Short-Term Nitrogen Enrichment Reshapes Carbon Allocation and Enhances Synergistic Ecosystem Services in Semi-Arid Sandy Grasslands in China
Abstract
1. Introduction
2. Results
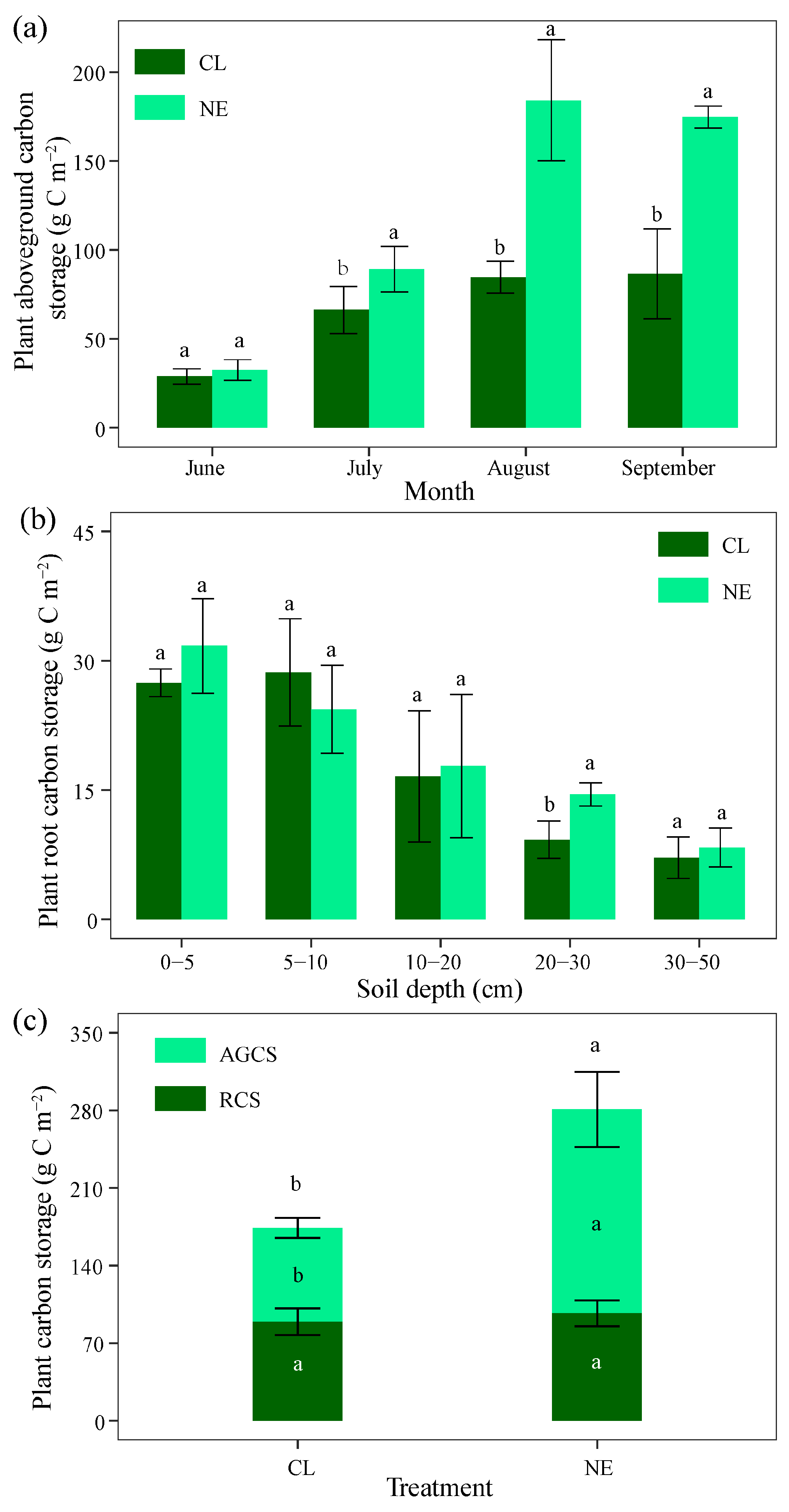
2.1. Changes in Plant Carbon Storage with Month, Soil Depth, and NE Treatment
2.2. Changes in Soil Carbon Storage with Depth
2.3. Changes in Multiple Grassland Services
3. Discussion
3.1. NE Increased Plant Carbon Storage Above Ground Compared to Below Ground
3.2. Contrasting Effects of Short-Term NE on Plant and Soil Carbon Storage
3.3. Synergistic Effects of NE on Grassland Forage Supply and Windbreak Services Under Light Grazing Scenario
4. Materials and Methods
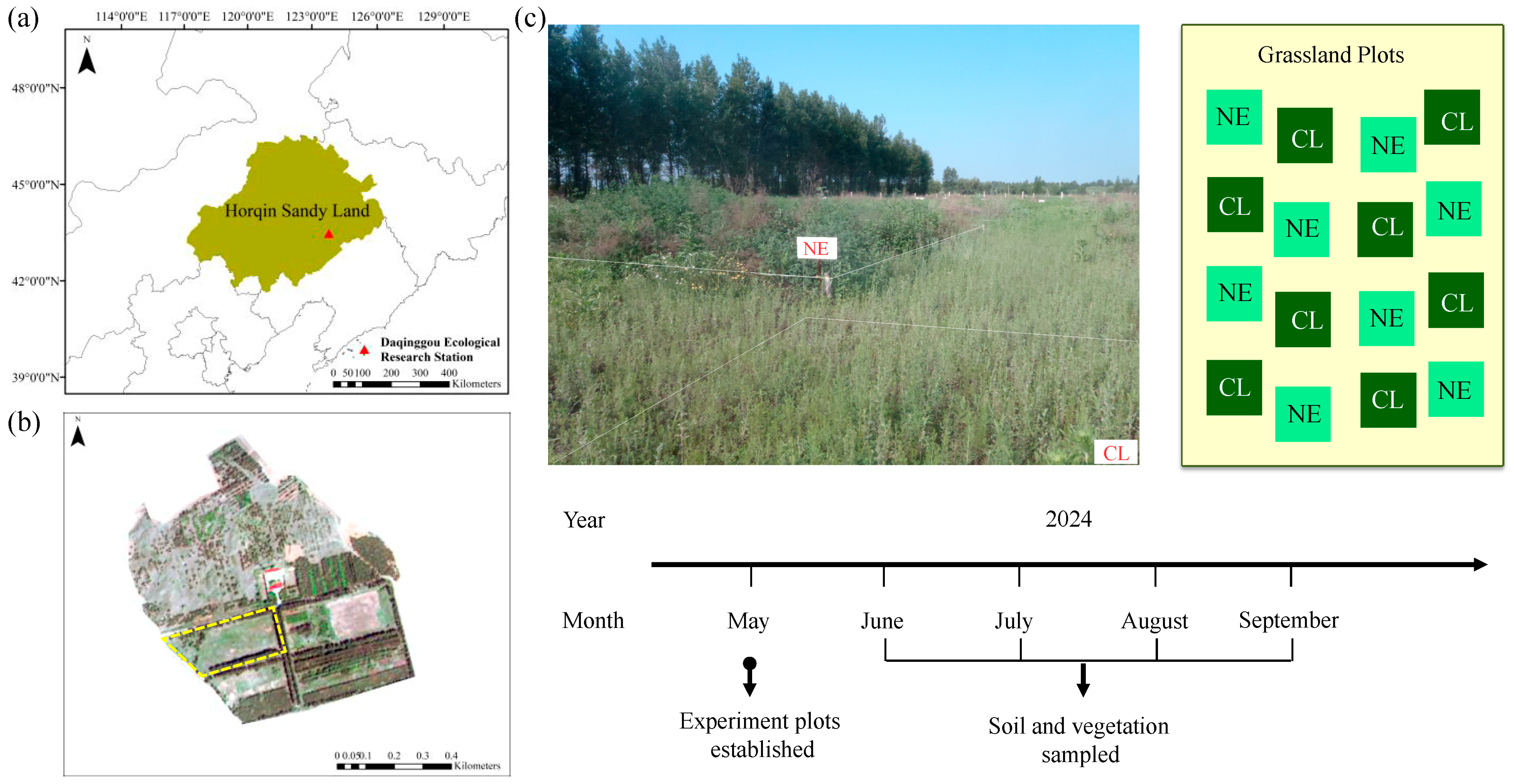
4.1. Site Description and Experiment Design
4.2. Field Investigation and Sample Collection
4.3. Laboratory Analysis of Plant and Soil Samples
4.4. Carbon Storage and Ecosystem Service Calculation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands-more important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Straffelini, E.; Luo, J.; Tarolli, P. Climate change is threatening mountain grasslands and their cultural ecosystem services. Catena 2024, 237, 107802. [Google Scholar] [CrossRef]
- Wang, D.L.; Wang, L.; Liu, J.S.; Zhu, H.; Zhong, Z.W. Grassland ecology in China: Perspectives and challenges. Front. Agric. Sci. Eng. 2018, 5, 24–43. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low–level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Debaba, G.H.; Li, K.Y.; Wang, X.W.; Wang, Y.A.; Bai, W.M.; Li, G.Y. Effect of nitrogen application rate on the relationships between multidimensional plant diversity and ecosystem production in a temperate steppe. Biology 2024, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Xu, Y.; Shan, Y.X.; Burgess, K.S.; Ge, X.J. Biotic and abiotic factors determine species diversity–productivity relationships in mountain meadows. J Plant Ecol. 2021, 14, 1175–1188. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.Z.; Zhang, Y.H.; Yang, J.J.; Smith, M.D.; Knapp, A.K.; Eissenstat, D.M.; Han, X.G. Asymmetry in above- and belowground productivity responses to N addition in a semi-arid temperate steppe. Glob. Change Biol. 2019, 25, 2958–2969. [Google Scholar] [CrossRef]
- He, W.Y.; Wei, H.J.; Liang, J.W.; Xu, T.Y.; Chen, H.; Hu, W.T.; Tang, M. How do ectomycorrhizal fungi regulate Eucalyptus biomass allocation through the stoichiometric changes in the plant-soil-microbe system under nitrogen addition? Ind. Crops Prod. 2024, 218, 118876. [Google Scholar] [CrossRef]
- Bai, Y.F.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef]
- Wang, D.; He, H.L.; Gao, Q.; Zhao, C.Z.; Zhao, W.Q.; Yin, C.Y.; Chen, X.L.; Ma, Z.L.; Li, D.D.; Sun, D.D.; et al. Effects of short-term N addition on plant biomass allocation and C and N pools of the Sibiraea angustata scrub ecosystem. Eur. J. Soil Sci. 2017, 68, 212–220. [Google Scholar] [CrossRef]
- Chen, D.M.; Lan, Z.C.; Hu, S.J.; Bai, Y.F. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Y.L.; Cheng, L.; Yang, H.L.; Ning, Z.Y.; Liang, R.Q. Effect of N addition and litter manipulation on plant community productivity in the semiarid sandy grassland. Ecol. Eng. 2024, 201, 107191. [Google Scholar] [CrossRef]
- Lu, X.F.; Gilliam, F.S.; Yue, X.; Wang, B.; Kuang, Y.W. Shifts in above- versus below-ground carbon gains to terrestrial ecosystems carbon sinks under excess nitrogen inputs. Glob. Biogeochem. Cycles 2023, 37, e2022GB007638. [Google Scholar] [CrossRef]
- Su, L.C.; Chen, X.S.; Luo, Z.Z.; Hu, Y.; Chen, Y.J.; Wu, D.M.; Zeng, S.C. Effects of nitrogen addition on the organic carbon sequestration and CO2 emissions in forest soils: A review. Acta Ecol. Sin. 2024, 44, 2717–2733. [Google Scholar]
- Wang, Z.F.; Jing, X.; Lin, L.T.; Wang, Y.G.; Feng, W.T. Divergent controls of exchangeable calcium and iron oxides in regulating soil organic carbon content across climatic gradients in arid regions. Agric. For. Meteorol. 2024, 349, 109939. [Google Scholar] [CrossRef]
- Okin, G.S.; Gillette, D.A.; Herrick, J.E. Multi-scale controls on and consequences of aeolian processes in landscape change in arid and semi-arid environments. J. Arid Environ. 2006, 65, 253–275. [Google Scholar] [CrossRef]
- Meng, Z.J.; Dang, X.H.; Gao, Y.; Ren, X.M.; Ding, Y.L.; Wang, M. Interactive effects of wind speed, vegetation coverage and soil moisture in controlling wind erosion in a temperate desert steppe, Inner Mongolia of China. J. Arid Land 2018, 10, 534–547. [Google Scholar] [CrossRef]
- Borrelli, P.; Boggio, F.; Sturzenbaum, P.; Paramidani, M.; Heinken, R.; Pague, C.; Stevens, M.; Nogués, A. Grassland Regeneration and Sustainability Standard (GRASS); The Nature Conservancy-Ovis: Arlington, VA, USA, 2013. [Google Scholar]
- Schönbach, P.; Wan, H.W.; Gierus, M.; Bai, Y.F.; Müller, K.; Lin, L.J.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Fryrear, D.; Sutherland, P.; Davis, G.; Hardee, G.; Dollar, M. Wind erosion estimates with RWEQ and WEQ. In Proceedings of the Conference Sustaining the Global Farm, 10th International Soil Conservation Organization Meeting, West Lafayette, IN, USA, 24–29 May 1999; Purdue University: West Lafayette, IN, USA, 1999. [Google Scholar]
- An, H.; Zhao, F.Y.; Li, H.N.; Meng, Z.J.; Ding, H.L.; Ding, Y.L.; Qin, L.; Xin, J. The typical sand-fixing plants in the Ulan Buh desert-oasis area significantly changed the distribution pattern of surface sediments. Front. Environ. Sci. 2025, 13, 1556083. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.T. Theoretical calculation of the minimum vegetation cover for fixing sand. J. Desert Res. 2025, 42, 97–101. (In Chinese) [Google Scholar] [CrossRef]
- Lu, X.F.; Hou, E.Q.; Guo, J.Y.; Gilliam, F.S.; Li, J.L.; Tang, S.B.; Kuang, Y.W. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta–analysis. Glob. Change Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.J.; Lind, E.M.; Hautier, Y.; Harpole, W.S.; Borer, E.T.; Hobbie, S.; Seabloom, E.W.; Ladwig, L.; Bakker, J.D.; Chu, C.J.; et al. Anthropogenic nitrogen deposition predicts local grassland primary production worldwide. Ecology 2015, 96, 1459–1465. [Google Scholar] [CrossRef]
- Hou, L.L.; Xia, F.; Chen, Q.H.; Huang, J.K.; He, Y.; Rose, N.; Rozelle, S. Grassland ecological compensation policy in China improves grassland quality and increases herders’ income. Nat. Commun. 2021, 12, 4683. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Sun, X.K.; Yu, Z.Y.; Huang, Y.; Zeng, D.H. Responses of soil respiration to N fertilization and grazing in a Keerqin sandy grassland in northeast China. Arid Land Res. Manag. 2021, 35, 230–245. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biot. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Wang, Y.X.; Li, J.L. Effects of nitrogen addition on plant-soil carbon dynamics in terrestrial ecosystems of China. Acta Ecol. Sin. 2022, 42, 4823–4833. (In Chinese) [Google Scholar] [CrossRef]
- Lin, L.T.; Liu, Y.J.; Feng, W.T.; Wang, Y.G.; Zhang, C.; Ma, B.; Han, F.; Wang, L.L.; Geng, J.Y.; Li, F.J. Effects of Soil Salinization on the Structure and Function of Rare and Common Bacteria. Res. Environ. Sci. 2024, 37, 2126–2137. (In Chinese) [Google Scholar] [CrossRef]
- He, Y.C.; Geng, Y.Y.; Han, B.; Shi, L.A.; Shao, X.Q.; Liu, K.S. Water controls the divergent responses of terrestrial plant photosynthesis under nitrogen enrichment. J. Ecol. 2024, 112, 2638–2651. [Google Scholar] [CrossRef]
- Feng, H.L.; Guo, J.H.; Peng, C.H.; Kneeshaw, D.; Roberge, G.; Pan, C.; Ma, X.H.; Zhou, D.; Wang, W.F. Nitrogen addition promotes terrestrial plants to allocate more biomass to aboveground organs: A global meta-analysis. Glob. Change Biol. 2023, 29, 3970–3989. [Google Scholar] [CrossRef]
- Yang, X.M.; Ma, S.H.; Huang, E.R.; Zhang, D.H.; Chen, G.P.; Zhu, J.L.; Ji, C.J.; Zhu, B.; Liu, L.L.; Fang, J.Y. Nitrogen addition promotes soil carbon accumulation globally. Sci. China Life Sci. 2025, 68, 284–293. [Google Scholar] [CrossRef]
- Li, L.J.; Zeng, D.H.; Yu, Z.Y.; Fan, Z.P.; Mao, R. Soil microbial properties under N and P additions in a semi-arid, sandy grassland. Biol. Fert. Soils 2010, 46, 653–658. [Google Scholar] [CrossRef]
- Jian, J.N.; Liu, W.C.; Zhu, Y.F.; Li, J.X.; Wen, Y.H.; Liu, F.H.; Ren, C.J.; Han, X.H. Effects of short-term nitrogen addition on soil organic carbon components in Robinia pseudoacacia L. Plantation. Environ. Sci. 2023, 44, 2767–2774. [Google Scholar] [CrossRef]
- Yang, H.; Liu, M.J.; Zhang, R.B.; Wang, Z.H. Effects of nitrogen addition on soil organic carbon and its fractions in China’s terrestrial ecosystems. J. Beijing For. Univ. 2025, 47. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.T.; Zhao, J.N.; Huang, J.J.; Wang, H.; Yu, Y.; Xiao, C.W. Root exudates mediate the processes of soil organic carbon input and efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhao, L.; Tan, W.B.; Wang, G.A.; Xi, B.D. Discrepant responses of soil organic carbon dynamics to nitrogen addition in different layers: A case study in an agroecosystem. Front. Agric. Sci. Eng. 2024, 11, 314–325. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Fan, J.W.; Cao, W.; Harris, W.; Li, Y.Z.; Chi, W.F.; Wang, S.Z. Response of wind erosion dynamics to climate change and human activity in Inner Mongolia, China during 1990 to 2015. Sci. Total Environ. 2018, 639, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.S.; Hättenschwiler, S.; Lü, X.T.; Kardol, P.; Zhang, Y.H.; Wei, C.Z.; Xu, C.Y.; Huang, J.H.; Li, A.; Yang, J.J.; et al. Carbon limitation overrides acidification in mediating soil microbial activity to nitrogen enrichment in a temperate grassland. Glob. Change Biol. 2021, 27, 5976–5988. [Google Scholar] [CrossRef]
- McSherry, M.E.; Ritchie, M.E. Effects of grazing on grassland soil carbon: A global review. Glob. Change Biol. 2013, 19, 1347–1357. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, X.B.; Liu, S.Y.; Li, X.; Lyu, X.; Dang, D.L.; Dou, H.S. Ecosystem services under different grazing intensities in typical grasslands in Inner Mongolia and their relationships. Glob. Ecol. Conserv. 2021, 26, e01526. [Google Scholar] [CrossRef]
- McNaughton, S.J. Ecology of a grazing ecosystem: The Serengeti. Ecol. Monogr. 1985, 55, 259–294. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 1993, 63, 327–366. [Google Scholar] [CrossRef]
- Li, L.J.; Zeng, D.H.; Yu, Z.Y.; Fan, Z.P.; Yang, D.; Liu, Y.X. Impact of litter quality and soil nutrient availability on leaf decomposition rate in a semi-arid grassland of Northeast China. J. Arid Environ. 2011, 75, 787–792. [Google Scholar] [CrossRef]
- Bai, J.; Sun, X.K.; Xu, C.B.; Ma, X.P.; Huang, Y.; Fan, Z.; Cao, X.Y. Effects of sewage sludge application on plant growth and soil characteristics at a Pinus sylvestris var. mongolica plantation in Horqin Sandy Land. Forests 2022, 13, 984. [Google Scholar] [CrossRef]
- Zeng, D.H.; Hu, Y.L.; Chang, S.C.; Fan, Z.P. Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil 2009, 317, 121–133. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org (accessed on 31 October 2024).
- Peterson, B.G.; Carl, P. Performance Analytics: Econometric Tools for Performance and Risk Analysis; R Package Version 2.0.8; R Core Team: Vienna, Austria, 2024; Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 9 December 2024).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package, R Package Version 2.6-8; 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 August 2024).


| Ecosystem Service | Price (USD t−1) | CL | NE |
|---|---|---|---|
| Forage supply (t ha−1) | 100.00 | 2.08 ± 0.20 b | 4.32 ± 0.66 a |
| GCSS (t C ha−1) | 12.57 | 6.94 ± 0.19 a | 7.25 ± 0.43 a |
| WBSFS (t ha−1) | 10.00 | 0.60 ± 0.01 a | 0.62 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Yu, H.; Sun, X.; Ai, G.; Bai, J. Short-Term Nitrogen Enrichment Reshapes Carbon Allocation and Enhances Synergistic Ecosystem Services in Semi-Arid Sandy Grasslands in China. Plants 2025, 14, 1915. https://doi.org/10.3390/plants14131915
Lin L, Yu H, Sun X, Ai G, Bai J. Short-Term Nitrogen Enrichment Reshapes Carbon Allocation and Enhances Synergistic Ecosystem Services in Semi-Arid Sandy Grasslands in China. Plants. 2025; 14(13):1915. https://doi.org/10.3390/plants14131915
Chicago/Turabian StyleLin, Litao, Huiyi Yu, Xuekai Sun, Guiyan Ai, and Jie Bai. 2025. "Short-Term Nitrogen Enrichment Reshapes Carbon Allocation and Enhances Synergistic Ecosystem Services in Semi-Arid Sandy Grasslands in China" Plants 14, no. 13: 1915. https://doi.org/10.3390/plants14131915
APA StyleLin, L., Yu, H., Sun, X., Ai, G., & Bai, J. (2025). Short-Term Nitrogen Enrichment Reshapes Carbon Allocation and Enhances Synergistic Ecosystem Services in Semi-Arid Sandy Grasslands in China. Plants, 14(13), 1915. https://doi.org/10.3390/plants14131915





