Essential Oils and Extracts from Epazote (Dysphania ambrosioides): A Phytochemical Treasure with Multiple Applications
Abstract
1. Introduction
2. Methodology
2.1. Bibliographic Search Conditions
2.2. Selection Process of Relevant Papers Assisted by the AI Tool
2.2.1. Preprocessing of Search Data
2.2.2. Text Clustering and Cluster Analyses
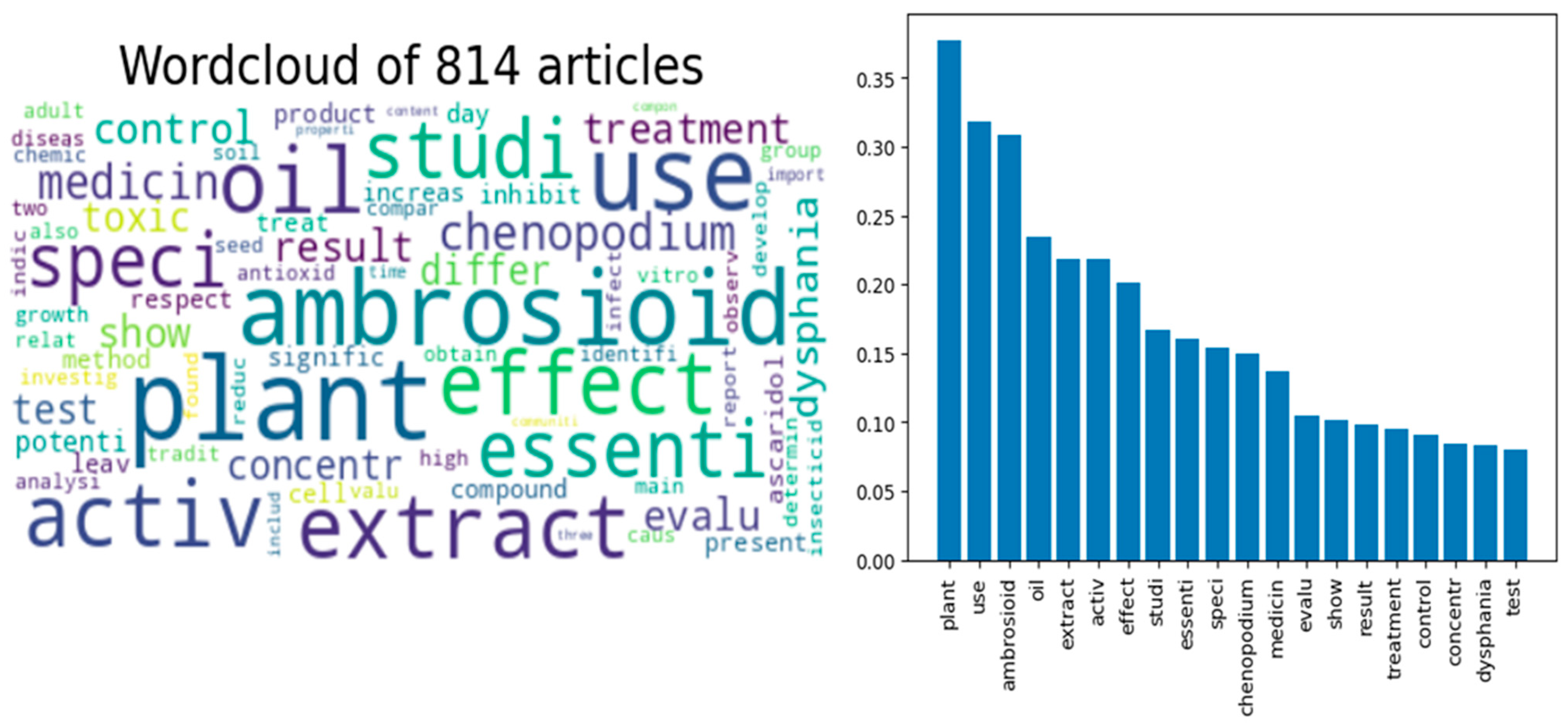
3. Selection of the Relevant Papers Assisted by the AI Tool
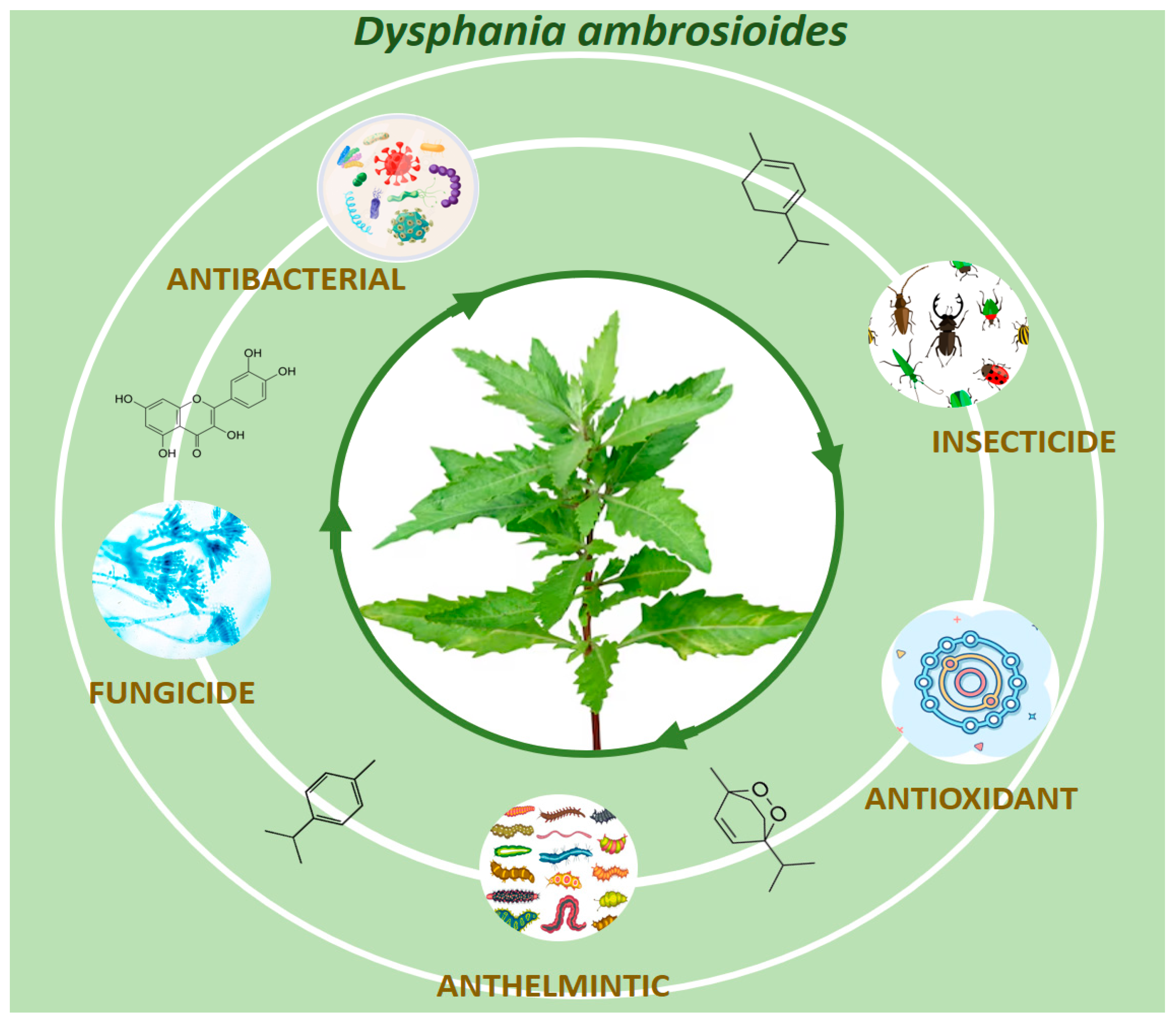
4. Traditional Review of the Selected Papers
4.1. Chemical Composition of Dysphania ambrosioides
4.2. Antimicrobial Properties of Dysphania ambrosioides
4.3. Antioxidant Properties of Dysphania ambrosioides
4.4. Insecticidal and Repellent Activities of Dysphania ambrosioides
4.5. Antiparasitic Activities of Dysphania ambrosioides
4.6. Other Biological Properties of Dysphania ambrosioides
4.7. Safety Margins of Dysphania ambrosioides Extracts and Essential Oils
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kandsi, F.; Conte, R.; Marghich, M.; Lafdil, F.Z.; Alajmi, M.F.; Bouhrim, M.; Mechchate, H.; Hano, C.; Aziz, M.; Gseyra, N. Phytochemical analysis, antispasmodic, myorelaxant, and antioxidant effect of Dysphania ambrosioides (L.) Mosyakin and Clemants flower hydroethanolic extracts and its chloroform and ethyl acetate fractions. Molecules 2021, 26, 7300. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandey, A.K. Dysphania ambrosioides essential oils: From pharmacological agents to uses in modern crop protection—A review. Phytochem. Rev. 2022, 21, 141–159. [Google Scholar] [CrossRef]
- Sá, R.D.; Santana, A.C.C.O.; Silva, F.C.L.; Soares, L.A.L.; Randau, K.P. Anatomical and histochemical analysis of Dysphania ambrosioides supported by light and electron microscopy. Rev. Bras. Farmacogn. 2016, 26, 533–543. [Google Scholar] [CrossRef][Green Version]
- Kamdem, B.P.; Le Doux Kamto, E.; Kamdem Paumo, H.; Katata-Seru, L.M.; Pegnyemb, D.E.; Igne, F.E. Chemical constituents, ethnomedicinal uses, pharmacology, and toxicity of Dysphania ambrosioides (L.) Mosyakin & Clemants, formerly Chenopodium Ambrosioides L. Nat. Prod. J. 2022, 12, 1. [Google Scholar] [CrossRef]
- Fatokun, O.T.; Diyaolu, A.H.; Esievo, K.B.; Adamu, A.; Aboh, M.O.; Okhale, S.E. Chemical Composition and Antibacterial Activity of the Essential Oil of Dysphania ambrosioides (L.) Mosyakin & Clemants from North Central Nigeria. J. Phytomed. Therap. 2019, 18, 304–313. [Google Scholar]
- Hewis, L.; Daeli, G.; Tanoto, K.; Carlos, C.; Sahamastuti, A. A Review of Botany, Phytochemical, and Pharmacological Effects of Dysphania ambrosioides. Indones. J. Life Sci. 2020, 2, 70–82. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Role of Ascaridole and p-Cymene in the Sleep-Promoting Effects of Dysphania ambrosioides Essential Oil via the GABAergic System in a ddY Mouse Inhalation Model. J. Nat. Prod. 2021, 84, 91–100. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Zidane, L. Ethnobotanical and Ethnomedicinal study of medicinal and aromatic plants used against dermatological diseases by the people of Rif Morocco. J. Herb. Med. 2022, 32, 100542. [Google Scholar] [CrossRef]
- Tlemcani, S.; Lahkimi, A.; Eloutassi, N.; Bendaoud, A.; Hmamou, A.; Hicham Bekkari, H. Ethnobotanical study of medicinal plants in the Fez-Meknes region of Morocco. J. Pharm. Pharmacog. Res. 2023, 11, 137–159. [Google Scholar] [CrossRef]
- do Nascimento Magalhães, K.; Guarniz, W.A.S.; Sá, K.M.; Freire, A.B.; Monteiro, M.P.; Nojosa, R.T.; Bieski, I.G.C.; Custódio, J.B.; Balogun, S.O.; Bandeira, M.A.M. Medicinal plants of the Caatinga, northeastern Brazil: Ethnopharmacopeia (1980-1990) of the late professor Francisco José de Abreu Matos. J. Ethnopharmacol. 2019, 237, 314–353. [Google Scholar] [CrossRef]
- Ogunleye, G.S.; Fagbohun, O.F.; Babalola, O.O. Chenopodium ambrosioides var. Ambrosioides leaf extracts possess regenerative and ameliorative effects against mercury-induced hepatotoxicity and nephrotoxicity. Ind. Crops Prod. 2020, 154, 112723. [Google Scholar] [CrossRef]
- Zohra, T.; Ovais, M.; Khalil, A.T.; Qasim, M.; Ayaz, M.; Shinwari, Z.K. Extraction optimization, total phenolic, flavonoid contents, HPLC-DAD analysis and diverse pharmacological evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat. Prod. Res. 2018, 33, 136–142. [Google Scholar] [CrossRef]
- Abdulkader, O.M.; Sharaf, A.E.A.; Fouda, H.M.; Elhaw, M.H. hytoconstitutes investigation of Chenopodium ambrosioides Linn. and gas chromatography with mass spectroscopy material analysis. Mater. Today Proc. 2022, 61, 992–997. [Google Scholar] [CrossRef]
- Shang, H.; He, D.; Li, B.; Chen, X.; Luo, K.; Li, G. Environmentally Friendly and Effective Alternative Approaches to Pest Management: Recent Advances and Challenges. Agronomy 2024, 14, 1807. [Google Scholar] [CrossRef]
- Niaz, A.; Muhammad, N.; Mohammad, S.; Ismail, S.; Ghayour, A.; Shakirullah; Ziauddi; Syed, W.A.S.; Mehreen, G.; Shahzeb, K.; et al. GC/MS analysis, anti-leishmanial and relaxant activity of essential oil of Chenopodium ambrosioides (L.) from Malakand region. Pak. J. Pharm. Sci. 2021, 34, 577–583. [Google Scholar] [CrossRef]
- Fernández-López, J.; Borrás-Rocher, F.; Viuda-Martos, M.; Pérez-Álvarez, J.Á. Using Artificial Intelligence-Based Tools to Improve the Literature Review Process: Pilot Test with the Topic “Hybrid Meat Products”. Informatics 2024, 11, 72. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Borrás-Rocher, F.; Micol, V.; Barrajón-Catalán, E. Artificial Intelligence Applied to Improve Scientific Reviews: The Antibacterial Activity of Cistus Plants as Proof of Concept. Antibiotics 2023, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Ikotun, A.M.; Ezugwu, E.E.; Abualigah, L.; Belal Abuhaija, B.; Heming, J. K-means clustering algorithms: A comprehensive review, variants analysis, and advances in the era of big data. Inf. Sci. 2023, 622, 178–210. [Google Scholar] [CrossRef]
- Syakur, M.A.; Khotimah, B.K.; Rochman, E.M.S.; Satoto, B.D. Integration K-Means Clustering Method and Elbow Method for Identification of The Best Customer Profile Cluster. IOP Conf. Ser. Mater. Sci. Eng. 2018, 336, 012017. [Google Scholar] [CrossRef]
- Punhani, A.; Faujdar, N.; Mishra, K.K.; Subramanian, M. Binning-Based Silhouette Approach to Find the Optimal Cluster Using K-Means. IEEE Access 2022, 10, 115025–115032. [Google Scholar] [CrossRef]
- Zefzoufi, M.; Smaili, A.; Fdil, R.; Rifai, L.A.; Faize, L.; Koussa, T.; Makroum, K.; Ben Ali, A.; Tabyaoui, M.; Mouzdahir, A.; et al. Composition of essential oil of Moroccan Dysphania ambrosioides and its antimicrobial activity against bacterial and fungal phytopathogens. J. Plant Pathol. 2020, 102, 47–58. [Google Scholar] [CrossRef]
- Maldaner, J.; Silva, F.S.; Santos, D.d.A.; Silva, S.Y.S.; Silva, S.d.C.; Lima, T.d.C.; Goulart, S.L.; Tomchinsky, B.; Oliveira, M.N. Bioherbicide potential of essential oils from plant species used in folk medicine and cuisine in the Amazon region. S. Afr. Bot. 2023, 162, 761–766. [Google Scholar] [CrossRef]
- Hsu, K.P.; Yang, M.L.; Wei, L.Y.; Ho, H.T.; Ho, C.L. Chemical Composition and In Vitro Anti-Wood-Decay Fungal Activities of Dysphania ambrosioides Leaf Essential Oil From Taiwan. Nat. Prod. Commun. 2022, 17, 1934578X2210999. [Google Scholar] [CrossRef]
- Pagotti, M.C.; Candido, A.C.B.B.; Marçal, M.G.; Vieira, T.M.; Groppo, M.; Silva, M.L.A.; Ferreira, D.S.; Esperandim, V.R.; Crotti, A.E.M.; Magalhães, L.G. Trypanocidal Activity of Dysphania ambrosioides, Lippia alba, and Tetradenia riparia Essential Oils against Trypanosoma cruzi. Chem. Biodivers. 2021, 18, e2100678. [Google Scholar] [CrossRef]
- Sounouvou, H.T.; Toukourou, H.; Catteau, L.; Toukourou, F.; Evrard, B.; Van Bambeke, F.; Gbaguidi, F.; Quetin-Leclercq, J. Antimicrobial potentials of essential oils extracted from West African aromatic plants on common skin infections. Sci. Afric. 2021, 11, e00706. [Google Scholar] [CrossRef]
- Ferreira Martins, S.H.; Ferreira-Silva, A.; Souza Cruz, R.; Lucchese, A.M.; Camilloto, G.P. Antifungal Film Incorporated with Chenopodium ambrosioides L. Essential Oil for Postharvest Storage. ACS Food Sci. Technol. 2022, 2, 1086–1095. [Google Scholar] [CrossRef]
- Degenhardt, R.T.; Farias, I.V.; Grassi, L.T.; Franchi, G.C., Jr.; Nowill, A.E.; Bittencourt, C.M.S.; Wagner, T.M.; Souza, M.M.D.; Cruz, A.B.; Malheiros, A. Characterization and evaluation of the cytotoxic potential of the essential oil of Chenopodium ambrosioides. Rev. Bras. 2016, 26, 56–61. [Google Scholar] [CrossRef]
- Almeida Bezerra, J.W.; Rodrigues Costa, A.; De Freitas, M.A.; Rodrigues, F.C.; De Souza, M.A.; Da Silva, A.R.P.; Dos Santos, A.T.L.; Linhares, K.V.; Melo Coutinho, H.D.; De Lima Silva, J.R.; et al. Chemical composition, antimicrobial, modulator and antioxidant activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ez-Zriouli, R.; ElYacoubi, H.; Imtara, H.; Mesfioui, A.; ElHessni, A.; Al Kamaly, O.; Zuhair Alshawwa, S.; Nasr, F.A.; Benziane Ouaritini, Z.; Rochdi, A. Chemical Composition, Antioxidant and Antibacterial Activities and Acute Toxicity of Cedrus atlantica, Chenopodium ambrosioides and Eucalyptus camaldulensis Essential Oils. Molecules 2023, 28, 2974. [Google Scholar] [CrossRef]
- Azghar, A.; Dalli, M.; Loukili, E.H.; Belbachir, Y.; Tahri, M.; Benaissa, E.; Ben Lahlou, Y.; Elouennass, M.; Maleb, A. Evaluation of the Antibacterial Activity of Essential Oil of Dysphania ambrosioides (L.) Mosyakin and Clemants Against Clinical Multidrug-Resistant Bacteria. As. J. Plant Sci. 2023, 22, 75–81. [Google Scholar] [CrossRef]
- Bisht, B.S.; Kumar, A. Terpenoid composition of Chenopodium ambrosioides L. and its antimicrobial activity from Uttarakhand Himalaya of India. J. Drug Del. Ther. 2019, 9, 612–617. [Google Scholar] [CrossRef]
- Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Meskali, N.; Azghar, A.; Addi, M.; Hano, C.; Maleb, A.; Gseyra, N. Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics 2022, 11, 482. [Google Scholar] [CrossRef]
- Kasrati, A.; Sakar, E.H.; Aljaiyash, A.; Hirri, A.; Tamegart, L.; Abbad, I.; Alaoui Jamali, C. Chemical Profiling, Insecticidal, and Phytotoxic Effect of Essential Oils from Leaves and Inflorescence of Moroccan Chenopodium ambrosioides (L.). Plants 2024, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Stappen, I.; Tabanca, N.; Ali, A.; Wanner, J.; Lal, B.; Jaitak, V.; Wedge, D.E.; Kaul, V.K.; Schmidt, E.; Jirovetz, L. Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya. Open Chem. 2018, 16, 306–316. [Google Scholar] [CrossRef]
- Ngan, T.T.K.; Quan, P.M.; Toan, T.Q. Characterization of Dysphania ambrosioides (L.) Mosyakin & Clemants essential oil from Vietnam. Nat. Volat. Essen. Oils 2020, 7, 34–40. [Google Scholar] [CrossRef]
- Black Solis, J.; Ventura Aguilar, R.I.; Barrera Necha, L.L.; Bautista Baños, S. Caracterización química, variabilidad composicional y modelamiento matemático del efecto de aceites esenciales en Alternaria alternata. Mex. J. Phytopathol. 2017, 35, 204–226. [Google Scholar] [CrossRef]
- Harraz, F.M.; Hammoda, H.M.; El Ghazouly, M.G.; Farag, M.A.; El-Aswad, A.F.; Bassam, S.M. Chemical composition, antimicrobial and insecticidal activities of the essential oils of Conyza linifolia and Chenopodium ambrosioides. Nat. Prod. Res. 2015, 29, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Almadiy, A.A. Chemical profile, mosquitocidal, and biochemical effects of essential oil and major components of Dysphania ambrosioides against Culex quinquefasciatus Say. Environ. Sci. Pollut. Res. 2020, 27, 41568–41576. [Google Scholar] [CrossRef]
- Monteiro, J.N.M.; Archanjo, A.B.; Passos, G.P.; Costa, A.V.; Porfirio, L.C.; Martins, I.V.F. Chenopodium ambrosioides L. essential oil and ethanol extract on control of canine Ancylostoma spp. Semin. Ciências Agrárias 2017, 38, 1947. [Google Scholar] [CrossRef]
- Ait Sidi Brahim, M.; Fadli, M.; Hassani, L.; Boulay, B.; Markouk, M.; Bekkouche, K.; Abbad, A.; Ait Ali, M.; Larhsini, M. Chenopodium ambrosioides var. Ambrosioides used in Moroccan traditional medicine can enhance the antimicrobial activity of conventional antibiotics. Ind. Crops Prod. 2015, 71, 37–43. [Google Scholar] [CrossRef]
- Zerrifi, S.E.A.; Kasrati, A.; Redouane, E.M.; Tazart, Z.; Khalloufi, F.E.; Abbad, A.; Oudra, B.; Campos, A.; Vasconcelos, V. Essential oils from Moroccan plants as promising ecofriendly tools to control toxic cyanobacteria blooms. Ind. Crops Prod. 2020, 143, 111922. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennett, R.; Mota, M.; Figueiredo, A.C.D.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016, 89, 207–217. [Google Scholar] [CrossRef]
- Mokni, R.E.; Youssef, F.S.; Jmii, H.; Khmiri, A.; Bouazzi, S.; Jlassi, I.; Jaidane, H.; Dhaouadi, H.; Ashour, M.L.; Hammami, S. The Essential Oil of Tunisian Dysphania ambrosioides and its Antimicrobial and Antiviral Properties. J. Essen. Oil Bear. Plants. 2019, 22, 282–294. [Google Scholar] [CrossRef]
- Wei, H.; Liu, J.; Li, B.; Zhan, Z.; Chen, Y.; Tian, H.; Lin, S.; Gu, X. The toxicity and physiological effect of essential oil from Chenopodium ambrosioides against the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Protect. 2015, 76, 68–74. [Google Scholar] [CrossRef]
- Barros, A.F.; Campos, V.P.; De Paula, L.L.; Oliveira, D.F.; De Silva, F.J.; Terra, W.C.; Silva, G.H.; Salimena, J.P. Nematicidal screening of essential oils and potent toxicity of Dysphania ambrosioides essential oil against Meloidogyne incognita in vitro and in vivo. J. Phytopathol. 2019, 167, 380–389. [Google Scholar] [CrossRef]
- Shameem, A.S.; Khan, K.Z.; Waza, A.A.; Shah, A.H.; Qadri, H.; Ganai, B.A. Bioactivities and chemoprofiling comparisons of Chenopodium ambrosioides L. and Chenopodium botrys L. growing in Kashmir India. Asian J. Pharm. Clin. Res. 2019, 12, 124–129. [Google Scholar] [CrossRef]
- Khan, M.H.; Dar, N.A.; Alie, B.A.; Dar, S.A.; Lone, A.A.; Mir, G.H.; Fayaz, U.; Ali, S.; Tyagi, A.; El-Sheikh, M.A.; et al. Unraveling the Variability of Essential Oil Composition in Different Accessions of Bunium persicum Collected from Different Temperate Micro-Climates. Molecules 2023, 28, 2404. [Google Scholar] [CrossRef]
- Fdil, R.; Derhalia, S.; Malikib, S.; Filali-Ansarib, N.; Zefzoufia, M.; Abbouyib, A.; Khyarib, S.; Sraidia, K.; Mouzdahira, A. Comparative analysis, antibacterial and antiradical activities of essential oils in leaves and fruits of Chenopodium ambrosioides of Morocco. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 1038–1044. [Google Scholar]
- Jesus, R.S.; Piana, M.; Freitas, R.B.; Brum, T.F.; Alves, C.F.S.; Belke, B.V.; Mossmann, N.J.; Cruz, R.C.; Santos, R.C.V.; Dalmolin, T.V.; et al. In vitro antimicrobial and antimycobacterial activity and HPLC–DAD screening of phenolics from Chenopodium ambrosioides L. Braz. J. Microbiol. 2018, 49, 296–302. [Google Scholar] [CrossRef]
- Li, J.; Si, C.; Hong, W.; Xia, C.; Yang, Y.; He, Y.; Su, M.; Long, X.; Zhang, H. Identification of the chemical components of ethanol extract of Chenopodium ambrosioides and evaluation of their in vitro antioxidant and anti tumor activities. Trop. J. Farmaceut. Res. 2022, 21, 1689–1697. [Google Scholar] [CrossRef]
- Figueroa-Merma, A.; Chirinos, R.; García-Rios, D.; Pedreschi, R.; Aguilar-Galvez, A.; Campos, D. Bioactive compounds characterisation of Peruvian Dysphania ambrosioides (L.) Mosyakin & Clemants leaves by GC/MS and UPLC–ESI–Q/TOF–MSN techniques. Int. J. Food Sci. Technol. 2023, 58, 1219–1229. [Google Scholar] [CrossRef]
- Kandsi, F.; Lafdil, F.Z.; Elbouzidi, A.; Bouknana, S.; Miry, A.; Addi, M.; Conte, R.; Hano, C.; Gseyra, N. Evaluation of Acute and Subacute Toxicity and LC-MS/MS Compositional Alkaloid Determination of the Hydroethanolic Extract of Dysphania ambrosioides (L.) Mosyakin and Clemants Flowers. Toxins 2022, 14, 475. [Google Scholar] [CrossRef]
- Shah, H.; Khan, A.A. Phytochemical characterisation of an important medicinal plant, Chenopodium ambrosioides Linn. Nat. Prod. Res. 2017, 31, 2321–2324. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Saad, A.M.; Abdou, A.M.; Refahy, L.A.G.; Ahmed, W.S. A new kaempferol glycoside with antioxidant activity from Chenopodium ambrosioides growing in Egypt. Orient. J. Chem. 2016, 32, 3054–3061. [Google Scholar] [CrossRef]
- De Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; Da Cunha, F.A.B.; Da Costa, R.H.S.; Pereira, P.S.; Lima, L.F.; De Matos, Y.M.L.S.; et al. Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018, 262, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Limaverde, P.W.; Campina, F.F.; Da Cunha, F.A.B.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; Oliveira-Tintino, C.D.M.; De Matos, Y.M.L.S.; Morais-Braga, M.F.B.; Menezes, I.R.A.; et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef]
- Gishen, N.Z.; Taddese, S.; Zenebe, T.; Dires, K.; Tedla, A.; Mengiste, B.; Shenkute, D.; Tesema, A.; Shiferaw, Y.; Lulekal, E. In vitro antimicrobial activity of six Ethiopian medicinal plants against Staphylococcus aureus, Escherichia coli and Candida albicans. Eur. J. Integrat. Med. 2020, 36, 101121. [Google Scholar] [CrossRef]
- Martínez-Alva, J.E.; Espinoza-Simón, E.; Bayona-Pérez, Y.; Ruiz-Pérez, N.C.; Ochoa, S.A.; Xicohtencatl-Cortes, J.; Torres, J.; Romo-Castillo, M. In Vitro Analysis of Extracts of Plant Used in Mexican Traditional Medicine, Which Are Useful to Combat Clostridioides difficile Infection. Pathogens 2022, 11, 774. [Google Scholar] [CrossRef]
- Knauth, P.; Acevedo-Hernández, G.J.; Cano, M.E.; Gutiérrez-Lomelí, M.; López, Z. In Vitro bioactivity of methanolic extracts from Amphipterygium adstringens (Schltdl.) Schiede ex Standl., Chenopodium ambrosioides L., Cirsium mexicanum DC., Eryngium carlinae F. Delaroche, and Pithecellobium dulce (Roxb.) Benth. Used in Traditional Medicine in Mexico. Evidence-Based Complement. Altern. Med. 2018, 1, 3610364. [Google Scholar] [CrossRef]
- Ajaib, M.; Hussain, T.; Farooq, S.; Ashiq, M. Analysis of Antimicrobial and Antioxidant Activities of Chenopodium ambrosioides: An Ethnomedicinal Plant. J. Chem. 2016, 1, 4827157. [Google Scholar] [CrossRef]
- de Andrade Santiago, J.; Cardoso, M.G.; Batista, L.R.; Castro, E.M.; Teixeira, M.L.; Pires, M.F. Essential oil from Chenopodium ambrosioides L.: Secretory structures, antibacterial and antioxidant activities. Acta. Sci. Biol. Sci. 2016, 38, 139–147. [Google Scholar] [CrossRef]
- Ouadja, B.; Katawa, G.; Toudji, G.A.; Layland, L.; Gbekley, E.H.; Ritter, M.; Anani, K.; Ameyapoh, Y.; Karou, S.D. Anti-inflammatory, antibacterial and antioxidant activities of Chenopodium ambrosioides L. (Chenopodiaceae) extracts. J. Appl. Biosci. 2021, 162, 16764–16794. [Google Scholar] [CrossRef]
- Bano, S.; Baig, M.W.; Okla, M.K.; Zahra, S.S.; Akhtar, N.; Al-Qahtani, W.H.; AbdElgawad, H.; Haq, I.U. Antioxidant, antimicrobial, and protein kinase inhibition profiling of C. ambrosioides seed extracts along with RP-HPLC. J. Chem. 2022, 6486717, 1–12. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Ajat, M.; Yap, W.S.; Erin Lim, S.H.; Lai, K.S. Antimicrobial activity and mode of action of terpene linalyl anthranilate against carbapenemase-producing Klebsiella pneumoniae. J. Pharmac. Anal. 2021, 11, 210–219. [Google Scholar] [CrossRef]
- Musa, A.; Međo, I.; Marić, I.; Marčić, D. Acaricidal and sublethal effects of a Chenopodium-based biopesticide on the two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol. 2017, 71, 211–226. [Google Scholar] [CrossRef]
- Drioua, S.; El-Guourrami, O.; Assouguem, A.; Ameggouz, M.; Kara, M.; Ullah, R.; Bari, A.; Zahidi, A.; Skender, A.; Benzeid, H.; et al. Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.). Open Chem. 2024, 22, 20230194. [Google Scholar] [CrossRef]
- Maldonado-Garcia, M.; Angulo, C.; Vazquez-Martinez, J.; Sanchez, V.; Lopez, M.G.; Reyes-Becerril, M. Antioxidant and immunostimulant potentials of Chenopodium ambrosioides L. in Pacific red snapper (Lutjanus peru). Aquaculture 2019, 513, 734414. [Google Scholar] [CrossRef]
- Tauchen, J.; Huml, L.; Bortl, L.; Doskocil, I.; Jarosova, V.; Marsik, P.; Frankova, A.; Clavo Peralta, Z.M.; Chuspe Zans, M.E.; Havlik, J.; et al. Screening of medicinal plants traditionally used in Peruvian Amazon for in vitro antioxidant and anticancer potential. Nat. Prod. Res. 2019, 33, 2718–2721. [Google Scholar] [CrossRef]
- Owokotomo, I.A. Chemical Analysis and Antioxidant Studies of the Essential Oil of Chenopodium ambrosioides (L.) Growing Wild in South-West Nigeria. Nig. Res. J. Chem. Sci. 2022, 10, 9–17. [Google Scholar]
- Villalobos-Delgado, L.H.; González-Mondragón, E.G.; Salazar Govea, A.Y.; Ramírez Andrade, J.; Santiago-Castro, J.T. Potential application of epazote (Chenopodium ambrosioides L.) as natural antioxidant in raw ground pork. LWT-Food Sci. Tecnol. 2017, 84, 306–313. [Google Scholar] [CrossRef]
- Pandiangan, D.; Lamlean, P.Y.V.; Nainggolan, N. Product quality test of pasote tea bags leaves pasote (Dysphania ambrosioides): Comparison of antioxidant activities of water extract with acetone extract. Eur. J. Mol. Clin. Med. 2020, 7, 878–886. [Google Scholar]
- Tchani, G.W.; Agbeme, K.S.; Agbodan, K.A.; Baba, G.; Kpegba, K. Phytochemical Study and Comparative Antioxidant Activity of Extracts from Aerial Parts of Chenopodium ambrosioides Linn. (Chenopodiaceae). Adv. Biol. Chem. 2021, 11, 220–233. [Google Scholar] [CrossRef]
- Yikinç, M.; Tunaz, H. Bazı Bitkisel Kökenli Uçucu Yağların Amerikan Hamamböceği (Periplaneta americana L.) Erginlerine Karşı Ölüm Etkisi. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa. Dergisi 2023, 26, 579–587. [Google Scholar] [CrossRef]
- Laghzaoui, E.M.; Aglagane, A.; Soulaimani, B.; Abbad, I.; Kimdil, L.; Er-rguibi, O.; Abbad, A.; El Mouden, E.H. Insecticidal activity of some plant essential oils against the Opuntia cochineal scale insect, Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae). Phytoparasitica 2022, 50, 901–911. [Google Scholar] [CrossRef]
- Oyeboade Adegorite, S.; Aladesida, A.A.; Bamidele John, I.; Modupe kayode-Isola, T. Bioefficacy of Chenopodium ambrosioides L. (Chenopodiaceae) on adult Callosobruchus maculatus F. (Coleoptera: Bruchidae). World J. Biol. Pharm. Health Sci. 2024, 17, 192–200. [Google Scholar] [CrossRef]
- Arena, J.S.; Omarini, A.B.; Zunino, M.P.; Peschiutta, M.L.; Defagó, M.T.; Zygadlo, J.A. Essential oils from Dysphania ambrosioides and Tagetes minuta enhance the toxicity of a conventional insecticide against Alphitobius diaperinus. Ind. Crops Prod. 2018, 122, 190–194. [Google Scholar] [CrossRef]
- Velez, B.A.D.A.; Diniz, A.G.; Barbosa, L.F.S.; Santos, A.C.D.S.; Da Costa, A.F.; Tiago, P.V. Potential of Fusarium incarnatum-equiseti species complex isolates with Chenopodium ambrosioides and Enterolobium contortisiliquum extracts to control Dactylopius opuntiae. Int. J. Trop. Insect Sci. 2019, 39, 131–138. [Google Scholar] [CrossRef]
- Vite-Vallejo, O.; Barajas-Fernández, M.G.; Saavedra-Aguilar, M.; Cardoso-Taketa, A. Insecticidal Effects of Ethanolic Extracts of Chenopodium ambrosioides, Piper nigrum, Thymus vulgaris, and Origanum vulgare against Bemisia tabaci. Southwest. Entomol. 2018, 43, 383–393. [Google Scholar] [CrossRef]
- Trindade, R.C.P.; Ferreira, E.S.; Gomes, I.B.; Silva, L.; Sant’Ana, A.E.G.; Broglio, S.M.F.; Silva, M.S. Extratos aquosos de inhame (Dioscorea rotundataPoirr.) e de mastruz (Chenopodium ambrosioides L.) no desenvolvimento da lagarta-do-cartucho-do-milho Spodoptera frugiperda (J.E. Smith, 1797). Rev. Bras. Plantas Med. 2015, 17, 291–296. [Google Scholar] [CrossRef]
- Valdés-Estrada, M.E.; Aldana-Llanos, L.; Salinas-Sánchez, D.O.; Figueroa-Brito, R.; Hernández-Reyes, M.C.; Valladares-Cisneros, M.G. Toxicity of Plant Extracts to Scyphophorus acupunctatus (Coleoptera: Curculionidae). Fla. Entomologist. 2016, 99, 226–230. [Google Scholar] [CrossRef]
- Langsi, D.J.; Tofel, H.K.; Fokunang, C.N.; Suh, C.; Eloh, K.; Caboni, P.; Nukenine, E.N. Insecticidal activity of essential oils of Chenopodium ambrosioides and Cupressus sempervirens and their binary combinations on Sitophilus zeamais. GSC Biol. Pharmac. Sci. 2018, 3, 24–34. [Google Scholar] [CrossRef]
- Guimarães, N.N.; Silva, R.V.; Guimarães, L.N.; Santos, A.S.; Campos, I.C.A.; Inoue, T.Y. Potencial de extratos de plantas e manipueira no controle de Meloidogyne javanica em jiloeiro. Holos 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Monzote, L.; Geroldinger, G.; Tonner, M.; Scull, R.; De Sarkar, S.; Bergmann, S.; Bacher, M.; Staniek, K.; Chatterjee, M.; Rosenau, T.; et al. Interaction of ascaridole, carvacrol, and caryophyllene oxide from essential oil of Chenopodium ambrosioides L. with mitochondria in Leishmania and other eukaryotes. Phytoth. Res. 2018, 32, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Illescas, C.; Quijada, M.; Martínez-Díaz, R.A.; Ochoa, E.; Gómez-Muñoz, M.T.; Navarro-Rocha, J.; González-Coloma, A. Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants. Molecules 2023, 28, 1467. [Google Scholar] [CrossRef]
- Kouam, M.K.; Payne, V.K.; Miégoué, E.; Tendonkeng, F.; Lemoufouet, J.; Kana, J.R.; Boukila, B.; Pamo, E.T.; Mnm, B. Evaluation of In Vivo Acaricidal Effect of Soap Containing Essential Oil of Chenopodium ambrosioides Leaves on Rhipicephalus lunulatus in the Western Highland of Cameroon. J. Pathog. 2015, 2015, 516869. [Google Scholar] [CrossRef] [PubMed]
- Ajaib, M.; Farooq, S.; Abid, S.; Ajaib, M.; Qamar, M.; Zahid, M.T. Assessment of in vitro anthelmintic activity of crude extracts of Dysphania ambrosioides against Haemonchus contortus a sheep pathogenic parasite. Biosci. Res. 2020, 17, 2760–2769. [Google Scholar]
- Zamilpa, A.; García-Alanís, C.; López-Arellano, M.E.; Hernández-Velázquez, V.M.; Valladares-Cisneros, M.G.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. In vitro nematicidal effect of Chenopodium ambrosioides and Castela tortuosa n-hexane extracts against Haemonchus contortus (Nematoda) and their anthelmintic effect in gerbils. J. Helminthol. 2019, 93, 434–439. [Google Scholar] [CrossRef]
- Oliveira, E.; Silva, M.D.; Sprenger, L.; Pedrassani, D. In vitro activity of the hydroalcoholic extract of Chenopodium ambrosioides against engorged females of Rhipicephalus (Boophilus) microplus. Arq. Inst. Biológico 2018, 84, e0222016. [Google Scholar] [CrossRef][Green Version]
- Freire, M.D.S.; Santos, C.D.G. Reação de espécies vegetais a Meloidogyne enterolobii e eficiência de seus extratos aquosos no controle do patógeno. Semin. Ciências Agrárias 2018, 39, 2385. [Google Scholar] [CrossRef]
- Bernardes, W.A.; Silva, E.O.; Crotti, A.E.M.; Baldin, L.L. Bioactivity of selected plant-derived essential oils against Zabrotes subfasciatus (Coleoptera: Bruchidae). J. Stored Prod. Res. 2018, 77, 16–19. [Google Scholar] [CrossRef]
- Soares, M.H.; Dias, H.J.; Vieira, T.M.; de Souza, M.G.M.; Cruz, A.F.F.; Badoco, F.R.; Nicolella, H.D.; Cunha, W.R.; Groppo, M.; Martins, C.H.G.; et al. Chemical Composition, Antibacterial, Schistosomicidal, and Cytotoxic Activities of the Essential Oil of Dysphania ambrosioides (L.) Mosyakin & Clemants (Chenopodiaceae). Chem. Biodiv. 2017, 14, e1700149. [Google Scholar] [CrossRef]
- Pandiangan, D.; Lamlean, P.Y.; Maningkas, P.F.; Nainggolan, N.; Unitly, A.J.A. Antioxidant and anticancer activity tests of “pasote” leaf water extracts (Dysphania ambrosioides L.) by in vitro method in leukemia cancer cells. J. Phys. Conf. Ser. IOP Publ. 2020, 1463, 012020. [Google Scholar] [CrossRef]
- Huang, J.; Hao, J.; Nie, J.; Qian, R.; Li, H.; Zhao, J.; Wang, Y. Possible Mechanism of Dysphania ambrosioides (L.) Mosyakin & Clemants Seed Extract Suppresses the Migration and Invasion of Human Hepatocellular Carcinoma Cells SMMC-7721. Chem. Biodiver. 2023, 20, e202200768. [Google Scholar] [CrossRef]
- Rios, C.E.P.; Abreu, A.G.; Braga Filho, J.A.; Nascimento, J.R.; Guerra, R.N.; Amaral, F.M.; Maciel, M.C.; Nascimento, F.R. Chenopodium ambrosioides L. improves phagocytic activity and decreases bacterial growth and the systemic inflammatory response in sepsis induced by cecal ligation and puncture. Front. Microbiol. 2017, 8, 148. [Google Scholar] [CrossRef]
- Drioua, S.; Ameggouz, M.; Assouguem, A.; Kara, M.; Ullah, R.; Bari, A.; Lahlali, R.; Fidan, H.; El-Guourrami, O.; Benkhouili, F.Z.; et al. Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions. Open Life Sci. 2024, 19, 20220895. [Google Scholar] [CrossRef]
- Adinci Kossi, J.; Sessou, P.; Dougnon Tamegnon, V.; Assogba Mahoudo, F.; Towanou, R.; Komagbe, G.; Dougnon Tossou, J.; Laleye, A.; Souaibou, F. Acute Toxicity of Chenopodium ambrosioides and Annona muricata oils with Acaricidal Potentials. Asian J. Biolog. Sci. 2020, 13, 1–8. [Google Scholar]

| Extracts | Part of the Plant | Compound | Concentration (%) | References |
|---|---|---|---|---|
| Essential oil | Leaves | Ascaridole-glycol Linalool acetate Dihydrocitronellol acetate | 10.58 11.26 19.53 | [22] |
| Essential oil | Leaves | α-Terpinene p-Cymene Carvacrol Ascaridole | 30.50 17.30 16.20 15.10 | [23] |
| Essential oil | Leaves | Cis-Piperitone oxide Trans-Isoascaridole p-Cymene | 30.30 18.20 13.20 | [24] |
| Essential oil | Leaves | β-Cymene 5-Isopropyl-6-methyl-hepta-3,5-dien-2-ol | 47.10 19.20 | [25] |
| Essential oil | Leaves and Stems | α-Terpinene p-Cymene Trans-Chrysanthenyl acetate | 59.7 22.8 13.8 | [26] |
| Essential oil | Leaves | Ascaridole p-Cymene | 49.77 42.32 | [27] |
| Essential oil | Leaves | Ascaridole α-Terpinene Ascaridole epoxide | 15.13 54.09 9.77 | [28] |
| Essential oil | Leaves | Ascaridole p-Cymene | 35.50 47.20 | [7] |
| Essential oil | Aerial Parts | α-Terpinene Ascaridole p-Cymene | 53.4 17.7 12.1 | [46] |
| Essential oil | Aerial Parts | p-Cymene 4-Carene α-Cyclogeraniol acetate | 31.72 27.34 16.90 | [30] |
| Essential oil | Aerial Parts | α-Terpinene p-Cymene | 72.5 20.6 | [31] |
| Essential oil | Aerial Parts | 4-Carene α-Cyclogeraniol acetate Trans-β-Terpinyl butanoate | 50.50 22.64 31.13 | [32] |
| Essential oil | Leaves | δ-3-Carene p-Cymene | 61.51 14.67 | [33] |
| Essential oil | Aerial Parts | Ascaridole Trans-Ascaridole glycol p-Cymene | 31.20 5.60 36.30 | [34] |
| Essential oil | Aerial Parts | Isoascaridole α-Terpinene 2,3-Dehydro-1,4-cineole | 15.30 15.20 55.00 | [35] |
| Essential oil | Aerial Parts | Isoascaridole Trans-Ascaridole glycol p-Cymene | 6.33 10.07 22.40 | [36] |
| Essential oil | Aerial Parts | o-Cymene α-Terpinene Nona-3,5-dien-2-ol | 39.20 36.80 10.00 | [19] |
| Essential oil | Aerial Parts | p-Cymene α-Terpinene cis-Ascaridole | 19.30 13.20 38.10 | [37] |
| Essential oil | Aerial Parts | (Z)-Ascaridole (E)-Ascaridole p-Cymene | 87 5.04 4.83 | [38] |
| Essential oil | Aerial Parts | α-Terpinene Ascaridole p-Cymene | 23.77 14.48 12.22 | [39] |
| Essential oil | Aerial Parts | p-Cymene δ-3-Carene | 14.70 61.50 | [40] |
| Essential oil | Aerial Parts | Ascaridole Isoascaridole p-Cymene | 16.30 51.00 6.70 | [41] |
| Essential oil | Aerial Parts | o-Cymene (+)-4-Carene | 41.46 56.59 | [14] |
| Essential oil | Aerial Parts | Ascaridole m-Cymene | 60.33 22.17 | [42] |
| Essential oil | Whole Plant | p-Cymene α-Terpinene | 49.60 26.81 | [43] |
| Essential oil | Whole Plant | Ascaridole Isoascaridole p-Cymene | 87.30 8.40 3.30 | [44] |
| Essential oil | Aerial Parts | α-Terpinene Isoascaridole Ascaridole | 37.17 20.48 14.83 | [45] |
| Extracts | Part of the Plant | Main Compound | Microorganism | MIC (µg/mL) | Ref. |
|---|---|---|---|---|---|
| Essential oil | Leaves | o-Cymene α-Terpinene | Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa Bacillus subtilis | 10 10 20 20 | [5] |
| Essential oil | Aerial Parts | 4-Carene Trans-β-Terpinyl butanoate | E. coli S. aureus Enterococcus faecalis | 6 12 18 | [32] |
| Essential oil | Leaves | β-Cymene | S. aureus P. aeruginosa | 10 10 | [25] |
| Essential oil | Aerial Parts | o-Cymene α-Terpinene | E. coli B. subtilis | 7.8 3.9 | [37] |
| Essential oil | Leaves | α-Terpinene Ascaridole | E. coli S. aureus P. aeruginosa | 1024 256 512 | [28] |
| Essential oil | Aerial Parts | α-Terpinene Ascaridole | E. coli S. aureus P. aeruginosa Klebsiella pneumoniae | 310 1250 10,000 20,000 | [40] |
| Essential oil | Aerial Parts | δ-3-Carene p-Cymene | Microcystis aeruginosa | 3120 | [41] |
| Essential oil | Aerial Parts | p-Cymene 4-Carene | E. coli S. aureus P. aeruginosa | 90 120 120 | [30] |
| Essential oil | Leaves | No Determined | S. aureus | 1024 | [55] |
| Essential oil | Leaves | α-Terpinene | S. aureus | 1024 | [56] |
| Essential oil | Aerial Parts | cis-Ascaridole m-Cymene | P. aeruginosa Bacillus subtilis | 19 19 | [43] |
| Ethanolic extract | Stem | Rutin Quercetin | B. subtilis | 11.1 | [12] |
| Ethanolic extract | Leaves | No determined | E. coli S. aureus | 25,000 25,000 | [57] |
| Ethanolic extract | Leaves | No determined | Clostridioides difficile | 3900 | [58] |
| Chloroform extract | Leaves | Quercetin Chrysin | S. aureus Enterococcus faecalis | 4290 4290 | [49] |
| Ethanolic extract | Aeria Parts | No determined | E. coli E. faecalis | 1094 4375 | [59] |
| Ethanolic and methanolic extracts | Aeria Parts | No determined | E. coli S. aureus P. aeruginosa B. subtilis | 9 9 43 9 | [60] |
| Extracts | Part of the Plant Used | Main Component | Methodology | Quantity | Reference |
|---|---|---|---|---|---|
| Ethyl Acetate extract | Aerial Part | No determined | FRAP ABTS | 12.90 mg/mL 4.56 mg/mL | [66] |
| Aqueous extract | Leaves | 16-methyl-heptadecane-1,2-diol Phytol | FIC FRAP DPPH | IC50 20.98 mg/mL 64.19 mg/AAE g IC50 1.39 mg/mL | [11] |
| Methanolic extract | Seeds | Rutin | DPPH ABTS FRAP | IC50 110.7 µg/mL 110.6 µg AAE/mg 94.30 µg AAE/mg | [63] |
| Aqueous extract | Fruits | No determined | ABTS FIC | 8.25 mM TE/g 78% quelation | [60] |
| n-Butanol ethyl acetate extracts | Leaves | Caffeic acid Coumarin Kaempferol | DPPH | IC50 2.98 mg/mL IC50 16.48 mg/mL | [54] |
| Methanolic extract | Leaves | Rutin Quercetin | DPPH | IC50 130.7 µg/mL | [12] |
| Methanolic extract | Leaves | No determined | FRAP ABTS | 0.141 µM TE/g 0.224 mg AAE/g | [67] |
| Hydroethanolic extract | Flowers | Syringic acid Quercetin Kaempferol | DPPH β-Carotene/linoleic acid FRAP | IC50 166.47 µg/mL IC50 57.04 µg/mL IC50 231.05 µg/mL | [1] |
| Aqueous extract | Aerial Part | No determined | DPPH ORAC | IC50 80.6 µg TE /mL IC50 687.3 µg TE/mL | [68] |
| Essential oil | Aerial Part | α-Terpinene Ascaridole | DPPH | 30.182 mg TE/g oil | [29] |
| Essential oil | Leaves | No determined | DPPH FRAP ABTS | 1.59 mg AAE/g 8.36 mg AAE/g 2.11 mg AAE/g | [59] |
| Essential oil | Stems | 4-Carene α-Cyclogeraniol Acetate | FRAP ABTS | IC50 309.45 µg/mL IC50 147.99 µg/mL | [32] |
| Flower | trans-β-TerpinylButanoate 4-Carene | DPPH β-Carotene/linoleic acid | IC50 158.15 µg/mL IC50 266.25 µg/mL | ||
| Essential oil | Leaves | α-Terpinene Ascaridole | DPPH | IC50 1024 µg/mL | [28] |
| Essential oil | Aerial Part | α-Terpinene Ascaridole | DPPH β-Carotene/linoleic acid FRAP | IC50 4.00 mg/mL IC50 3.03 µg/mL IC50 6.02 µg/mL | [40] |
| Essential oil | Leaves | α-Terpinene α-Terpinenyl Acetate | DPPH | IC50 1.74 mg/mL | [69] |
| Extracts | Part of the Plant Used | Main Compounds | Quantity | Insects | Reference |
|---|---|---|---|---|---|
| Essential oil | Leaves | No determined | 1 µL/L air | Periplaneta americana | [73] |
| Essential oil | Aerial Parts | Terpinolene p-Cymene | 1.02 µL/L air | Dactylopius opuntiae | [74] |
| Essential oil | Aerial Parts | cis-Ascaridole p-Cymene | 3.125 µL/L | Culex quinquefasciatus | [38] |
| Essential oil | Aerial Parts | p-Cymene Ascaridole | 62.5 mg/L 10 mg/L | Aedes aegypti Anopheles gambiae | [34] |
| Essential oil | Aerial Parts | o-Cymene α-Terpinene | 0.75 mg/mL | Culex pipiens | [37] |
| Essential oil | Whole Plant | No determined | 66.81 mg/L | Plutella xylostella | [44] |
| Essential oil | Leaves | δ-3-Carene p-Cymene | 0.04 µL/cm2 | Tribolium confusum | [33] |
| Inflorescences | δ-3-Carene p-Cymene | 0.05 µL/cm2 | |||
| Essential oil | Leaves | No determined | 0.50 mg/m2 | Callosobruchus maculatus | [75] |
| Essential oil | Fresh Leaves | Ascaridole p-Cymene | LC50 17.74 µg/cm2 | Alphitobius diaperinus | [76] |
| Extracts | Aerial Parts | No determined | 50 g/L | Dactylopius opuntiae | [77] |
| Extracts | Aerial Parts | No determined | 10 g/L | Bemisia tabaci | [78] |
| Extracts | Aerial Parts | No determined | 200 g/L | Spodoptera frugiperda | [79] |
| Extract | Stem and Leaves | No determined | 500 mg/mL | Scyphophorus scupunctatus | [80] |
| Extracts | Part of the Plant | Main Compound | Concentration | Parasites | Reference |
|---|---|---|---|---|---|
| Essential oil | Fruit and Seeds | (Z)-Ascaridole E-Ascaridole | 307 µg/mL | Meloidogyne incognita | [45] |
| Essential oil | Aerial Part | 4-Carene o-Cymene | 4.74 mg/mL | Leishmania tropica | [15] |
| Essential oil | Aerial Part | cis-Piperitone oxide trans-Isoascaridole | 8.7 µg/mL | Trypanosoma cruzi | [24] |
| Essential oil | Aerial Part | No determined | 50 µL/mL | Ancylostoma spp. | [39] |
| Essential oil | Aerial Part | p-Cymene α-terpinene | 0.037 µL/g | Rhipicephalus lunulatus | [85] |
| Essential oil | Flower | Isoascaridole Ascaridole | 0.041 µL/mL | Meloidogyne chitwoodi | [42] |
| Extracts | Aerial Part | No determined | 20 mg/mL | Haemonchus contortus | [86] |
| Extracts | Aerial Part | No determined | 0.6 mg/mL | Haemonchus contortus | [87] |
| Extracts | Aerial Part | No determined | 400 mg/mL | Rhipicephalus microplus | [88] |
| Extracts | Leaves | No determined | 300 mg/mL | Meloidogyne javanica | [82] |
| Extracts | Leaves | No determined | 50 mg/mL | Meloidogyne enterolobi | [89] |
| Extracts | Whole Plant | Rutin Quercetin | 1 mg/mL | Leishmania tropica | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia Severino, A.; Fernández-López, J.; Borrás-Rocher, F.; Viuda-Martos, M. Essential Oils and Extracts from Epazote (Dysphania ambrosioides): A Phytochemical Treasure with Multiple Applications. Plants 2025, 14, 1903. https://doi.org/10.3390/plants14131903
Heredia Severino A, Fernández-López J, Borrás-Rocher F, Viuda-Martos M. Essential Oils and Extracts from Epazote (Dysphania ambrosioides): A Phytochemical Treasure with Multiple Applications. Plants. 2025; 14(13):1903. https://doi.org/10.3390/plants14131903
Chicago/Turabian StyleHeredia Severino, Arsenio, Juana Fernández-López, Fernando Borrás-Rocher, and Manuel Viuda-Martos. 2025. "Essential Oils and Extracts from Epazote (Dysphania ambrosioides): A Phytochemical Treasure with Multiple Applications" Plants 14, no. 13: 1903. https://doi.org/10.3390/plants14131903
APA StyleHeredia Severino, A., Fernández-López, J., Borrás-Rocher, F., & Viuda-Martos, M. (2025). Essential Oils and Extracts from Epazote (Dysphania ambrosioides): A Phytochemical Treasure with Multiple Applications. Plants, 14(13), 1903. https://doi.org/10.3390/plants14131903









