Rhizosphere Growth-Promoting Bacteria Enhance Oat Growth by Improving Microbial Stability and Soil Organic Matter in the Saline Soil of the Qaidam Basin
Abstract
1. Introduction
2. Results
2.1. Oat Growth Performance and Root Development
2.2. Rhizosphere Soil Physicochemical Properties
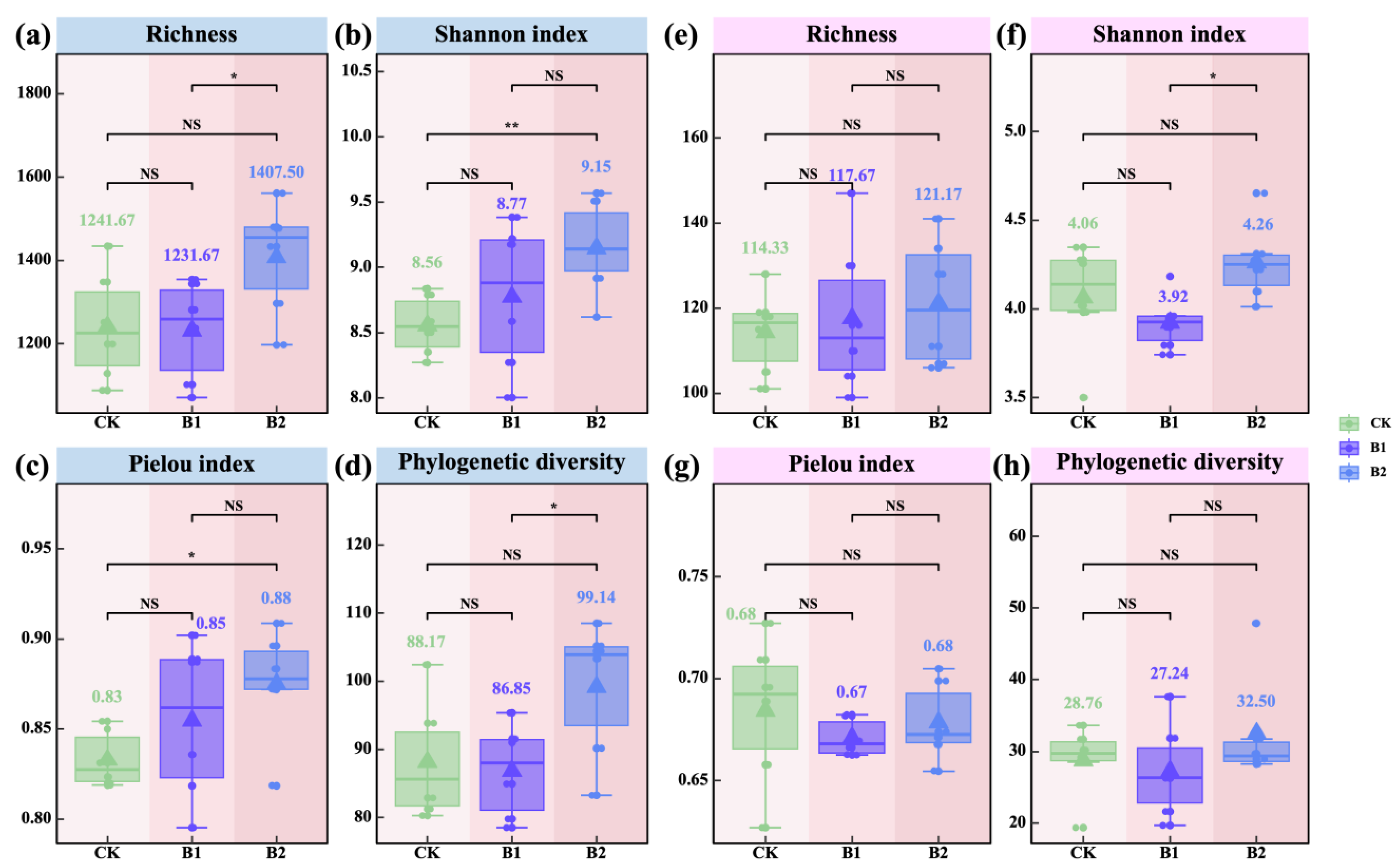
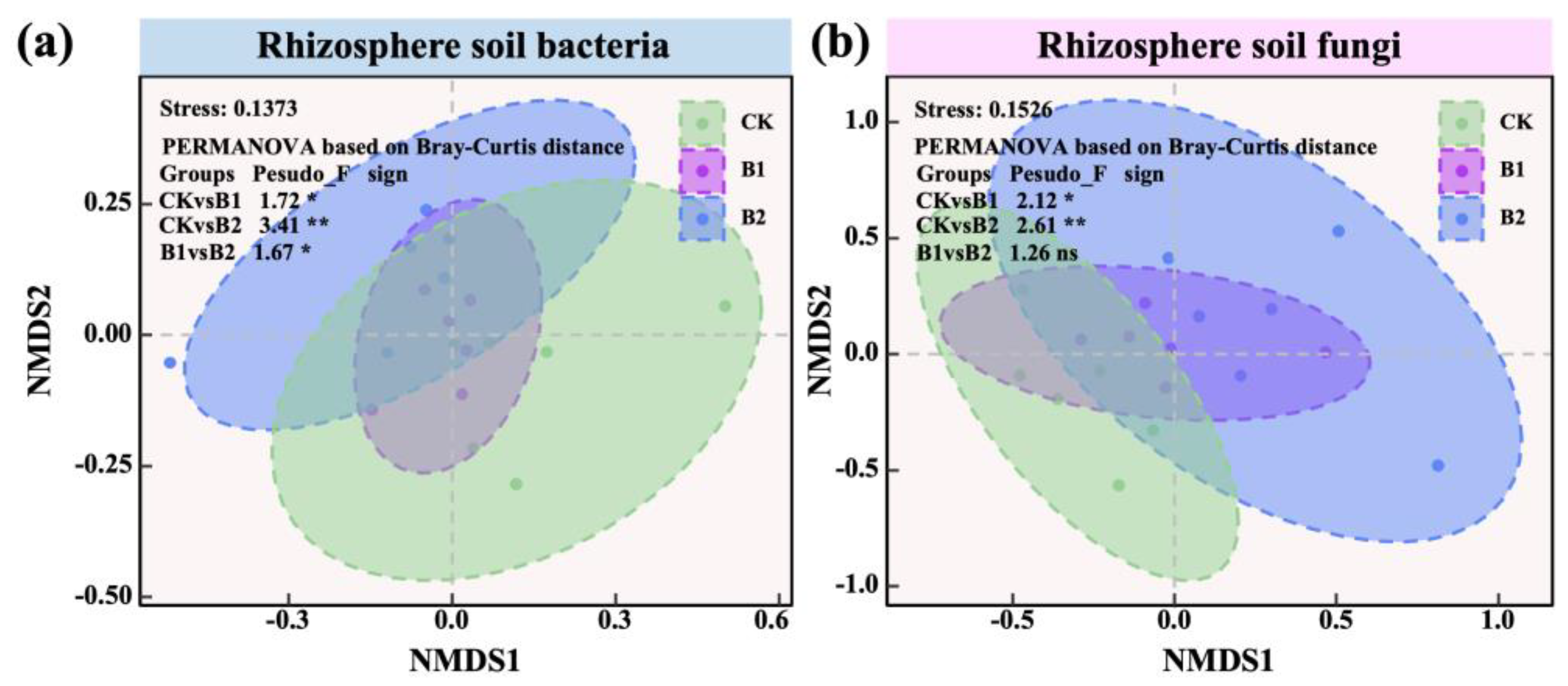
2.3. Diversity, Structure, and Stability of Rhizosphere Microbial Communities
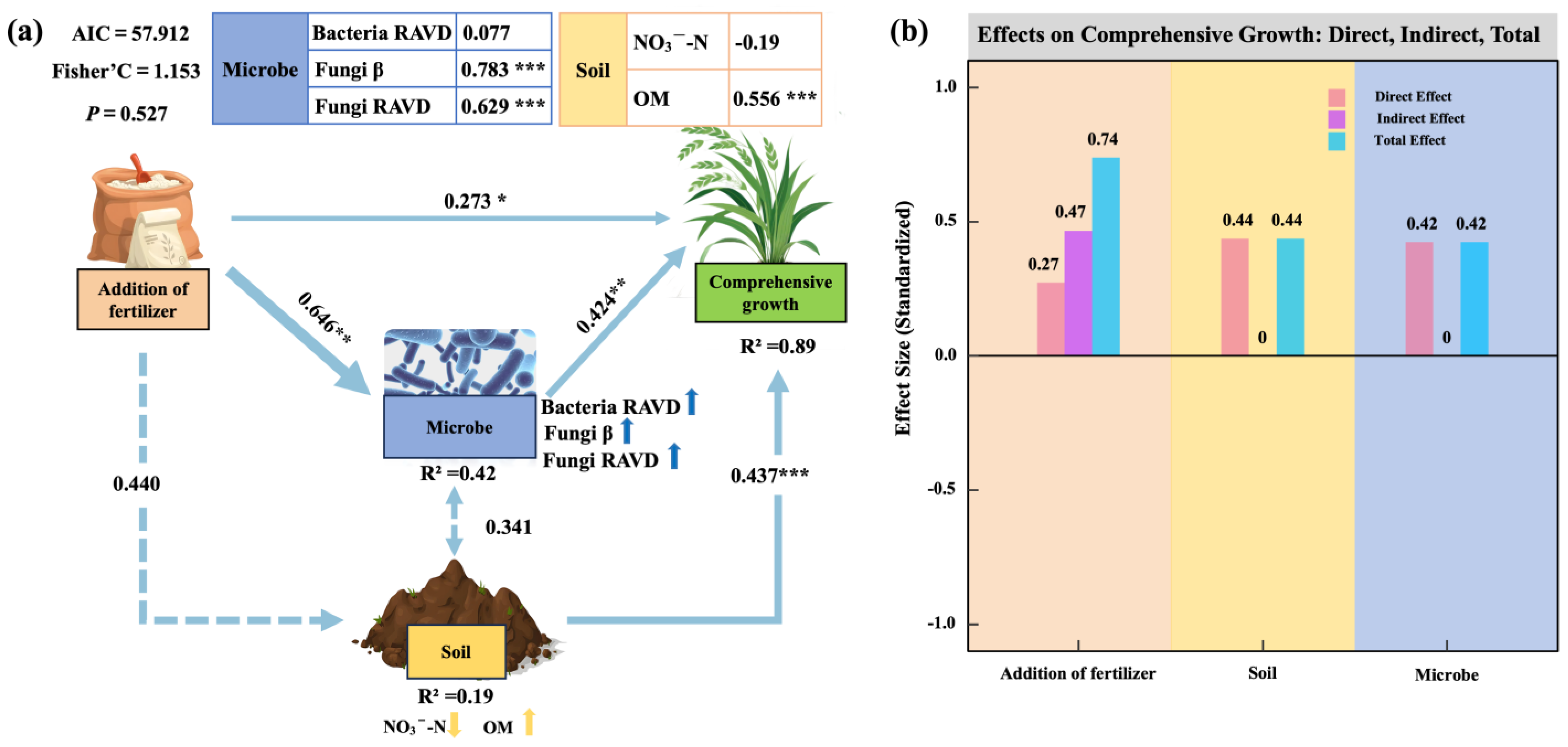
2.4. Comprehensive Oat Growth and Regulatory Pathways of Key Driving Factors
3. Discussion
3.1. Effects of PGPR on Oat Growth and Root Development
3.2. Effects of PGPR on Rhizosphere Soil Nutrients and pH
3.3. Effects of PGPR on the Diversity, Structure, and Stability of Rhizosphere Microbial Communities
3.4. Effects of PGPR on Comprehensive Oat Growth Through Coordinated Regulation of Soil and Microbial Communities
4. Materials and Methods
4.1. Experimental Site Description and PGPR Preparation
4.2. Experimental Design and Field Management
4.3. Plant Growth Assessment
4.4. Soil and Rhizosphere Sampling
4.5. Rhizosphere DNA Sample Preparation
4.6. Rhizosphere Microbial DNA Extraction, Amplicon Library Construction, and Bioinformatic Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Dong, S.; Wen, L.; Wang, X.; Wu, Y. Three-Dimensional Framework of Vigor, Organization, and Resilience (VOR) for Assessing Rangeland Health: A Case Study from the Alpine Meadow of the Qinghai-Tibetan Plateau, China. Ecohealth 2013, 10, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Tan, F.; Liao, W.; Wang, Q.; Mu, J.; Zhou, X.; Yang, Z.; Zhao, X. Isolation and Identification of Lactobacillus Plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms 2019, 7, 530. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Pei, J.; Wang, X.; Guo, S.; Guo, X.; Yan, P. Lipidomics and Transcriptome Reveal the Effects of Feeding Systems on Fatty Acids in Yak’s Meat. Foods 2022, 11, 2582. [Google Scholar] [CrossRef]
- Ren, C.Z.; Cui, L.; Yang, C.; Tian, C.Y.; Fu, X.F.; Liu, Y.M.; Zhao, G.Q.; Guo, L. Establishment and Application of High Efficient Breeding Technology System of Oat in China. J. Agric. Sci. Technol. 2016, 18, 1–6. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Wang, J.; Zhang, H. Comparative Study on Production Performance and Nutritional Quality of Eight Importeooat Varieties in the Shioatse Redion of Tibet. China Pratacult. Sci. 2019, 36, 1117–1125. [Google Scholar]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Hui, R.; Tan, H.; Li, X.; Wang, B. Variation of Soil Physical-Chemical Characteristics in Salt-Affected Soil in the Qarhan Salt Lake, Qaidam Basin. J. Arid Land 2022, 14, 341–355. [Google Scholar] [CrossRef]
- Wang, X.; Kong, F.; Kong, W.; Xu, W. Edaphic Characterization and Plant Zonation in the Qaidam Basin, Tibetan Plateau. Sci. Rep. 2018, 8, 1822. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant Growth-Promoting Rhizobacteria Allow Reduced Application Rates of Chemical Fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; García de Salamone, I.E.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and Arbuscular Mycorrhizal Colonization Enhance Rice Growth and Physiological Traits under Well-Watered and Drought Conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Wang, X.; Sun, K.-N.; Wang, K.-A.; Gao, J.-W.; Zhang, W.; Yang, N. Effects of Different Forms of Microbial Agents on the Growth and Quality of Brassica rapa L. ssp. chinensis Makino (Non-Heading Chinese Cabbage). Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2021, 32, 1777–1782. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Parra Cota, F.I.; Cira Chávez, L.A.; García Ortega, L.F.; Estrada Alvarado, M.I.; Santoyo, G.; de los Santos-Villalobos, S. Microbial Inoculants in Sustainable Agriculture: Advancements, Challenges, and Future Directions. Plants 2025, 14, 191. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial Inoculants for Soil Quality and Plant Health. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 281–307. ISBN 978-3-319-48006-0. [Google Scholar]
- Trabelsi, D.; Mhamdi, R. Microbial Inoculants and Their Impact on Soil Microbial Communities: A Review. Biomed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Yue, L.; Uwaremwe, C.; Zhao, X.; Zhou, Q.; Wang, Y.; Wang, R. Bacterial Inoculant and Sucrose Amendments Improve the Growth of Rheum palmatum L. by Reprograming Its Metabolite Composition and Altering Its Soil Microbial Community. Int. J. Mol. Sci. 2022, 23, 1694. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does Plant—Microbe Interaction Confer Stress Tolerance in Plants: A Review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Youseif, S.H.; Abd El-Megeed, F.H.; Abu Zeid, A.Z.A.; Abd-Elrahman, R.A.; Mohamed, A.H.; Khalifa, M.A.; Saleh, S.A. Alleviating the Deleterious Effects of Soil Salinity and Alkalinity on Faba Bean (Vicia faba L.) Production Using Rhizobium/Agrobacterium Inoculants. Arch. Agron. Soil Sci. 2021, 67, 577–593. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, C.; Zeng, W.; Hou, M.; Wang, Z.; Xu, G.; Huang, J.; Ao, C. Application of Rhizobacteria to Improve Microbial Community Structure and Maize (Zea mays L.) Growth in Saline Soil. Environ. Sci. Pollut. Res. 2024, 31, 2481–2494. [Google Scholar] [CrossRef]
- do Amaral, F.P.; Tuleski, T.R.; Pankievicz, V.C.S.; Melnyk, R.A.; Arkin, A.P.; Griffitts, J.; Tadra-Sfeir, M.Z.; Maltempi de Souza, E.; Deutschbauer, A.; Monteiro, R.A.; et al. Diverse Bacterial Genes Modulate Plant Root Association by Beneficial Bacteria. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- FAjilogba, C.; Babalola, O.O.; Adebola, P.; Adeleke, R. Bambara Groundnut Rhizobacteria Antimicrobial and Biofertilization Potential. Front. Plant Sci. 2022, 13, 854937. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; El-Saadony, M.T.; Abdelaziz, S.; Abdou, N.M. Plant Growth-Promoting Rhizobacteria Improve Growth, Morph-Physiological Responses, Water Productivity, and Yield of Rice Plants under Full and Deficit Drip Irrigation. Rice 2022, 15, 16. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Wei, Y.; Yao, W.; Lei, Y.; Sun, Y. Soil Microbes Drive the Flourishing Growth of Plants from Leucocalocybe mongolica Fairy Ring. Front. Microbiol. 2022, 13, 893370. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clement, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo-and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial Competition: Surviving and Thriving in the Microbial Jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Çakmakçi, R.; Dönmez, F.; Aydın, A.; Şahin, F. Growth Promotion of Plants by Plant Growth-Promoting Rhizobacteria under Greenhouse and Two Different Field Soil Conditions. Soil Biol. Biochem. 2006, 38, 1482–1487. [Google Scholar] [CrossRef]
- Yu, Z.; Liang, K.; Huang, G.; Wang, X.; Lin, M.; Chen, Y.; Zhou, Z. Soil Bacterial Community Shifts Are Driven by Soil Nutrient Availability along a Teak Plantation Chronosequence in Tropical Forests in China. Biology 2021, 10, 1329. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant Growth-Promoting Rhizobacteria (Pgpr) Increase Yield, Growth and Nutrition of Strawberry under High-Calcareous Soil Conditions. J. Plant Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Tang, L.; Shi, Y.; Zhang, Y.; Yang, D.; Guo, C. Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions. Diversity 2023, 15, 537. [Google Scholar] [CrossRef]
- Renoud, S.; Abrouk, D.; Prigent-Combaret, C.; Wisniewski-Dyé, F.; Legendre, L.; Moënne-Loccoz, Y.; Muller, D. Effect of Inoculation Level on the Impact of the PGPR Azospirillum lipoferum CRT1 on Selected Microbial Functional Groups in the Rhizosphere of Field Maize. Microorganisms 2022, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, G.; Caravaca, F.; Fernández-González, A.J.; Alguacil, M.M.; Fernández-López, M.; Roldán, A. Arbuscular Mycorrhizal Fungi Inoculation Mediated Changes in Rhizosphere Bacterial Community Structure While Promoting Revegetation in a Semiarid Ecosystem. Sci. Total Environ. 2017, 584–585, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Liu, Y.-Q.; Li, Y.-J.; Han, H.; Zhang, H.; Ji, M.-F.; Chen, Z.-J. Enhancing Mechanisms of the Plant Growth-Promoting Bacterial Strain Brevibacillus sp. SR-9 on Cadmium Enrichment in Sweet Sorghum by Metagenomic and Transcriptomic Analysis. Int. J. Environ. Res. Public Health 2022, 19, 16309. [Google Scholar] [CrossRef]
- Garrido-Sanz, D.; Čaušević, S.; Vacheron, J.; Heiman, C.M.; Sentchilo, V.; van der Meer, J.R.; Keel, C. Changes in Structure and Assembly of a Species-Rich Soil Natural Community with Contrasting Nutrient Availability upon Establishment of a Plant-Beneficial Pseudomonas in the Wheat Rhizosphere. Microbiome 2023, 11, 214. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Self-Generated Diversity Produces “Insurance Effects” in Biofilm Communities. Proc. Natl. Acad. Sci. USA 2004, 101, 16630–16635. [Google Scholar] [CrossRef]
- Nie, H.; Shi, Y.; Yang, X.; Zeng, J.; Tang, Y.; Liu, X.; Sun, L.; Zhou, Y.; Xu, X.; Liu, M.; et al. Microbial Inoculant-Induced Modifications of Rhizospheric Metabolites and Microbial Communities Enhance Plant Growth. Plant Soil 2024. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus Subtilis against Infection of Arabidopsis Roots by Pseudomonas Syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef]
- Pac Ocirc Me, A.N.; Nad Egrave Ge, A.E.A.; Farid, B.M.; Adolphe, A.; Lamine, B.M. Plant Growth Promoting Rhizobacteria: Beneficial Effects for Healthy and Sustainable Agriculture. Afr. J. Biotechnol. 2016, 15, 1452–1463. [Google Scholar] [CrossRef]
- Halim, M.A.; Rahman, M.M.; Megharaj, M.; Naidu, R. Cadmium Immobilization in the Rhizosphere and Plant Cellular Detoxification: Role of Plant-Growth-Promoting Rhizobacteria as a Sustainable Solution. J. Agric. Food Chem. 2020, 68, 13497–13529. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal Basis for the Plant Growth Promoting Action of Naturally Occurring Biostimulators. J. Sci. Food Agric. 2014, 94, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Fu, S.; Guo, X.; Sun, Z.; Liu, F.; Chen, Q.; Yu, T.; Gao, Y.; Zhang, L.; Yang, L.; et al. Plant Growth-Promoting Rhizobacteria Microbial Fertilizer Changes Soils’ Microbial Structure and Promotes Healthy Growth of Cigar Tobacco Plants. Agronomy 2023, 13, 2895. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Chang, F.; Wang, X.; Zhang, X.; Luan, H.; Qi, G.; Guo, S. Effects of Nitrogen Reduction Combined with Bio-Organic Fertilizer on Soil Bacterial Community Diversity of Red Raspberry Orchard. PLoS ONE 2023, 18, e0283718. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D. The Effect of Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR) on the Soil Properties, Root Yield, and Technological Quality of Sugar Beet. Agronomy 2020, 10, 1262. [Google Scholar] [CrossRef]
- Ding, C.; Lv, C.; Chen, H.; Zhou, J.; Ren, H. Evaluation of Soil Total Nitrogen as an Indicator of Soil Bacterial Community Response to Biochar and Plant Growth-Promoting Rhizobacteria Applications. Agronomy 2024, 14, 428. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Packer, A.; Bever, J.D.; Clay, K. Grassroots Ecology: Plant–Microbe–Soil Interactions as Drivers of Plant Community Structure and Dynamics. Ecology 2003, 84, 2281–2291. [Google Scholar] [CrossRef]
- Zeng, W.; Hou, Y.; Ao, C.; Huang, J. Effects of PGPR and γ-PGA on Maize Growth and Rhizosphere Microbial Community in Saline Soil. Agric. Water Manag. 2024, 295, 108736. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, R.; Qi, D.; Xu, T.; Chang, W.; Li, K.; Fang, X.; Song, F. The Effect of AMF Combined with Biochar on Plant Growth and Soil Quality under Saline-Alkali Stress: Insights from Microbial Community Analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Feng, S.; Guo, P.; Wang, Y.; Hao, B.; Guo, W.; Li, F.Y. Synergistic Effects of AMF and PGPR on Improving Saline-Alkaline Tolerance of Leymus chinensis by Strengthening the Link between Rhizosphere Metabolites and Microbiomes. Environ. Technol. Innov. 2024, 36, 103900. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Li, Y.; He, K. The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China. Sustainability 2019, 11, 828. [Google Scholar] [CrossRef]
- Huang, M.; Lu, R.; Zhao, J.; Ma, L. Assessment of Soil Quality in Typical Wind Erosion Area of Qaidam Basin. J. Desert Res. 2023, 43, 199–209. [Google Scholar]
- Wang, X.; Tang, C. The Role of Rhizosphere pH in Regulating the Rhizosphere Priming Effect and Implications for the Availability of Soil-Derived Nitrogen to Plants. Ann. Bot. 2018, 121, 143–151. [Google Scholar] [CrossRef]
- Tagliavini, M.; Masia, A.; Quartieri, M. Bulk Soil pH and Rhizosphere pH of Peach Trees in Calcareous and Alkaline Soils as Affected by the Form of Nitrogen Fertilizers. Plant Soil 1995, 176, 263–271. [Google Scholar] [CrossRef]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The Effects of Chemical and Organic Fertilizer Usage on Rhizosphere Soil in Tea Orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- Barakah, F.N.; Salem, S.H.; Heggo, A.M.; Bin-Shiha, M.A. Activities of Rhizosphere Microorganisms as Affected by Application of Organic Amendments in a Calcareous Loamy Soil. 2. Nitrogen Transformations. Arid Soil Res. Rehabil. 1995, 9, 467–480. [Google Scholar] [CrossRef]
- Thomson, C.J.; Marschner, H.; Römheld, V. Effect of Nitrogen Fertilizer Form on pH of the Bulk Soil and Rhizosphere, and on the Growth, Phosphorus, and Micronutrient Uptake of Bean. J. Plant Nutr. 1993, 16, 493–506. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Z.T.; Wu, Z.C.; Zhan, X.M.; Zhang, K.; Zhao, E.F.; Han, X.R. Adsorption Characteristics of Ammonium Nitrogen by Biochar from Diverse Origins in Water. Adv. Mater. Res. 2013, 664, 305–312. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The Multifaceted Roles of Arbuscular Mycorrhizal Fungi in Peanut Responses to Salt, Drought, and Cold Stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Dixon, P. Vegan, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized Metabolic Functions of Keystone Taxa Sustain Soil Microbiome Stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Wang, C.; Pan, X.; Yu, W.; Ye, X.; Erdenebileg, E.; Wang, C.; Ma, L.; Wang, R.; Huang, Z.; Indree, T.; et al. Aridity and Decreasing Soil Heterogeneity Reduce Microbial Network Complexity and Stability in the Semi-Arid Grasslands. Ecol. Indic. 2023, 151, 110342. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing Aridity Reduces Soil Microbial Diversity and Abundance in Global Drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- García-Palacios, P.; Gross, N.; Gaitán, J.; Maestre, F.T. Climate Mediates the Biodiversity–Ecosystem Stability Relationship Globally. Proc. Natl. Acad. Sci. USA 2018, 115, 8400–8405. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. Glmm.Hp: An R Package for Computing Individual Effect of Predictors in Generalized Linear Mixed Models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Lefcheck, J.S. PIECEWISESEM: Piecewise Structural Equation Modelling in r for Ecology, Evolution, and Systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Yang, X.; Liu, W.; Feng, B.; Sun, S.; Dong, Q. Yak and Tibetan Sheep Mixed Grazing Enhances Plant Functional Diversity in Alpine Grassland. J. Integr. Agric. 2024, 24, 936–948. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Liu, X.; Wang, J.; Chang, J.; Li, C.; Lu, G. Rhizosphere Growth-Promoting Bacteria Enhance Oat Growth by Improving Microbial Stability and Soil Organic Matter in the Saline Soil of the Qaidam Basin. Plants 2025, 14, 1926. https://doi.org/10.3390/plants14131926
Jin X, Liu X, Wang J, Chang J, Li C, Lu G. Rhizosphere Growth-Promoting Bacteria Enhance Oat Growth by Improving Microbial Stability and Soil Organic Matter in the Saline Soil of the Qaidam Basin. Plants. 2025; 14(13):1926. https://doi.org/10.3390/plants14131926
Chicago/Turabian StyleJin, Xin, Xinyue Liu, Jie Wang, Jianping Chang, Caixia Li, and Guangxin Lu. 2025. "Rhizosphere Growth-Promoting Bacteria Enhance Oat Growth by Improving Microbial Stability and Soil Organic Matter in the Saline Soil of the Qaidam Basin" Plants 14, no. 13: 1926. https://doi.org/10.3390/plants14131926
APA StyleJin, X., Liu, X., Wang, J., Chang, J., Li, C., & Lu, G. (2025). Rhizosphere Growth-Promoting Bacteria Enhance Oat Growth by Improving Microbial Stability and Soil Organic Matter in the Saline Soil of the Qaidam Basin. Plants, 14(13), 1926. https://doi.org/10.3390/plants14131926




