Threatened Aquatic Plants of the Southern Tigris-Euphrates Basin: Status, Threats, and Conservation Priorities
Abstract
1. Introduction
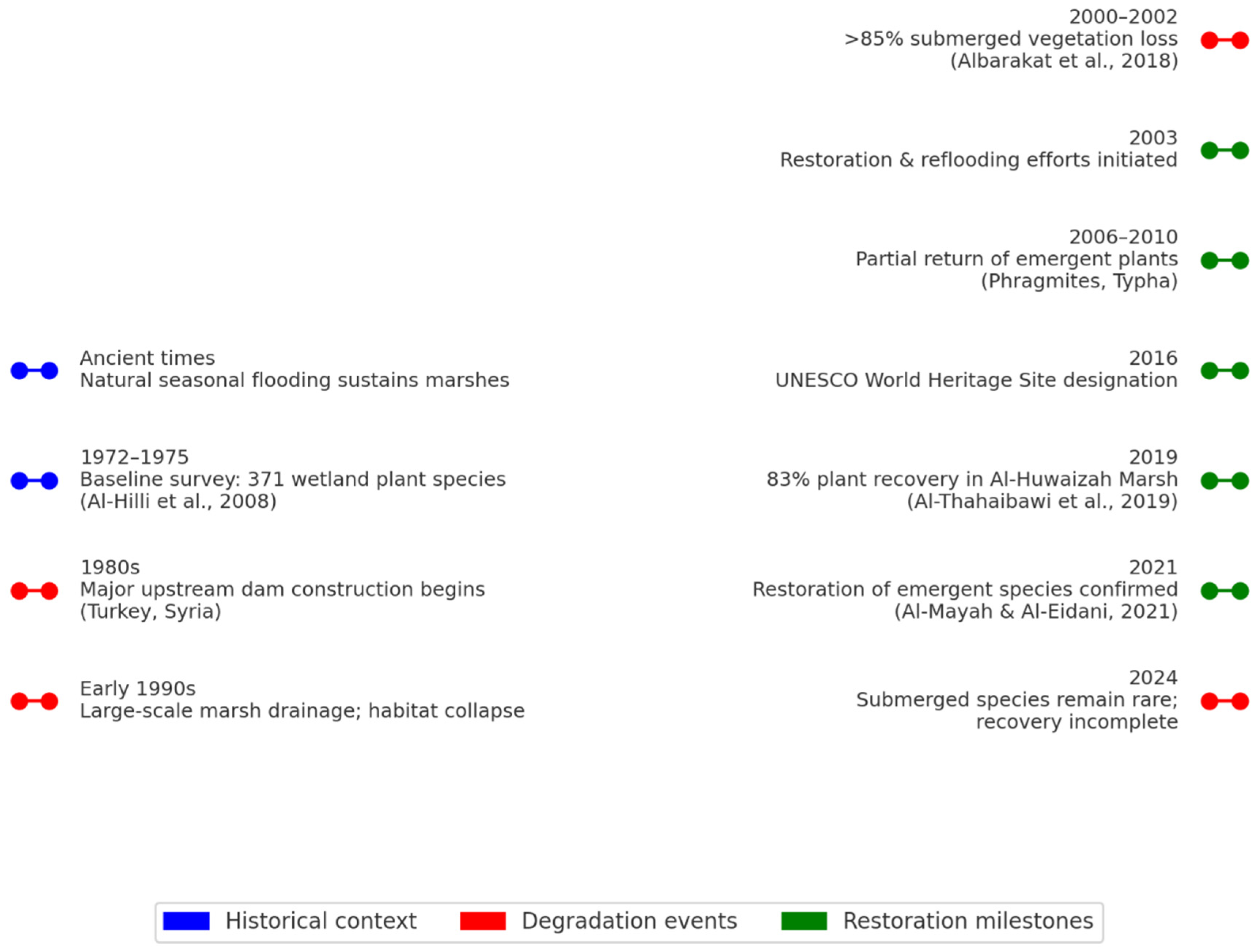
2. Historical Overview of Aquatic Flora in the Tigris-Euphrates Basin
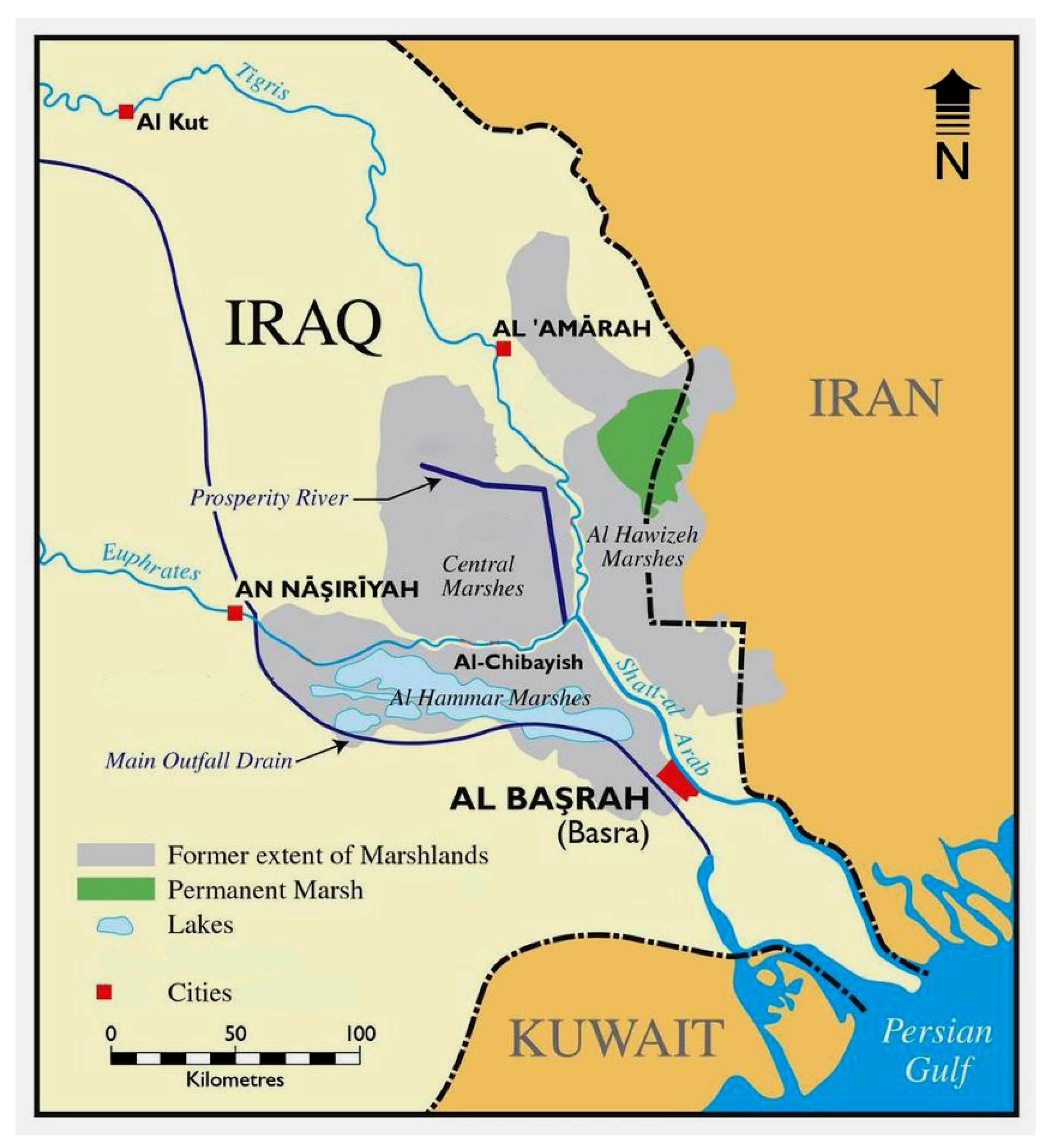
3. List of Threatened Aquatic Plant Species
4. Threats
4.1. Hydrological Alterations
4.2. Pollution and Water Quality Degradation
4.3. Intrusion of Saline Waters
4.4. Wetland Drainage
4.5. Climate Change
4.6. Spread of Invasive Alien and Expanding Native Species
5. Conservation Efforts and Needs
5.1. Marshland Restoration Projects
5.2. International Recognition
5.3. Research and Monitoring Initiatives
5.4. Gaps in Current Conservation Strategies
6. Recommendations for the Conservation of Native Aquatic Plants
6.1. Develop and Implement Species-Specific Recovery Plans
6.2. Establish Aquatic Plant Seed Banks and Propagation Programs
6.3. Integrate Aquatic Plants into Wetland Management Frameworks
6.4. Enhance Water Quality Management
6.5. Promote Cross-Border Hydrological Cooperation
6.6. Conduct Regular Monitoring and Research
6.7. Raise Public Awareness and Engage Local Communities
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chambers, P.A.; Lacoul, P.; Murphy, K.J.; Thomaz, S.M. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 2008, 595, 9–26. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: Methods of measurement, causes, and consequences on animal assemblages’ composition and abundance. Acta Limnol. Bras. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Richardson, C.J.; Hussain, N.A. Restoring the Garden of Eden: An ecological assessment of the marshes of Iraq. BioScience 2006, 56, 477–489. [Google Scholar] [CrossRef]
- UNEP (United Nations Environment Programme). The Mesopotamian Marshlands: Demise of an Ecosystem; UNEP Regional Office for West Asia: Manama, Bahrain, 2001. [Google Scholar]
- Bozkurt, D.; Sen, O.L. Climate change impacts in the Euphrates–Tigris Basin based on different model and scenario simulations. J. Hydrol. 2013, 480, 149–161. [Google Scholar] [CrossRef]
- Becker, R.H. The stalled recovery of the Iraqi marshes. Remote Sens. 2014, 6, 1260–1274. [Google Scholar] [CrossRef]
- Bijnens, T. Hydrologic structures in the Tigris–Euphrates basin and their impact on the vitality of the marshes. In Southern Iraq’s Marshes: Their Environment and Conservation; Springer: Cham, Switzerland, 2021; pp. 113–125. [Google Scholar]
- Montazeri, A.; Mazaheri, M.; Morid, S.; Mosaddeghi, M.R. Effects of upstream activities of Tigris–Euphrates River Basin on water and soil resources of Shatt al-Arab Border River. Sci. Total Environ. 2023, 858, 159751. [Google Scholar] [CrossRef]
- Albarakat, R.; Lakshmi, V.; Tucker, C.J. Using satellite remote sensing to study the impact of climate and anthropogenic changes in the Mesopotamian Marshlands, Iraq. Remote Sens. 2018, 10, 1524. [Google Scholar] [CrossRef]
- Cunningham, B. The Régime of the Rivers Euphrates and Tigris. Nature 1938, 142, 373–374. [Google Scholar] [CrossRef]
- Teclaff, L.A.; Teclaff, L.A. The river basin as the basis of water control for agriculture in antiquity. In The River Basin in History and Law; Teclaff, L.A., Ed.; FAO: Rome, Italy, 1967; pp. 15–25. [Google Scholar]
- Ghadiri, H. Marshlands of Mesopotamia and the rivers which feed them. In 8th River Symposium 2005; Riverfestival: Brisbane, Australia, 2005. [Google Scholar]
- Chen, Z.R.; Kavvas, M.L.; Ohara, N.; Anderson, M.L.; Yoon, J. Impact of water resources utilization on the hydrology of Mesopotamian marshlands. J. Hydrol. Eng. 2011, 16, 1083–1092. [Google Scholar] [CrossRef]
- Rzóska, J. (Ed.) Euphrates and Tigris, Mesopotamian Ecology and Destiny; Springer: Dordrecht, The Netherlands, 2012; Volume 38. [Google Scholar]
- Salim, S.M. The Marsh Arabs; Allen & Unwin: London, UK, 1962. [Google Scholar]
- Al-Hilli, M.R.A.; Warner, B.G.; Asada, T.; Douabul, A. An assessment of vegetation and environmental controls in the 1970s of the Mesopotamian wetlands of southern Iraq. Wetl. Ecol. Manag. 2008, 17, 207–223. [Google Scholar] [CrossRef]
- Nature Iraq; Iraq Ministry of Environment. Key Biodiversity Areas of Iraq: 2010 Site Review (Partial); Nature Iraq: Sulaimani, Iraq, 2011.
- Fitzpatrick, R.W. Changes in Soil and Water Characteristics of Natural, Drained and Re-Flooded Soils in the Mesopotamian Marshlands: Implications for Land Management Planning; CSIRO Land Water Report; CSIRO Land and Water: Canberra, Australia, 2004. [Google Scholar]
- Abdullah, D.S.; Al-Mayah, S.H.; Al-Saad, H.T. Ecological and Environmental Monitoring Report of the East Hammar Marsh after Restoration; Marine Science Centre, University of Basrah: Basrah, Iraq, 2007. [Google Scholar]
- Abdulhasan, N.A.; Salim, M.A.; Al-Obaidi, G.S.; Ali, H.J.; Al-Saffar, M.A.; Abd, I.M.; Minjil, M.S. Habitat Mapping and Monitoring Project: Classification and Description of Southern Iraqi Marshlands; Nature Iraq Report: Sulaimani, Iraq, 2009. [Google Scholar]
- Al-Abbawy, D.A.H.; Al-Mayah, A.A. Ecological survey of aquatic macrophytes in restored marshes of southern Iraq during 2006 and 2007. Marsh Bull. 2010, 5, 177–196. [Google Scholar]
- Al-Quraishi, A.K.; Kaplan, D.A. Connecting changes in Euphrates River flow to hydropattern of the Western Mesopotamian Marshes. Sci. Total Environ. 2021, 768, 144445. [Google Scholar] [CrossRef] [PubMed]
- Bobbink, R.; Beltman, B.; Verhoeven, J.T.A.; Whigham, D.F. (Eds.) Wetlands: Functioning, Biodiversity Conservation, and Restoration; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bachmann, A.; Tice, V.; Al-Obeidi, L.A.; Kılıç, D.T. Tigris–Euphrates River ecosystem: A status report. In Proceedings of the Mesopotamia Water Forum, Sulaymaniyah, Iraq, 6–8 April 2019; pp. 6–8. [Google Scholar]
- Al-Abbawy, D.A.; Al-Zaidi, S.A. Spatial distribution and population density of submerged aquatic vegetation in Shatt Al-Arab River. Dynamics 2023, 1, 2. [Google Scholar]
- Richardson, C.J.; Reiss, P.; Hussain, N.A.; Alwash, A.J.; Pool, D.J. The restoration potential of the Mesopotamian marshes of Iraq. Science 2005, 307, 1307–1311. [Google Scholar] [CrossRef]
- Adriansen, H.K. The Iraqi marshlands: Is environmental rehabilitation possible? Pap. Proc. Appl. Geogr. Conf. 2006, 29, 215–221. [Google Scholar]
- Al-Zaidy, K.J.; Parisi, G. Re-extrapolation for the Iraq marshes which fall within the world heritage list (a literature review). Al-Qadisiyah J. Agric. Sci. 2018, 8, 65–82. [Google Scholar]
- Hussein, A.A.K.; Abd Asal, A. Drying water and its impact on the vital system in the marshes of southern Iraq. IOP Conf. Ser. Earth Environ. Sci. 2023, 1129, 012032. [Google Scholar] [CrossRef]
- Al-Mayah, A.R.; Al-Eidani, T.Y. Macrophytes. In Biodiversity of the Inland Waters of Basrah, Following the 2003 Marshlands Restoration Project; Ali, M.H., Ed.; Marine Science Centre, University of Basrah: Basrah, Iraq, 2021; pp. 55–67. [Google Scholar]
- Alwan, A.R.A. Past and present status of the aquatic plants of the Marshlands of Iraq. J. Marsh Bull. 2006, 1, 120–172. [Google Scholar]
- Al-Thahaibawi, B.M.H.; Al-Mayaly, I.K.A.; Younis, K.H. Ecological survey of aquatic macrophytes in Al-Huwaizah Marsh southern Iraq after inclusion in the World Heritage List. Plant Arch. 2019, 19 (Suppl. 2), 294–302. [Google Scholar]
- Al-Mayah, A.R.; Al-Abbawy, D.A.H.; Al-Assadi, W.M.T.; Al-Saadi, S.A.A.; Al-Edany, T.Y. Status of aquatic macrophytes in Saffia Nature Reserve, south of Huwaiza Marsh, Iraq. Marsh Bull. 2012, 7, 1–16. [Google Scholar]
- Salim, M.A.; Al-Sudani, I.M.; Haloob, A.; Abed, S.A. Invasive alien species in Al-Dalmaj Protected Area, Iraq: Conservation and wildlife management approach. IOP Conf. Ser. Earth Environ. Sci. 2021, 790, 012088. [Google Scholar] [CrossRef]
- Al-Rawi, S.M. Contribution of man–made activities to the pollution of the Tigris within Mosul Area, Iraq. Int. J. Environ. Res. Public Health 2005, 2, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.L. Civilization’s Drying Cradle: Water Politics in the Tigris–Euphrates River Basin; United States Army War College: Carlisle, PA, USA, 2012.
- Jawad, L.A. The effects of thermal pollution on the aquatic life in the southern marshes of Iraq. In Southern Iraq’s Marshes: Their Environment and Conservation; Springer: Cham, Switzerland, 2021; pp. 559–571. [Google Scholar]
- Stevens, M.L.; Salman, N. Application of international water law in Eden: Environment protection of the Mesopotamian Marshes in southern Iraq. Wetlands Sci. Policy 2015, 32, 17–27. [Google Scholar] [CrossRef]
- Al-Yassin, A.M. The impact of climate change on water resources in Iraq. J. Earth Environ. Sci. 2015, 2, 81–92. [Google Scholar]
- Al-Salihi, Z.A.; Kamel, A.H.; Abdulhameed, I.M. Effect of climate changes on water resources in Iraq: A review study. AIP Conf. Proc. 2024, 3009, 030079. [Google Scholar]
- Riis, T.; Sand-Jensen, K. Historical changes of species composition and richness accompanying perturbation and eutrophication in Danish lowland streams over 100 years. Freshw. Biol. 2001, 46, 269–284. [Google Scholar] [CrossRef]
- Goldenberg-Vilar, A.; Delgado, C.; Penas, F.J.; Barquin, J. The effect of altered flow regimes on aquatic primary producer communities: Diatoms and macrophytes. Ecohydrology 2022, 15, e2353. [Google Scholar] [CrossRef]
- Reitsema, R.; Preiner, S.; Meire, P.; Hein, T.; De Boeck, G.; Blust, R.; Schoelynck, J. Implications of climate change for submerged macrophytes: Effects of CO2, flow velocity and nutrient availability on Berula erecta. Aquat. Ecol. 2020, 54, 775–793. [Google Scholar] [CrossRef]
- Reitsema, R.; Wolters, J.; Preiner, S.; Meire, P.; Hein, T.; De Boeck, G.; Blust, R.; Schoelynck, J. Response of submerged macrophyte growth, morphology, chlorophyll content and nutrient stoichiometry to increased flow velocity and elevated CO2 and dissolved organic carbon concentrations. Front. Environ. Sci. 2020, 11, 527801. [Google Scholar] [CrossRef]
- Naser, M.D. First record of the freshwater crab, Potamon mesopotamicum Brandis, Storch & Türkay, 1998 (Decapoda, Brachyura, Potamidae) from the Al-Huwaizah marshes, Iraq. Crustaceana 2009, 82, 1599–1602. [Google Scholar]
- Alsaadoon, D.W.K.; Hassan, F.M.; Mahdi, W.M. Assessment of water quality of Diyala River using overall index of pollution (OIP) in Iraq. Iraqi J. Agric. Sci. 2023, 54, 682–690. [Google Scholar] [CrossRef]
- Yasser, A.; Al-Kaaby, I.; Shabeeb, A.; Naser, M.; Auda, N.; Ajeel, S.; Yesser, A.; Al-Hello, A.-Z.; Lebepe, J. Histopathology and micronuclei induction as pollution biomarkers in common carp, Cyprinus carpio from southern Iraq. J. Biol. Stud. 2024, 7, 11–30. [Google Scholar] [CrossRef]
- Yasser, A.G.; Naser, M.D. Impact of pollutants on fish collected from different parts of Shatt Al-Arab River: A histopathological study. Environ. Monit. Assess. 2011, 181, 175–182. [Google Scholar] [CrossRef]
- Yasser, A.G.; Naser, M. Acute toxicity and histopathological effects of Malathion on shrimp Macrobrachium nipponense (De Haan, 1849) (Caridea: Palaemonidae). J. Biol. Stud. 2023, 5, 774–779. [Google Scholar] [CrossRef]
- Bakker, E.S.; Van Donk, E.; Declerck, S.A.J.; Helmsing, N.R.; Hidding, B.; Nolet, B.A. Effect of macrophyte community composition and nutrient enrichment on plant biomass and algal blooms. Basic Appl. Ecol. 2010, 11, 432–439. [Google Scholar] [CrossRef]
- Zhi, Y.; Wang, W.; Li, W.; Cao, Y.; Xia, M. Increased nutrient levels induce different allocation strategies between canopy-forming and rosette-like submerged macrophytes. Water 2024, 16, 3196. [Google Scholar] [CrossRef]
- Al-Asadi, W.M.T.; Sabbar, A.A.; Al-Saadi, S.A.A.M.; Al-Zewar, J.M.M. Distribution of elements in four species of submergent plants in East Hammar and Al-Chebiyesh Marshes, Iraq. Egypt. J. Aquat. Biol. Fish. 2024, 28, 529–541. [Google Scholar] [CrossRef]
- Jaweir, H.J.; Radhi, M.M. Naididae (Clitellata: Oligochaeta) and Aeolosomatidae (Polychaeta: Aphanoneura) species associated with aquatic plants in Tigris River/Baghdad/Iraq. Baghdad Sci. J. 2013, 10, 116–125. [Google Scholar] [CrossRef]
- Salman, M.D.; Shebli, M.K.; Alfalahi, M.J.O.; Aenab, A.M.; Singh, S.K. Sorting of Glossiphonia complanata (Linnaeus, 1758) (Rhynchobdellida: Glossiphoniidae) from three aquatic plants in Tigris River within Baghdad City. Egypt. J. Pet. 2017, 26, 851–853. [Google Scholar] [CrossRef][Green Version]
- Al-Ani, R.R.; Al Obaidy, A.M.J.; Hassan, F.M. Multivariate analysis for evaluation of the water quality of Tigris River within Baghdad City in Iraq. Iraqi J. Agric. Sci. 2019, 50, 331–342. [Google Scholar]
- Salman, I.R.; Rasheed, A.A.; Hassan, S.A.H.; Hussein, R.A.; Al-Saady, M. Automated aquatic biodiversity monitoring using deep learning on the Tigris River: Species identification and ecosystem assessment. Int. J. Aquat. Biol. 2025, 13, 30–40. [Google Scholar]
- Middelboe, A.L.; Markager, S. Depth limits and minimum light requirements of freshwater macrophytes. Freshw. Biol. 1997, 37, 553–568. [Google Scholar] [CrossRef]
- Abdullah, A.D.; Gisen, J.I.; van der Zaag, P.; Savenije, H.H.; Karim, U.F.; Masih, I.; Popescu, I. Predicting the salt water intrusion in the Shatt al-Arab estuary using an analytical approach. Hydrol. Earth Syst. Sci. 2016, 20, 4031–4042. [Google Scholar] [CrossRef]
- Shihab, H.F.A.; Mohammed, A.A.H.; Kannah, A.M.A. Environmental factors and their impact on the abundance of aquatic plants in Iraq. J. Res. Appl. Sci. Biotechnol. 2023, 2, 58–65. [Google Scholar] [CrossRef]
- Partow, H. The Mesopotamian Marshlands: Demise of an Ecosystem; United Nations Environment Programme (UNEP): Geneva, Switzerland, 2001. [Google Scholar]
- Ning, N.S.; Nielsen, D.L.; Baldwin, D.S. Assessing the potential for biotic communities to recolonise freshwater wetlands affected by sulfidic sediments. Freshw. Biol. 2011, 56, 2299–2315. [Google Scholar] [CrossRef]
- Abdul Jabbar, M.F.; Al-Ma’amar, A.F.; Shehab, A.T. Change detections in marsh areas, South Iraqi using remote sensing and GIS application. Iraqi Bull. Geol. Min. 2010, 6, 17–39. [Google Scholar]
- International Energy Agency (IEA). National Climate Resilience Assessment for Iraq; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/national-climate-resilience-assessment-for-iraq (accessed on 22 April 2025).
- Nakhaei Ashtari, M.; Della Ventura, G.; Correia, M. From drought to deluge: The complex impacts of climate change on earthen heritage. J. Cult. Herit. Manag. Sustain. Dev. 2025, in press. [Google Scholar]
- Zimmermann, K.; Abadi, A.M.; Brauman, K.A.; Maestu, J.; Oude Essink, G.; Schuster-Wallace, C.; Gribble, M.O. Addressing water scarcity to support climate resilience and human health. Integr. Environ. Assess. Manag. 2025, 21, 291–300. [Google Scholar] [CrossRef]
- Döll, P.; Zhang, J. Impact of climate change on freshwater ecosystems: A global-scale analysis of ecologically relevant river flow alterations. Hydrol. Earth Syst. Sci. 2010, 14, 783–799. [Google Scholar] [CrossRef]
- Yildiz, D. Natural diminishing trend of the Tigris and Euphrates streamflows is alarming for the Middle East future. World Sci. News 2016, 47, 279. [Google Scholar]
- Adamo, N.; Al-Ansari, N.; Sissakian, V. How dams can affect freshwater issues in the Euphrates–Tigris basins. J. Earth Sci. Geotech. Eng. 2020, 10, 43–76. [Google Scholar]
- Al-Hasani, A.A. Trend analysis and abrupt change detection of streamflow variations in the lower Tigris River Basin, Iraq. Int. J. River Basin Manag. 2021, 19, 523–534. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Busico, G.; Colombani, N.; Usai, A.; Ruberti, D. Seasonal salinity variations in a coastal wetland induced by complex interactions between sea, river and evapoconcentration processes. In Estuaries and Coastal Zones in Times of Global Change: Proceedings of ICEC-2018; Springer: Singapore, 2020; pp. 77–88. [Google Scholar]
- Lorrain-Soligon, L.; Robin, F.; Bertin, X.; Jankovic, M.; Rousseau, P.; Lelong, V.; Brischoux, F. Long-term trends of salinity in coastal wetlands: Effects of climate, extreme weather events, and sea water level. Environ. Res. 2023, 237, 116937. [Google Scholar] [CrossRef] [PubMed]
- La Fuente, S.; Jennings, E.; Lenters, J.D.; Verburg, P.; Kirillin, G.; Shatwell, T.; Couture, R.M.; Côté, M.; Vinnå, C.L.R.; Woolway, R.I. Increasing warm-season evaporation rates across European lakes under climate change. Clim. Change 2024, 177, 173. [Google Scholar] [CrossRef]
- Hart, B.T.; Lake, P.S.; Webb, J.A.; Grace, M.R. Ecological risk to aquatic systems from salinity increases. Aust. J. Bot. 2003, 51, 689–702. [Google Scholar] [CrossRef]
- Bernstein, N. Plants and salt: Plant response and adaptations to salinity. In Model Ecosystems in Extreme Environments; Academic Press: Cambridge, MA, USA, 2019; pp. 101–112. [Google Scholar]
- Barbafieri, M.; Bretzel, F.; Scartazza, A.; Di Baccio, D.; Rosellini, I.; Grifoni, M.; Pini, R.; Clementi, A.; Franchi, E. Response to hypersalinity of four halophytes growing in hydroponic floating systems: Prospects in the phytomanagement of high saline wastewaters and extreme environments. Plants 2023, 12, 1737. [Google Scholar] [CrossRef]
- Hualpa-Ramirez, E.; Carrasco-Lozano, E.C.; Madrid-Espinoza, J.; Tejos, R.; Ruiz-Lara, S.; Stange, C.; Norambuena, L. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol. Biochem. 2024, 208, 108507. [Google Scholar] [CrossRef] [PubMed]
- Neckles, H.A.; Guntenspergen, G.R.; Rizzo, W.M.; Michot, T.C. Global Change and Submerged Aquatic Vegetation Research; U.S. Geological Survey Open-File Report; U.S. Geological Survey: Reston, VA, USA, 1997.
- Bly, P.; Vick, C.; Jefferson, M.; Brinker, E.; Branch, B. Submerged aquatic vegetation habitat product development: On-screen digitizing and spatial analysis of Core Sound. In Proceedings of the 2010 IEEE International Geoscience and Remote Sensing Symposium, Honolulu, HI, USA, 25–30 July 2010; pp. 1122–1124. [Google Scholar]
- McBride, J.; Cohen, M.J. Controls on productivity of submerged aquatic vegetation in two spring-fed rivers. Freshw. Sci. 2020, 39, 1–17. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Liping, X.; Schoelynck, J. Hydrogen peroxide variation patterns as abiotic stress responses of Egeria densa. Front. Plant Sci. 2022, 13, 855477. [Google Scholar] [CrossRef]
- Asaeda, T.; Wilfert, K.; Schoelynck, J. The identification of abiotic stress by hydrogen peroxide concentration in submerged macrophyte tissues. Aquat. Bot. 2024, 198, 103868. [Google Scholar] [CrossRef]
- Akter, S.; Asselberghs, J.; Kibor, S.; de Boeck, G.; Schoelynck, J. Interactive effects of nitrate pollution and heatwaves on aquatic macrophytes. Hydrobiologia 2025, in press. [Google Scholar]
- Borgnis, E.; Boyer, K.E. Salinity tolerance and competition drive distributions of native and invasive submerged aquatic vegetation in the Upper San Francisco Estuary. Estuaries Coasts 2016, 39, 707–717. [Google Scholar] [CrossRef]
- Douglass, J.G.; Chamberlain, R.H.; Wan, Y.; Doering, P.H. Submerged vegetation responses to climate variation and altered hydrology in a subtropical estuary: Interpreting 33 years of change. Estuaries Coasts 2020, 43, 1406–1424. [Google Scholar] [CrossRef]
- Khwarahm, N.R. MaxEnt-based distribution modeling of the invasive species Phragmites australis under climate change conditions in Iraq. Plants 2025, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Long, A.L.; Kettenring, K.M.; Hawkins, C.P.; Neale, C.M. Distribution and drivers of a widespread, invasive wetland grass, Phragmites australis, in wetlands of the Great Salt Lake, Utah, USA. Wetlands 2017, 37, 45–57. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Ahmad, N.S.; Salih, S. Invasive alien species in Iraq. In Invasive Alien Species: Observations and Issues from Around the World. Volume 2: Issues and Invasions in Asia and the Pacific Region; Pullaiah, T., Ielmini, M.R., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 126–146. [Google Scholar]
- Piñero-Rodríguez, M.J.; Fernández-Zamudio, R.; Arribas, R.; Gomez-Mestre, I.; Díaz-Paniagua, C. The invasive aquatic fern Azolla filiculoides negatively impacts water quality, aquatic vegetation and amphibian larvae in Mediterranean environments. Biol. Invasions 2021, 23, 755–769. [Google Scholar] [CrossRef]
- da Costa, L.; Vieira, L.A.; Michelan, T.S.; Vale, A.H.; Chiba de Castro, W.A. Growth allocation shifts in the invasive Hydrilla verticillata under interspecific competition with native submerged macrophytes. Plants 2024, 13, 3500. [Google Scholar] [CrossRef]
- Reitsema, R. Effects of Climate Change on Growth and Development of Berula erecta as Model Species for Freshwater Macrophytes. Ph.D. Thesis, University of Antwerp, Antwerpen, Belgium, 2021. [Google Scholar]
- Rodrigo, M.A. Wetland restoration with hydrophytes: A review. Plants 2021, 10, 1035. [Google Scholar] [CrossRef]
- Al-Mudaffar Fawzi, N.; Ali, M. Restoring the Marshlands of Iraq. In Ecological Restoration for Protected Areas; Keenleyside, K.A., Dudley, N., Cairns, S., Eds.; IUCN: Gland, Switzerland, 2012; pp. 93–95. [Google Scholar]
- Hasab, H.A.; Jawad, H.A.; Dibs, H.; Hussain, H.M.; Al-Ansari, N. Evaluation of water quality parameters in marshes zone southern of Iraq based on remote sensing and GIS techniques. Water Air Soil Pollut. 2020, 231, 1–11. [Google Scholar] [CrossRef]
- Bedair, H.M.; Al-Saad, H.T.; Salman, N.A. Iraq’s southern marshes—Something special to be conserved: A case study. Marsh Bull. 2006, 2, 99–126. [Google Scholar]
- Guarasci, B.L. The national park: Reviving Eden in Iraq’s marshes. Arab Stud. J. 2015, 23, 128–153. [Google Scholar]
- Tocchetto, D.; Rubenstein, M.; Nelson, M.; Al-Asadi, J. Circular economy in the Mesopotamian Marshes: The Eden in Iraq wastewater garden project. In Circular Economy and Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–198. [Google Scholar]
- Zhou, Y.; Zhang, L.; Zhao, C. Plant adaptation to climate change: Phenotypic plasticity and ecological consequences. Front. Plant Sci. 2024, 15, 1380466. [Google Scholar]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.K.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.M.; Dick, G.O. Propagation and Establishment of Aquatic Plants: A Handbook for Ecosystem Restoration Projects; US Army Corps of Engineers, Waterways Experiment Station: Vicksburg, MI, USA, 1999.
- Kettenring, K.M.; Tarsa, E.E. Need to seed? Ecological, genetic, and evolutionary keys to seed-based wetland restoration. Front. Environ. Sci. 2020, 8, 109. [Google Scholar] [CrossRef]
- Castelnuovo, N.; Villa, B.; Boldrocchi, G.; Iotti, P.; Bettinetti, R. Lake shore restoration with Vallisneria spiralis in Lake Como (Northern Italy) to improve sustainability. Sustainability 2024, 16, 10048. [Google Scholar] [CrossRef]
- Walusiak, E.; Krztoń, W.; Cieślak, E.; Szczepaniak, M.; Wilk-Woźniak, E. Native recovery or expansive threat? Past and predicted distribution of Trapa natans L. sl on northern limit of species’ range–Handout for species management. Ecol. Indic. 2024, 158, 111349. [Google Scholar] [CrossRef]
- Van der Valk, A.G.; Pederson, R.L.; Davis, C.B. Restoration and creation of freshwater wetlands using seed banks. Wetl. Ecol. Manag. 1992, 1, 191–197. [Google Scholar] [CrossRef]
- Neff, K.P.; Rusello, K.; Baldwin, A.H. Rapid seed bank development in restored tidal freshwater wetlands. Restor. Ecol. 2009, 17, 539–548. [Google Scholar] [CrossRef]
- Zepeda, G.C.; Lot, A.; Nemiga, X.A.; Manjarrez, J. Seed bank and established vegetation in the last remnants of the Mexican Central Plateau wetlands: The Lerma marshes. Rev. Biol. Trop. 2014, 62, 455–472. [Google Scholar] [CrossRef]
- Goodale, U.M.; Antonelli, A.; Nelson, C.R.; Chau, M.M. Seed banks needed to restore ecosystems. Science 2023, 379, 147. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.A. Australian wetland plants and wetlands in the landscape: Conservation of diversity and future management. Aquat. Ecosyst. Health Manag. 2003, 6, 29–40. [Google Scholar] [CrossRef]
- Jellinek, S.; Te, T.; Gehrig, S.L.; Stewart, H.; Nicol, J.M. Facilitating the restoration of aquatic plant communities in a Ramsar wetland. Restor. Ecol. 2016, 24, 528–537. [Google Scholar] [CrossRef]
- Gell, P.A.; Davidson, N.C.; Finlayson, C.M.; Herb, A.M.; McInnes, R.J.; Pittock, J.; Pritchard, D. Wetlands and future change—Implications and opportunities with the Ramsar Convention. In Ramsar Wetlands; Elsevier: Amsterdam, The Netherlands, 2023; pp. 555–561. [Google Scholar]
- Dubey, S. Ramsar wetlands: Critical zones for maintenance for ecological equilibrium. J. Pharm. Biol. Sci. 2024, 12, 127–134. [Google Scholar] [CrossRef]
- Strifling, D.A. Integrated water resources management and effective intergovernmental cooperation on watershed issues. Mercer Law Rev. 2018, 70, 399. [Google Scholar]
- Lindenschmidt, K.E.; Akomeah, E.; Baulch, H.; Boyer, L.; Davies, J.M.; Hassanzadeh, E.; Marin, L.M.; Strickert, G.; Wauchope, M. Interfacing stakeholder involvement into a surface water-quality modelling system for water management and policy development. In New Trends in Urban Drainage Modelling: UDM 2018; Springer: Cham, Switzerland, 2019; pp. 312–316. [Google Scholar]
- Kumar, D.; Sharma, U.; Singh, V.; Yadav, A.K.; Anita; Kumar, S.; Kumar, N. Efficiency of aquatic plants for remediation of wastewater. In Aquatic Macrophytes: Ecology, Functions and Services; Springer Nature: Singapore, 2023; pp. 159–174. [Google Scholar]
- Altinbilek, D. Development and management of the Euphrates–Tigris basin. Int. J. Water Resour. Dev. 2004, 20, 15–33. [Google Scholar] [CrossRef]
- Mazlum, I. Transboundary water management in the Euphrates–Tigris Basin: Dynamics of regional cooperation, sustainability and governance. In The Jordan River and Dead Sea Basin; Springer: Dordrecht, The Netherlands, 2009; pp. 139–164. [Google Scholar]
- Kibaroglu, A. Transboundary water relations in the Euphrates and Tigris region. In Water Law and Cooperation in the Euphrates–Tigris Region; Brill Nijhoff: Leiden, The Netherlands, 2013; pp. 61–81. [Google Scholar]
- Kibaroglu, A. State-of-the-art review of transboundary water governance in the Euphrates–Tigris river basin. Int. J. Water Resour. Dev. 2019, 35, 4–29. [Google Scholar] [CrossRef]
- Hartig, J.H.; Zarull, M.A.; Ciborowski, J.J.; Gannon, J.E.; Wilke, E.; Norwood, G.; Vincent, A.N. Long-term ecosystem monitoring and assessment of the Detroit River and Western Lake Erie. Environ. Monit. Assess. 2009, 158, 87–104. [Google Scholar] [CrossRef]
- Hampton, S.E.; Scheuerell, M.D.; Church, M.J.; Melack, J.M. Long-term perspectives in aquatic research. Limnol. Oceanogr. 2019, 64 (Suppl. S1), S2–S10. [Google Scholar] [CrossRef]
- Galatowitsch, S.; Bohnen, J. Long-term recovery of a restored palustrine wetland: The role of monitoring and adaptive management. Wetlands 2021, 41, 80. [Google Scholar] [CrossRef]
- Gökçe, D. The importance and effectiveness of aquatic biomonitoring. In New Paradigms in Environmental Biomonitoring Using Plants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–72. [Google Scholar]
- Zedler, J.B. Integrating traditional ecological knowledge with adaptive restoration. Ecosyst. Health Sustain. 2016, 2, e01222. [Google Scholar] [CrossRef]
- Reyes-García, V.; Fernández-Llamazares, Á.; McElwee, P.; Molnár, Z.; Öllerer, K.; Wilson, S.J.; Brondizio, E.S. The contributions of Indigenous Peoples and local communities to ecological restoration. Restor. Ecol. 2019, 27, 3–8. [Google Scholar] [CrossRef]
- Owusu-Achiaw, R.; Osei-Owusu, Y. Community-based approach to wetland restoration: Case study of the Songor Wetland, Ghana. In Governing Sustainability in the Global South; Nishi, M., Ed.; Routledge: London, UK, 2023; p. 157. [Google Scholar]
| Species | References | Historic Status | Current Status |
|---|---|---|---|
| Cyperus papyrus | [22,32] | Historically scattered | Now critically reduced due to habitat alteration and marsh drainage |
| Myriophyllum spicatum | [22,32] | Historically present in deep or semi-permanent water | Still locally present but declining; sensitive to turbidity and organic pollution |
| Najas marina | [32,33] | Historically recorded in shallow submerged zones | Now rare or possibly extirpated; affected by increased salinity and habitat desiccation |
| Potamogeton crispus | [22,32,33] | Historically widespread | Declining; attributed to pollution, turbidity, and potential heavy metal accumulation |
| Stuckenia pectinata | [32,33] | Historically present in low-salinity marshes | Still persists in some degraded systems; considered tolerant to eutrophication and moderate pollution, but may decline under extreme salinity or desiccation |
| Schoenoplectus litoralis | [22,32,34] | Historically abundant in shallow zones | Now declining; driven by wetland desiccation and increasing salinity |
| Trapa natans | [32,35] | Historically present in freshwater marshes | Now likely extinct or extremely rare; vulnerable to salinity and drainage |
| Typha domingensis | [22,34] | Historically common emergent species | Still present, but with patchy distribution; affected by pollution and marsh fragmentation |
| Vallisneria spiralis | [22,32,33] | Historically dominant submerged macrophyte | Currently rare or absent; impacted by turbidity, flow alteration, and pollution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naser, M.; Yasser, A.; Schoelynck, J.; Essl, F. Threatened Aquatic Plants of the Southern Tigris-Euphrates Basin: Status, Threats, and Conservation Priorities. Plants 2025, 14, 1914. https://doi.org/10.3390/plants14131914
Naser M, Yasser A, Schoelynck J, Essl F. Threatened Aquatic Plants of the Southern Tigris-Euphrates Basin: Status, Threats, and Conservation Priorities. Plants. 2025; 14(13):1914. https://doi.org/10.3390/plants14131914
Chicago/Turabian StyleNaser, Murtada, Amaal Yasser, Jonas Schoelynck, and Franz Essl. 2025. "Threatened Aquatic Plants of the Southern Tigris-Euphrates Basin: Status, Threats, and Conservation Priorities" Plants 14, no. 13: 1914. https://doi.org/10.3390/plants14131914
APA StyleNaser, M., Yasser, A., Schoelynck, J., & Essl, F. (2025). Threatened Aquatic Plants of the Southern Tigris-Euphrates Basin: Status, Threats, and Conservation Priorities. Plants, 14(13), 1914. https://doi.org/10.3390/plants14131914






