos). Molecules 2021, 26, 3961. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M.; Romagnoli, C.; Sacchetti, G. Chemical Composition and Biological Activities of Ishpingo Essential Oil, a Traditional Ecuadorian Spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) Flower Calices. Food Chem. 2004, 85, 415–421. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana jatamansi Jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Karban, R.; Wetzel, W.C.; Shiojiri, K.; Ishizaki, S.; Ramirez, S.R.; Blande, J.D. Deciphering the Language of Plant Communication: Volatile Chemotypes of Sagebrush. New Phytol. 2014, 204, 380–385. [Google Scholar] [CrossRef]
- Farias, K.D.S.; Delatte, T.; Arruda, R.D.C.D.O.; Alves, F.M.; Silva, D.B.; Beekwilder, J.; Carollo, C.A. In Depth Investigation of the Metabolism of Nectandra megapotamica Chemotypes. PLoS ONE 2018, 13, e0201996. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Mssillou, I.; Touhtouh, J.; Aanniz, T.; Chamkhi, I.; El Omari, N.; Khalid, A.; Abdalla, A.N.; Aboulagras, S.; et al. Natural Sources and Pharmacological Properties of Santalenes and Santalols. Ind. Crops Prod. 2024, 214, 118567. [Google Scholar] [CrossRef]
- Silva, D.T.; Pinheiro, C.G.; Bianchini, N.H.; Batista, B.F.; Diefenthaeler, J.; Brião Muniz, M.D.F.; Heinzmann, B.M. Microbiological Damage Influences the Content, Chemical Composition and the Antifungal Activity of Essential Oils in a Wild-Growing Population of Ocotea lancifolia (Schott) Mez. J. Essent. Oil Res. 2018, 30, 265–277. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Lin, P.-A.; Waterman, J.M.; Erb, M. Dynamic Environmental Interactions Shaped by Vegetative Plant Volatiles. Nat. Prod. Rep. 2023, 40, 840–865. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.D.P.; Tondolo, J.S.M.; Schindler, B.; Silva, D.T.D.; Pinheiro, C.G.; Longhi, S.J.; Mallmann, C.A.; Heinzmann, B.M. Seasonal Influence on the Essential Oil Production of Nectandra megapotamica (Spreng.) Mez. Braz. Arch. Biol. Technol. 2015, 58, 12–21. [Google Scholar] [CrossRef]
- Ferraz, E.D.O.; Vieira, M.A.R.; Ferreira, M.I.; Fernandes Junior, A.; Marques, M.O.M.; Minatel, I.O.; Albano, M.; Sambo, P.; Lima, G.P.P. Seasonality Effects on Chemical Composition, Antibacterial Activity and Essential Oil Yield of Three Species of Nectandra. PLoS ONE 2018, 13, e0204132. [Google Scholar] [CrossRef]
- Silva, D.T.D.; Bianchini, N.H.; Amaral, L.D.P.; Longhi, S.J.; Heinzmann, B.M. Análise do Efeito da Sazonalidade Sobre o Rendimento do Óleo Essencial das Folhas de Nectandra grandiflora Nees. Rev. Árvore 2015, 39, 1065–1072. [Google Scholar] [CrossRef]
- de Lima, R.F.; Aparecido, L.E.d.O.; Torsoni, G.B.; Rolim, G.d.S. Climate Change Assessment in Brazil: Utilizing the Köppen-Geiger (1936) Climate Classification. Rev. Bras. Meteorol. 2024, 38, e38230001. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From Plants to Antimicrobials: Natural Products against Bacterial Membranes. Phytother. Res. 2022, 36, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Ruhal, R.; Kataria, R. Biofilm Patterns in Gram-Positive and Gram-Negative Bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—the “accidental” Pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and Its Dual Lifestyle in Skin Health and Infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; ben Salem, Y.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; et al. Chemical Composition of 8 Eucalyptus Species’ Essential Oils and the Evaluation of Their Antibacterial, Antifungal and Antiviral Activities. BMC Complement Altern. Med. 2012, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Verma, S.K.; Chauhan, A.; Venkatesha, K.; Verma, R.S.; Singh, V.R.; Darokar, M.P.; Chanotiya, C.S.; Padalia, R.C. Chemical Composition and Antibacterial Activity of Melaleuca bracteata Essential Oil from India: A Natural Source of Methyl Eugenol. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Joshi, R. Chemical Composition and Antimicrobial Activity of the Essential Oil of Ocimum basilicum L. (Sweet Basil) from Western Ghats of North West Karnataka, India. Ancient Sci. Life 2014, 33, 149. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Selestino Neta, M.C.; Vittorazzi, C.; Guimarães, A.C.; Martins, J.D.L.; Fronza, M.; Endringer, D.C.; Scherer, R. Effects of β-Caryophyllene and Murraya paniculata Essential Oil in the Murine Hepatoma Cells and in the Bacteria and Fungi 24-h Time–Kill Curve Studies. Pharm. Biol. 2017, 55, 190–197. [Google Scholar] [CrossRef]
- Merghni, A.; Marzouki, H.; Hentati, H.; Aouni, M.; Mastouri, M. Antibacterial and Antibiofilm Activities of Laurus nobilis L. Essential Oil against Staphylococcus aureus Strains Associated with Oral Infections. Curr. Res. Transl. Med. 2016, 64, 29–34. [Google Scholar] [CrossRef]
- Dib, J.R.; Liebl, W.; Wagenknecht, M.; Farías, M.E.; Meinhardt, F. Extrachromosomal Genetic Elements in Micrococcus. Appl. Microbiol. Biotechnol. 2013, 97, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nava, G.; Mohamed, A.; Yanez-Bello, M.A.; Trelles-Garcia, D.P. Advances in Medicine and Positive Natural Selection: Prosthetic Valve Endocarditis Due to Biofilm Producer Micrococcus luteus. IDCases 2020, 20, e00743. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, E.E.; Macedo, J.; Vieira, T.M.; Valsecchi, J.; Calvimontes, J.; Marmontel, M.; Queiroz, H.L. Ciclo Hidrológico nos Ambientes de Várzea da Reserva de Desenvolvimento Sustentável Mamirauá–Médio Rio Solimões, Período De 1990 A 2008. Sci. Mag. UAKARI 2009, 5, 61–87. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Trentin, D.d.S.; Giordani, R.B.; Zimmer, K.R.; da Silva, A.G.; da Silva, M.V.; Correia, M.T.d.S.; Baumvol, I.J.R.; Macedo, A.J. Potential of Medicinal Plants from the Brazilian Semi-Arid Region (Caatinga) against Staphylococcus epidermidis Planktonic and Biofilm Lifestyles. J. Ethnopharmacol. 2011, 137, 327–335. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2003; Available online: https://www.R-project.org/ (accessed on 10 September 2024).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7, 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 10 September 2024).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4, 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 September 2024).
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
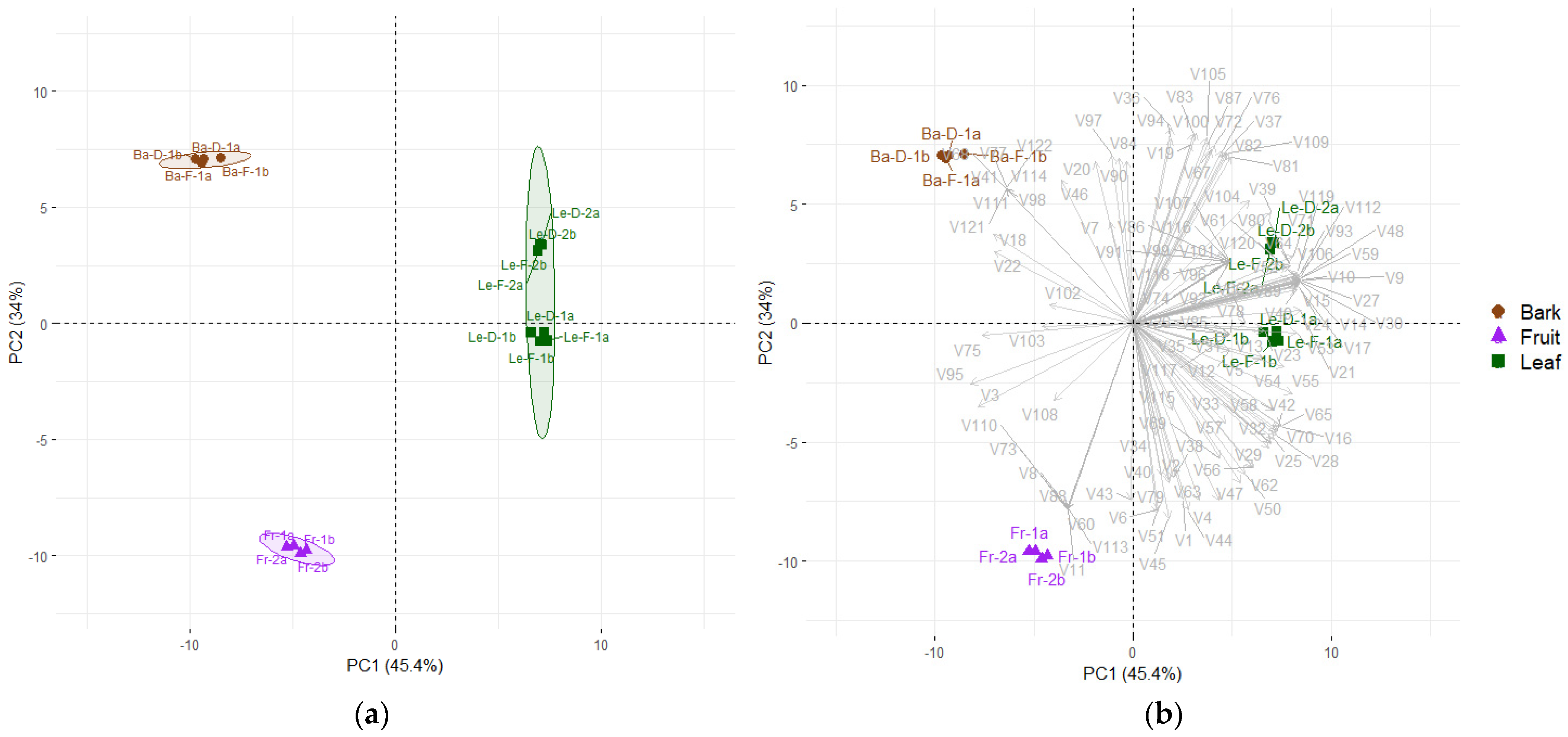
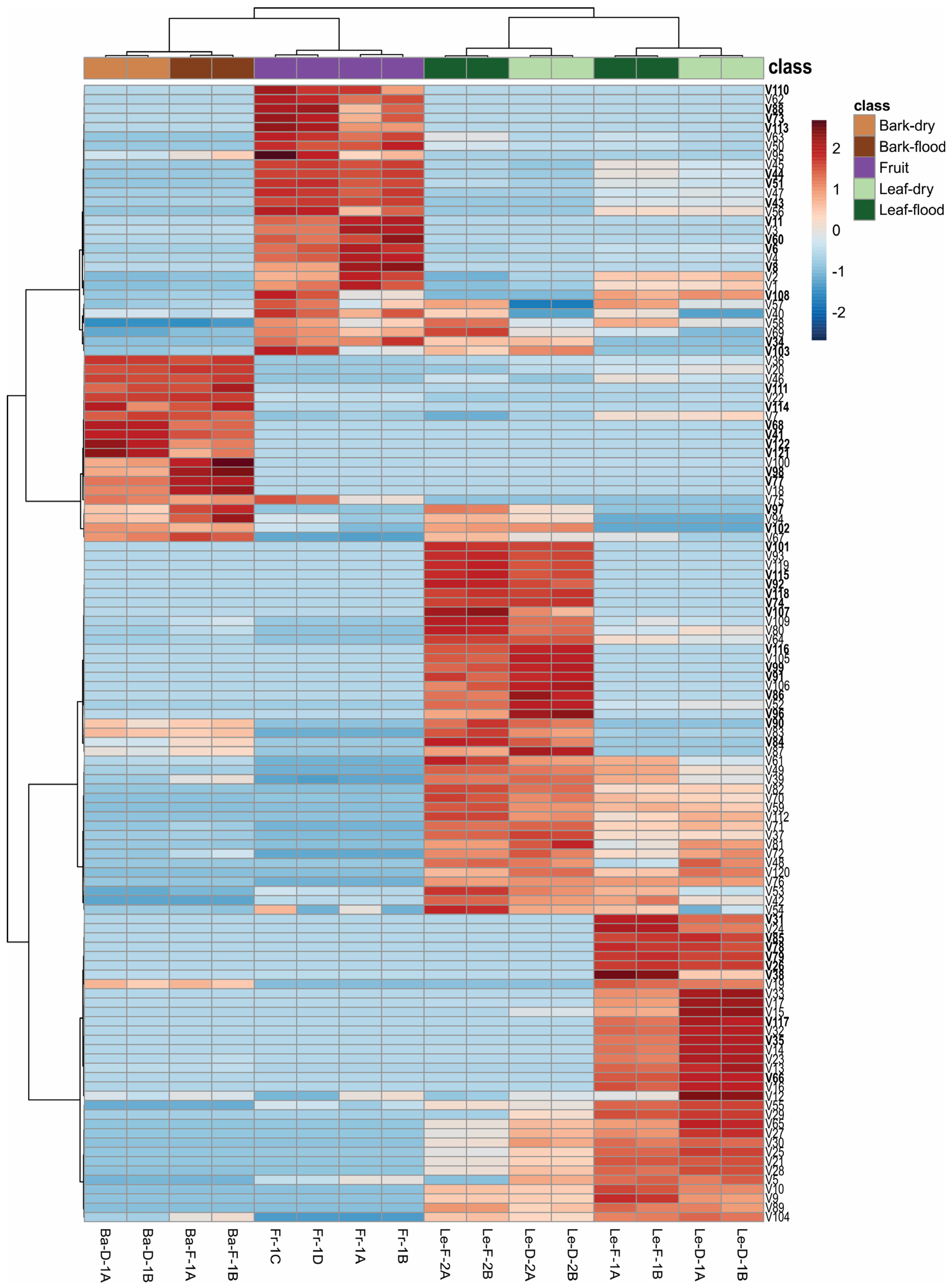
| VOC | IR * | Compound Name | Le-F-1 | Le-D-1 | Le-F-2 | Le-D-2 | Fr-1 | Ba-F-1 | Ba-D-1 | Class ** |
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 938 | α-Pinene | 1.71 ± 0.021 | 1.88 ± 0.247 | 0.1 ± 0.007 | 0.38 ± 0.007 | 3.32 ± 0.583 | 0.21 ± 0.021 | 0.08 ± 0.001 | HM |
| V2 | 979 | β-Pinene | 0.83 ± 0.007 | 0.87 ± 0.078 | <0.1 | 0.26 ± 0.007 | 1.2 ± 0.289 | 0.13 ± 0.007 | 0.08 ± 0.007 | HM |
| V3 | 990 | Myrcene | - | - | - | - | 0.6 ± 0.134 | <0.1 | <0.1 | HM |
| V4 | 1004 | α-Phellandrene | 0.11 ± 0.007 | 0.11 ± 0.007 | <0.1 | <0.1 | 1.76 ± 0.246 | - | - | HM |
| V5 | 1026 | p-Cymene | 2.64 ± 0.078 | 2.59 ± 0.198 | 0.2 ± 0.007 | 2.04 ± 0.042 | 0.96 ± 0.237 | <0.1 | <0.1 | HM |
| V6 | 1031 | Limonene | 2.37 ± 0.042 | 2.89 ± 0.233 | 0.33 ± 0.007 | 1.38 ± 0.028 | 19.32 ± 2.79 | 0.17 ± 0 | 0.22 ± 0.014 | HM |
| V7 | 1034 | 1,8-Cineole | 1.73 ± 0.007 | 1.9 ± 0.106 | 0.22 ± 0.007 | 0.76 ± 0.021 | 0.62 ± 0.114 | 3.33 ± 0.177 | 3.44 ± 0.184 | OM |
| V8 | 1050 | trans-β-Ocimene | - | - | - | - | 0.54 ± 0.179 | - | - | HM |
| V9 | 1074 | cis-Linalool oxide | 0.38 ± 0.007 | 0.28 ± 0.007 | 0.21 ± 0.002 | 0.21 ± 0.007 | - | - | - | OM |
| V10 | 1088 | trans-Linalool oxide | 0.27 ± 0.007 | 0.22 ± 0.001 | 0.17 ± 0.001 | 0.16 ± 0.001 | - | - | - | OM |
| V11 | 1088 | Terpinolene | - | - | - | - | 0.5 ± 0.074 | - | - | HM |
| V12 | 1098 | Linalool | 0.11 ± 0.007 | 0.34 ± 0.007 | <0.1 | 0.1 ± 0.007 | <0.1 | 0.1 ± 0.007 | 0.08 ± 0.014 | OM |
| V13 | 1141 | trans-Pinocarveol | 0.86 ± 0.021 | 1.13 ± 0.113 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | OM |
| V14 | 1144 | cis-Verbenol | 0.26 ± 0.007 | 0.37 ± 0.001 | <0.1 | <0.1 | - | - | - | OM |
| V15 | 1148 | trans-Verbenol | 0.63 ± 0.014 | 1.3 ± 0.014 | <0.1 | 0.2 ± 0.007 | - | - | - | OM |
| V16 | 1153 | NI (m/z 138) | 0.67 ± 0.035 | 0.8 ± 0.007 | <0.1 | <0.1 | <0.1 | - | - | |
| V17 | 1165 | Pinocarvone | 0.27 ± 0.007 | 0.49 ± 0.001 | <0.1 | <0.1 | - | - | - | OM |
| V18 | 1169 | Borneol | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.88 ± 0.049 | 0.65 ± 0.396 | OM |
| V19 | 1170 | p-Mentha-1,5-dien-8-ol | 0.55 ± 0.021 | 0.5 ± 0.007 | <0.1 | <0.1 | - | <0.1 | 0.35 ± 0.042 | OM |
| V20 | 1179 | Terpinen-4-ol | 0.55 ± 0.007 | 0.76 ± 0.007 | 0.18 ± 0.007 | 0.41 ± 0.021 | 0.18 ± 0.031 | 2.07 ± 0.014 | 1.97 ± 0.021 | OM |
| V21 | 1188 | Cryptone | 5.03 ± 0.014 | 5.08 ± 0.134 | 1.94 ± 0.028 | 2.64 ± 0.035 | <0.1 | <0.1 | <0.1 | Ke |
| V22 | 1192 | α-Terpineol | 0.42 ± 0.014 | 0.64 ± 0.007 | 0.23 ± 0.007 | 0.29 ± 0.007 | 0.75 ± 0.049 | 4.5 ± 0.078 | 4.38 ± 0.042 | OM |
| V23 | 1197 | Myrtenal | 0.72 ± 0.014 | 1.1 ± 0.007 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | OM |
| V24 | 1210 | Verbenone | 1.29 ± 0.021 | 0.86 ± 0.007 | <0.1 | <0.1 | - | - | - | OM |
| V25 | 1221 | trans-Carveol | 0.62 ± 0.007 | 0.7 ± 0.007 | 0.22 ± 0.007 | 0.34 ± 0.014 | <0.1 | - | - | OM |
| V26 | 1226 | NI | 0.57 ± 0.007 | 0.56 ± 0.007 | - | - | - | - | - | |
| V27 | 1243 | p-Isopropylbenzaldehyde (p-Cumic aldehyde) | 1.94 ± 0.057 | 2.58 ± 0.028 | 0.95 ± 0.035 | 1.62 ± 0.049 | - | - | - | OM |
| V28 | 1247 | Carvone | 0.8 ± 0.028 | 0.89 ± 0.007 | 0.35 ± 0.007 | 0.52 ± 0.014 | <0.1 | - | - | OM |
| V29 | 1276 | p-Menth-1-en-7-al | 0.63 ± 0.007 | 0.67 ± 0.001 | <0.1 | 0.26 ± 0.014 | <0.1 | - | - | OM |
| V30 | 1292 | p-Cymen-7-ol | 0.61 ± 0.028 | 0.64 ± 0.014 | 0.32 ± 0.007 | 0.52 ± 0.035 | - | - | - | OM |
| V31 | 1308 | NI | 0.74 ± 0.014 | 0.59 ± 0.007 | - | - | - | - | - | |
| V32 | 1331 | NI | 1.04 ± 0.014 | 1.39 ± 0.014 | <0.1 | <0.1 | <0.1 | - | - | |
| V33 | 1334 | NI | 0.61 ± 0.007 | 1.05 ± 0.028 | <0.1 | <0.1 | <0.1 | - | - | |
| V34 | 1337 | δ-Elemene | - | - | 0.26 ± 0.007 | 0.26 ± 0.014 | 0.37 ± 0.045 | - | - | HS |
| V35 | 1339 | NI | 1.57 ± 0.007 | 2.12 ± 0.014 | - | - | - | - | - | |
| V36 | 1351 | α-Cubebene | 0.18 ± 0.007 | 0.21 ± 0.014 | <0.1 | <0.1 | - | 1.1 ± 0.049 | 1.12 ± 0.014 | HS |
| V37 | 1368 | Cyclosativene | 0.32 ± 0.007 | 0.33 ± 0.007 | 0.62 ± 0.007 | 0.68 ± 0.007 | - | <0.1 | <0.1 | HS |
| V38 | 1370 | NI | 1.07 ± 0.035 | 0.35 ± 0.001 | - | - | <0.1 | - | - | |
| V39 | 1376 | α-Copaene | 1.39 ± 0.021 | 0.91 ± 0.014 | 1.65 ± 0.021 | 1.7 ± 0.014 | 0.26 ± 0.024 | 0.95 ± 0.049 | 0.53 ± 0.014 | HS |
| V40 | 1391 | β-Elemene | 0.18 ± 0.007 | <0.1 | 0.22 ± 0.007 | <0.1 | 0.32 ± 0.051 | <0.1 | <0.1 | HS |
| V41 | 1411 | Methyl eugenol | - | - | - | - | - | 37.49 ± 1.29 | 43.61 ± 0.49 | Ph |
| V42 | 1419 | β-Caryophyllene | 7.91 ± 0.926 | 4.77 ± 0.106 | 8.99 ± 0.071 | 7.61 ± 0.113 | 2.97 ± 0.093 | - | - | HS |
| V43 | 1421 | α-Santalene | 6.17 ± 0.085 | 6.29 ± 0.226 | <0.1 | <0.1 | 26.41 ± 0.50 | 1.69 ± 0.028 | 1.11 ± 0.028 | HS |
| V44 | 1437 | trans-α-Bergamotene | 2.85 ± 0.042 | 1.94 ± 0.014 | 0.79 ± 0.007 | 0.33 ± 0.007 | 8.18 ± 0.181 | 0.27 ± 0.021 | 0.2 ± 0.014 | HS |
| V45 | 1439 | α-Guaiene | 0.68 ± 0.007 | 0.45 ± 0.001 | 0.21 ± 0.001 | <0.1 | 1.95 ± 0.054 | <0.1 | <0.1 | HS |
| V46 | 1444 | 6,9-Guaiadiene | 0.29 ± 0.007 | 0.15 ± 0.021 | 0.16 ± 0.001 | <0.1 | <0.1 | 0.79 ± 0.042 | 0.79 ± 0.014 | HS |
| V47 | 1448 | epi-β-Santalene | 0.3 ± 0.007 | 0.37 ± 0.049 | <0.1 | <0.1 | 1.61 ± 0.107 | - | - | HS |
| V48 | 1451 | Allo-Aromadendrene | <0.1 | 0.3 ± 0.035 | 0.31 ± 0.007 | 0.28 ± 0.021 | - | - | - | HS |
| V49 | 1454 | α-Humulene | 0.72 ± 0.007 | 0.45 ± 0.021 | 0.92 ± 0.007 | 0.85 ± 0.021 | <0.1 | 0.16 ± 0.007 | <0.1 | HS |
| V50 | 1458 | trans-β-Farnesene | 0.24 ± 0.007 | 0.14 ± 0.021 | 0.35 ± 0.007 | 0.22 ± 0.001 | 1.47 ± 0.115 | - | - | HS |
| V51 | 1462 | β-Santalene | 3.77 ± 0.014 | 2.74 ± 0.021 | 2.05 ± 0.007 | 1.24 ± 0.014 | 12.02 ± 0.64 | 0.78 ± 0.049 | 0.45 ± 0.021 | HS |
| V52 | 1470 | Naphthalene, 1,2,3,4,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methylethenyl)-, (1R,7S,8aS)- | 0.58 ± 0.007 | 0.9 ± 0.001 | 2.89 ± 0.014 | 3.73 ± 0.085 | <0.1 | <0.1 | <0.1 | HS |
| V53 | 1477 | γ-Muurolene | 1.45 ± 0.014 | 0.71 ± 0.007 | 2.19 ± 0.007 | 1.75 ± 0.071 | 0.65 ± 0.092 | 0.25 ± 0.007 | <0.1 | HS |
| V54 | 1480 | α-Amorphene | 0.31 ± 0.021 | <0.1 | 0.54 ± 0.007 | 0.36 ± 0.007 | 0.14 ± 0.158 | <0.1 | 0.07 ± 0 | HS |
| V55 | 1485 | β-Selinene | 2.85 ± 0.021 | 3.14 ± 0.007 | 1.49 ± 0.021 | 1.34 ± 0.021 | 0.84 ± 0.241 | 0.06 ± 0.014 | 0.05 ± 0 | HS |
| V56 | 1488 | Eremophilene | 0.3 ± 0.007 | 0.27 ± 0.007 | <0.1 | <0.1 | 0.66 ± 0.18 | - | - | HS |
| V57 | 1491 | β-cadinene | 0.45 ± 0.014 | 0.27 ± 0.001 | 0.44 ± 0.007 | <0.1 | 0.42 ± 0.132 | 0.2 ± 0.014 | 0.16 ± 0.014 | HS |
| V58 | 1494 | Viridiflorene | 1.09 ± 0.007 | 0.73 ± 0.014 | 1.3 ± 0.014 | 0.67 ± 0.014 | 1 ± 0.202 | 0.19 ± 0.035 | 0.17 ± 0.007 | HS |
| V59 | 1498 | NI | 0.64 ± 0.021 | 0.58 ± 0.028 | 0.9 ± 0.021 | 0.74 ± 0.007 | - | - | - | |
| V60 | 1498 | α-Selinene | - | - | - | - | 0.38 ± 0.065 | - | - | HS |
| V61 | 1499 | α-Muurolene | 0.51 ± 0.007 | 0.23 ± 0.001 | 0.78 ± 0.049 | 0.56 ± 0.014 | <0.1 | 0.17 ± 0.007 | 0.16 ± 0.007 | HS |
| V62 | 1505 | α-Bulnesene | <0.1 | <0.1 | <0.1 | <0.1 | 0.39 ± 0.056 | - | - | HS |
| V63 | 1509 | β-Bisabolene | 0.43 ± 0.014 | 0.26 ± 0.021 | 0.66 ± 0.007 | 0.26 ± 0.001 | 2.12 ± 0.221 | <0.1 | 0.01 ± 0 | HS |
| V64 | 1514 | γ-Cadinene | 1.28 ± 0.028 | 0.88 ± 0.014 | 3.1 ± 0.014 | 2.93 ± 0.007 | <0.1 | 0.34 ± 0.007 | 0.21 ± 0.007 | HS |
| V65 | 1517 | NI | 0.63 ± 0.007 | 0.93 ± 0.014 | 0.32 ± 0.007 | 0.51 ± 0.014 | <0.1 | - | - | |
| V66 | 1521 | NI | 2.61 ± 0.007 | 3.02 ± 0.035 | - | - | - | - | - | |
| V67 | 1525 | δ-Cadinene | 3.37 ± 0.049 | 1.93 ± 0.021 | 5.14 ± 0.014 | 3.62 ± 0.028 | 0.52 ± 0.119 | 7.41 ± 0.332 | 6.29 ± 0.304 | HS |
| V68 | 1528 | Myristicin | - | - | - | - | - | 7.37 ± 0.205 | 9.6 ± 0.219 | Ph |
| V69 | 1534 | trans-γ-Bisabolene | 0.98 ± 0.014 | 0.37 ± 0.007 | 2.61 ± 0.028 | 1.21 ± 0.021 | 1.97 ± 0.234 | 0.43 ± 0.028 | 0.21 ± 0.014 | HS |
| V70 | 1540 | α-Cadinene | 0.27 ± 0.021 | 0.22 ± 0.014 | 0.45 ± 0.021 | 0.38 ± 0.021 | <0.1 | - | - | HS |
| V71 | 1543 | NI | 1.26 ± 0.007 | 1.46 ± 0.113 | 2.06 ± 0.021 | 2.14 ± 0.007 | <0.1 | 0.32 ± 0.064 | 0.19 ± 0 | |
| V72 | 1545 | α-Calacorene | 0.49 ± 0.028 | 0.75 ± 0.057 | 0.79 ± 0.007 | 0.87 ± 0.078 | - | 0.3 ± 0.042 | 0.16 ± 0.007 | HS |
| V73 | 1545 | trans-α-bisabolene | - | - | - | - | 0.42 ± 0.113 | - | - | HS |
| V74 | 1547 | NI | - | - | 2.99 ± 0.014 | 3.08 ± 0.035 | - | - | - | |
| V75 | 1551 | α-Elemol | <0.1 | <0.1 | <0.1 | <0.1 | 0.54 ± 0.318 | 0.7 ± 0.042 | 0.79 ± 0.028 | OS |
| V76 | 1558 | NI | 3.35 ± 0.007 | 3.36 ± 0.071 | 3.32 ± 0.028 | 3.45 ± 0.007 | - | 0.5 ± 0.028 | 0.54 ± 0.014 | |
| V77 | 1564 | Elemicin | - | - | - | - | - | 11.89 ± 0.17 | 8.82 ± 0.014 | Ph |
| V78 | 1565 | NI | 1.53 ± 0.028 | 1.47 ± 0.057 | - | - | - | - | - | |
| V79 | 1567 | NI | 0.83 ± 0.014 | 0.8 ± 0.007 | - | - | <0.1 | - | - | |
| V80 | 1579 | Spathulenol | 0.19 ± 0.014 | 0.3 ± 0.028 | 0.83 ± 0.014 | 0.66 ± 0.014 | <0.1 | <0.1 | <0.1 | OS |
| V81 | 1584 | Caryophyllene oxide | 1.39 ± 0.205 | 2.92 ± 0.113 | 2.88 ± 0.007 | 3.96 ± 0.389 | - | <0.1 | 0.22 ± 0.007 | OS |
| V82 | 1589 | NI (m/z = 234) | 5.38 ± 0.191 | 5.87 ± 0.134 | 10.99 ± 0.057 | 9.84 ± 0.134 | - | 0.47 ± 0.042 | 0.58 ± 0.007 | |
| V83 | 1603 | Rosifoliol | 0.26 ± 0.021 | 0.3 ± 0.007 | 1.41 ± 0.071 | 1.11 ± 0.049 | - | 0.87 ± 0.049 | 0.91 ± 0.049 | OS |
| V84 | 1605 | NI | - | - | 1.04 ± 0.007 | 0.83 ± 0.099 | - | 0.45 ± 0.035 | 0.24 ± 0.021 | |
| V85 | 1605 | NI | 0.85 ± 0.021 | 0.86 ± 0.035 | - | - | - | - | - | |
| V86 | 1608 | NI | - | - | 0.54 ± 0.014 | 0.74 ± 0.078 | - | - | - | |
| V87 | 1611 | Humulene epoxide II | <0.1 | <0.1 | 0.46 ± 0.021 | 0.79 ± 0.035 | - | 0.29 ± 0.007 | 0.2 ± 0.028 | OS |
| V88 | 1614 | Tetradecanal | - | - | - | - | 0.21 ± 0.065 | - | - | |
| V89 | 1614 | Viridiflorol | 0.53 ± 0.028 | 0.49 ± 0.035 | 0.47 ± 0.002 | 0.36 ± 0.028 | - | - | - | OS |
| V90 | 1618 | 1,10-di-epi-Cubenol | - | - | 1.29 ± 0.156 | 1.19 ± 0.064 | - | 0.81 ± 0.042 | 0.73 ± 0.134 | OS |
| V91 | 1620 | NI | - | - | 1.84 ± 0.17 | 2.06 ± 0.042 | - | - | - | |
| V92 | 1622 | epi-γ-Eudesmol | - | - | 0.77 ± 0.014 | 0.68 ± 0.035 | - | - | - | OS |
| V93 | 1623 | NI (m/z = 236) | <0.1 | <0.1 | 1.04 ± 0.014 | 0.96 ± 0.021 | - | - | - | |
| V94 | 1630 | 1-epi-Cubenol | <0.1 | <0.1 | 0.52 ± 0.007 | 0.39 ± 0.007 | 0.2 ± 0.082 | 0.84 ± 0.156 | 0.48 ± 0.021 | OS |
| V95 | 1634 | γ-Eudesmol | - | - | - | - | 0.5 ± 0.258 | 0.23 ± 0.064 | 0.1 ± 0.014 | OS |
| V96 | 1638 | Hinesol | - | - | 0.65 ± 0.021 | 1.17 ± 0.035 | - | - | - | OS |
| V97 | 1643 | 1,2,3,4,4a,7,8,8a-octahydro-1,6-dimethyl-4-(1-methylethyl)-1-Naphthalenol, | - | - | 0.33 ± 0.007 | 0.18 ± 0.014 | - | <0.1 | <0.1 | OS |
| V98 | 1645 | epi-α-Cadinol | - | - | - | - | - | 1.19 ± 0.064 | 0.61 ± 0.014 | OS |
| V99 | 1645 | NI | - | - | 0.59 ± 0.028 | 0.7 ± 0.028 | - | - | - | |
| V100 | 1649 | α-Muurolol (=Torreyol) | <0.1 | <0.1 | <0.1 | <0.1 | - | 0.33 ± 0.057 | 0.17 ± 0.014 | OS |
| V101 | 1651 | NI | - | - | 3.01 ± 0.042 | 2.87 ± 0.001 | - | - | - | |
| V102 | 1652 | β-Eudesmol | - | - | 2.43 ± 0.057 | 2.62 ± 0.092 | 0.62 ± 0.398 | 2.27 ± 0.12 | 2.52 ± 0.028 | OS |
| V103 | 1655 | α-Eudesmol | - | - | 0.5 ± 0.071 | 0.73 ± 0.021 | 0.62 ± 0.419 | <0.1 | <0.1 | OS |
| V104 | 1657 | α-Cadinol | 2.74 ± 0.028 | 2.9 ± 0.092 | 2.07 ± 0.049 | 1.8 ± 0.028 | <0.1 | 1.53 ± 0.085 | 0.73 ± 0.028 | OS |
| V105 | 1660 | NI | <0.1 | <0.1 | 1.28 ± 0.028 | 1.58 ± 0.021 | - | <0.1 | <0.1 | |
| V106 | 1664 | NI | <0.1 | <0.1 | 0.4 ± 0.057 | 0.56 ± 0.035 | - | - | - | |
| V107 | 1671 | NI | - | - | 0.49 ± 0.014 | 0.27 ± 0.049 | - | - | - | |
| V108 | 1671 | β-Bisabolol | 0.46 ± 0.042 | 0.51 ± 0.014 | - | - | 0.47 ± 0.242 | <0.1 | 0.07 ± 0.007 | OS |
| V109 | 1676 | Cadalene | 0.19 ± 0.078 | 0.13 ± 0.028 | 0.91 ± 0.014 | 0.7 ± 0.007 | - | 0.17 ± 0.035 | 0.08 ± 0.007 | HS |
| V110 | 1678 | cis-α-Santalol | - | - | - | - | 0.78 ± 0.161 | - | - | OS |
| V111 | 1678 | Bulnesol | - | - | - | - | - | 1.69 ± 0.247 | 1.47 ± 0.099 | OS |
| V112 | 1680 | NI | 1.04 ± 0.099 | 1.41 ± 0.014 | 2.08 ± 0.007 | 1.64 ± 0.035 | - | - | - | |
| V113 | 1680 | NI | - | - | - | - | 0.6 ± 0.171 | - | - | |
| V114 | 1680 | NI | - | - | - | - | - | 0.65 ± 0.085 | 0.6 ± 0.163 | |
| V115 | 1687 | α-Bisabolol | - | - | 0.61 ± 0.014 | 0.55 ± 0.014 | <0.1 | - | - | OS |
| V116 | 1708 | NI | - | - | 0.76 ± 0.001 | 0.87 ± 0.014 | - | - | - | |
| V117 | 1710 | NI | 0.85 ± 0.014 | 1.12 ± 0.057 | - | - | - | - | - | |
| V118 | 1729 | NI | - | - | 0.62 ± 0.014 | 0.62 ± 0.014 | - | - | - | |
| V119 | 1745 | NI | <0.1 | <0.1 | 0.86 ± 0.021 | 0.76 ± 0.021 | - | - | - | |
| V120 | 1751 | NI | 0.48 ± 0.007 | 0.67 ± 0.021 | 0.52 ± 0.014 | 0.69 ± 0.007 | - | - | - | |
| V121 | 1764 | Guaiol acetate | - | - | - | - | - | 0.28 ± 0.057 | 0.47 ± 0.028 | OS |
| V122 | 1806 | NI | - | - | - | - | - | 1.2 ± 0.071 | 1.8 ± 0.134 | |
| Hydrocarbon monoterpene | 7.6 | 8.3 | 0.6 | 4.1 | 28.2 | 0.6 | 0.5 | HM | ||
| Hydrocarbon sesquiterpene | 39.5 | 29.9 | 39.9 | 31.7 | 65.1 | 15.6 | 12.4 | HS | ||
| Ketone | 5.0 | 5.1 | 1.9 | 2.6 | 0.0 | 0.0 | 0.0 | Ke | ||
| Oxygenated monoterpene | 12.6 | 15.3 | 2.9 | 5.4 | 1.7 | 11.3 | 10.9 | OM | ||
| Oxygenated sesquiterpene | 5.1 | 6.9 | 14.9 | 15.8 | 3.8 | 11.8 | 9.7 | OS | ||
| Phenylpropanoid | - | - | - | - | - | 56.7 | 62.0 | Ph | ||
| Not identified (unknown) | 26.7 | 29.4 | 36.4 | 35.6 | 0.9 | 3.7 | 4.0 | NI | ||
| Total (%) | 96.08 | 94.45 | 96.28 | 94.90 | 99.60 | 99.75 | 99.55 | |||
| Yield (v/w %) | 0.91 | 0.91 | 0.93 | 1.2 | 3.6 | 0.6 | 0.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boaretto, A.G.; Gris, D.; Scherer, J.; Farias, K.S.; Quadros, J.C.; Macedo, A.J.; Carollo, C.A.; Silva, D.B. Chemotypic and Seasonal Variations in Essential Oils from Mespilodaphne cymbarum (Kunth) Trofimov and Their Antibacterial and Antibiofilm Activities. Plants 2025, 14, 1939. https://doi.org/10.3390/plants14131939
Boaretto AG, Gris D, Scherer J, Farias KS, Quadros JC, Macedo AJ, Carollo CA, Silva DB. Chemotypic and Seasonal Variations in Essential Oils from Mespilodaphne cymbarum (Kunth) Trofimov and Their Antibacterial and Antibiofilm Activities. Plants. 2025; 14(13):1939. https://doi.org/10.3390/plants14131939
Chicago/Turabian StyleBoaretto, Amanda Galdi, Darlene Gris, Jéssica Scherer, Katyuce Souza Farias, Jean Carlo Quadros, Alexandre José Macedo, Carlos Alexandre Carollo, and Denise Brentan Silva. 2025. "Chemotypic and Seasonal Variations in Essential Oils from Mespilodaphne cymbarum (Kunth) Trofimov and Their Antibacterial and Antibiofilm Activities" Plants 14, no. 13: 1939. https://doi.org/10.3390/plants14131939
APA StyleBoaretto, A. G., Gris, D., Scherer, J., Farias, K. S., Quadros, J. C., Macedo, A. J., Carollo, C. A., & Silva, D. B. (2025). Chemotypic and Seasonal Variations in Essential Oils from Mespilodaphne cymbarum (Kunth) Trofimov and Their Antibacterial and Antibiofilm Activities. Plants, 14(13), 1939. https://doi.org/10.3390/plants14131939







