1. Introduction
Eucalypts are a diverse group of large trees and shrubs classified within the tribe
Eucalypteae of the family
Myrtaceae. This group includes the genera
Eucalyptus,
Corymbia, and
Angophora, which are collectively referred to as gum trees, a name derived from their characteristic sap exudation. Eucalypts are native to Australia and the surrounding regions, and are highly adaptable, thriving in a wide range of climates, which contributes to their cultivation around the globe [
1]. They are extensively grown to produce pulp, plywood, and solid wood, with
E. globulus being the most cultivated species due to its rapid growth and desirable wood properties [
2]. In addition, eucalypts are recognized for their essential oils (EOs), which have gained global prominence in the pharmaceutical, cosmetic, and wellness industries. Applications include use in respiratory therapies, topical antiseptics, massage oils, and aromatherapy. Eucalypt EOs are mainly extracted from the leaves and comprise complex mixtures of bioactive compounds, including terpenes and phenylpropanoids, in variable amounts [
3]. The highly distinctive and easily recognizable fragrance of eucalypts is largely conveyed by their high content in the oxygen-containing monoterpene 1,8-cineole, commonly known as eucalyptol. However, eucalypts can exhibit a remarkable chemical diversity, allowing for the extraction of a wide range of EO compounds beyond eucalyptol [
4,
5]. Among the eucalypts,
Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Johnson, commonly known as lemon-scented gum, holds a prominent place due to its unique aromatic properties. Formerly classified within the genus
Eucalyptus as
Eucalyptus citriodora Hook., this species was later reclassified into the genus
Corymbia based on phylogenetic studies [
6]. The EO of
C. citriodora is characterized by a distinctive lemony aroma, attributed to high levels of the oxygenated monoterpene aldehyde citronellal and the monoterpene alcohols citronellol and isopulegol. The refined EO is widely used in the perfumery industry, where the refreshing lemon fragrance is a much sought-after ingredient in a variety of products, including perfumes, candles, and cosmetics [
7]. Additionally,
C. citriodora EO has demonstrated antimicrobial properties, showing activity against various bacteria and fungi, which supports its use in natural antiseptics and cleaning products [
3]. Furthermore, its anti-inflammatory and antioxidant effects have spurred interest in the therapeutic applications of
C. citriodora EO, particularly in skincare products aimed at reducing oxidative damage [
8]. Beyond these applications, it is extensively used as a natural insect repellent, with citronellal acting as the primary bioactive component in pest management [
9].
The development of novel active compounds for environmentally safer plant protection products has become an increasingly urgent priority in response to growing concerns about the adverse effects of conventional pesticides. As a result, new-generation pesticides have been introduced, demonstrating promising features such as reduced impacts on non-target organisms and diminished risks to human health. However, despite their improved safety profiles compared to traditional synthetic pesticides, many can still exhibit undesirable toxicological and ecotoxicological properties, highlighting the need for continued refinement and innovation [
10]. Even though in the EU, pesticide sales have dropped ca. 10%, the adoption of pest management strategies based on biopesticides is still not very extensive. Against plant parasitic nematodes, phytochemical volatiles, particularly those that compose EOs, have emerged as a compelling alternative, given that they are biodegradable, renewable, and often exhibit potent nematicidal activities while posing reduced risks to human health and the environment [
10]. The EOs of a large number of plant species have shown strong nematicidal activities against plant parasitic nematodes, inducing complete mortality at concentrations as low as 2 µg/mL, which underlines their vast potential for the formulation of biopesticides [
11]. Beyond the direct nematicidal action, volatile phytochemicals are also valued for their lower persistence in the soil, reducing risks of bioaccumulation and secondary environmental contamination. Regardless of their potential, there are challenges to the widespread adoption of EOs as nematicides, which include variability in chemical composition based on plant origin, seasonal factors, and the scalability and cost of production.
The in vitro production of plant material offers many advantages for obtaining nematicidal metabolites. Compared to in vivo cultivation, in vitro methods significantly reduce the time, space, and resources required for plant propagation [
12]. Additionally, in vitro tissue culture allows obtaining plant genetic clones under controlled laboratory conditions, indefinitely, enabling the rapid and large-scale production of plant material as well as a stable metabolite production [
12]. In vitro systems enable optimizing the production of volatile phytochemicals in a simplified and reproducible setting, free from microbial contamination. The production of bioactive compounds, including volatiles, can be scaled up using bioreactor systems, providing a sustainable alternative to harvesting plants from natural ecosystems [
13,
14].
The present work explores the nematicidal activity of C. citriodora EO against Bursaphelenchus xylophilus, the pinewood nematode (PWN), a destructive phytoparasite affecting Asian and European pine forests. Previous studies have analyzed the nematicidal activity of C. citriodora EOs; however, the contribution to the overall activity of their main compounds remains fairly unexplored. In the present work, the main compounds were assayed and compared to the conventional nematicide emamectin benzoate. Their toxicological and ecotoxicological safety was evaluated by comparing experimental data, freely available on online databases, and data estimated by predictive software. Finally, C. citriodora in vitro shoot cultures were established, and the volatile profile of their EO was determined and compared to that obtained from its in vivo mature mother plant, and tested against the PWN. The integration of EOs and other phytochemicals into nematode management strategies aligns with the broader goal of developing eco-friendly and sustainable approaches to crop protection.
3. Discussion
The development of novel biopesticides has become critical as the detrimental impacts of conventional pesticide overuse are being progressively revealed. Extensive and indiscriminate application of synthetic pesticides has led to persistent residues in ecosystems, resulting in significant damage to biodiversity, including the disruption of non-target organism populations and their essential ecological functions [
25]. Furthermore, these practices have accelerated the evolution of pesticide resistance among pest populations, undermining long-term pest management strategies and posing substantial challenges to agricultural sustainability [
25]. In this context, biopesticides, derived from natural biological sources, can offer a promising alternative.
Corymbia citriodora is a fast-growing tree species extensively cultivated for its high-quality timber. Its wood, characterized by its hardness and durability, is widely employed in heavy-duty construction projects and as a sustainable source of fuel [
26]. In addition to its timber value,
C. citriodora exhibits a substantial EO production, as the smaller shoots and leaves—often considered by-products—can yield a high content of EO, reaching up to 2% by weight [
27]. This high yield, coupled with its chemical composition, puts
C. citriodora as a promising candidate for the development of bio-based nematicides. The EO of
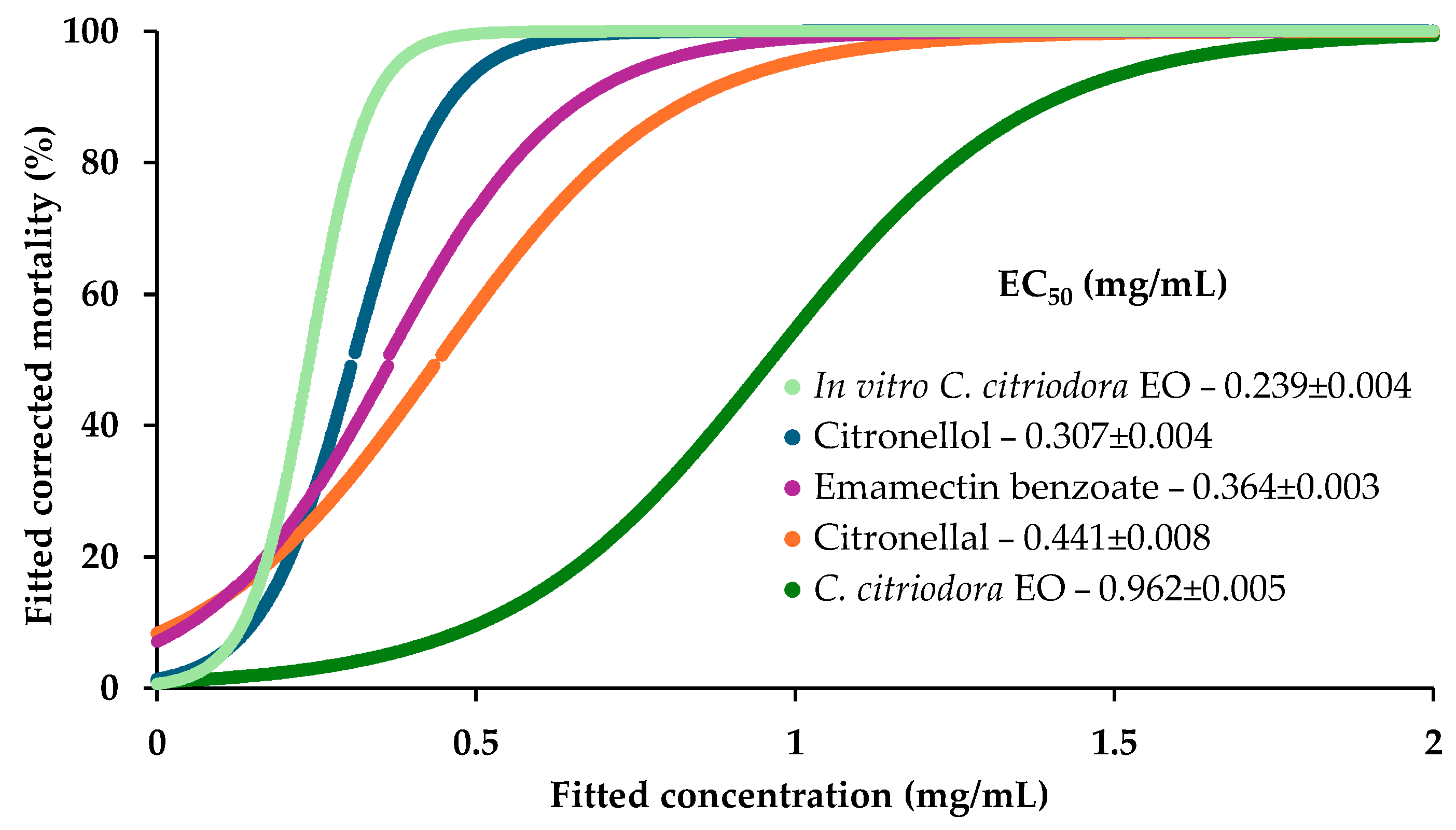
C. citriodora analyzed in this study exhibited a chemical profile dominated by the oxygenated monoterpenes citronellal, citronellol, 1,8-cineole, and isopulegol, which collectively accounted for approximately 80% of the total EO composition. This chemical profile is typical for
C. citriodora EOs obtained in several parts of the world, albeit with variations in compound proportions [
7]. At the highest concentration tested (2 mg/mL),
C. citriodora EO showed a strong nematicidal activity against the PWN. Moreover, when tested solely, its main components citronellal or citronellol induced complete mortality (100%), while 1,8-cineole or isopulegol displayed only marginal nematicidal activity under the same experimental conditions, highlighting the dominant role of citronellal and citronellol in the EO’s overall nematicidal strength. Although these compounds exhibited notable nematicidal activities individually, the observed overall bioactivity of the complete EO could not be entirely attributed to their simple additive effect, in their respective relative proportions. This suggests that synergistic interactions between EO components may play a critical role in enhancing their efficacy. This activity may arise from complex biochemical and biophysical interactions among the compounds, which can influence penetration into nematode cuticles, target-site binding, or interference with nematode physiological pathways [
28,
29,
30].
In other studies,
C. citriodora EOs were reported to demonstrate varying levels of nematicidal activity against the PWN. Reported activity ranges from less than 40% mortality, at a concentration of 2 mg/mL, to as high as 73% mortality at 10 mg/mL [
29,
31,
32]. These differences in activity can be attributed not only to the concentration of the EOs applied but also to the choice of solubilizing agent, which influences their dispersion and bioavailability [
33]. Most critically, the nematicidal potential can be strongly linked to the specific chemical composition of the EOs, which was not detailed in some works, but is known to vary depending on factors such as geographic origin, plant growth conditions, and extraction methods [
31,
32,
34].
C. citriodora EOs have demonstrated significant efficacy against other plant-parasitic nematodes, namely the root-knot nematodes, which are among the most destructive agricultural pests globally. For instance, complete mortality of second-stage juveniles (J2) of
Meloidogyne incognita was observed at a concentration of 0.25 mg/mL [
35]. In other studies, remarkably low lethal concentration values (LC
50) have been reported over a 24 h exposure period, such as 2.4 µg/mL [
36] or 746 µg/mL [
37], for EOs characterized by an exceptionally high citronellal content, ranging from 82% to 84%, which likely contributes to their enhanced nematicidal properties. In fact, in the present study, the nematicidal strength of citronellal (0.441 ± 0.008 mg/mL) seems to be only slightly lower than emamectin benzoate (0.364 ± 0.009 mg/mL), a conventional pesticide. Citronellol stood out for the low EC
50 value, 0.307 ± 0.004 mg/mL, showing very good properties against the PWN. In a previous study, the different nematicidal potencies of citronellal and citronellol were also reported. Citronellal was reported to exhibit an EC
50 of 0.245 mg/mL, 0.235 mg/mL, and 0.169 mg/mL against the male, female, and juvenile stages of the PWN, respectively. In contrast, citronellol demonstrated a lower nematicidal potency, with EC
50 values of 0.187 mg/mL, 0.139 mg/mL, and 0.253 mg/mL against the same PWN life stages [
38]. These findings highlight significant variations in the nematicidal activities of these two compounds, with citronellol showing superior efficacy against adults rather than juveniles, but citronellal displaying stronger effects against juveniles. This disparity in activity suggests that the developmental stage of the nematode plays a critical role in modulating the response to these compounds, a factor that may have influenced the outcomes observed in the present study, since the suspensions used were composed of mixed life stages of the PWN.
In terms of their environmental impacts, citronellal and citronellol are predicted to have more favorable environmental profiles compared to emamectin benzoate. Specifically, these compounds exhibited a higher predicted affinity for the air and water environmental compartments, in contrast to the strong affinity of emamectin benzoate for the soil compartment. Additionally, the predicted environmental persistence for emamectin benzoate is up to 40-fold higher than that of citronellal and citronellol. The environmental implications of this difference can be significant. While a high affinity for air is generally less hazardous, the increased affinity of citronellal and citronellol for the water compartment can be of greater concern. This suggests that these compounds may be more readily available for aquatic organisms, thereby posing a potential risk to aquatic ecosystems. However, it is important to note that citronellal and citronellol are predicted to volatilize from water bodies much more rapidly than emamectin benzoate. This could mitigate their environmental impact in aquatic environments to some extent, as their persistence in water may be significantly shorter than that of emamectin benzoate. This tendency is further supported when examining the respective bioaccumulation and bioconcentration factors for each compound. Citronellal and citronellol exhibited lower values than emamectin benzoate, suggesting a reduced potential for bioaccumulation in aquatic organisms. Additionally, the likelihood of biotransformation in an aquatic context appears to be higher for citronellal and citronellol, further diminishing their persistence and toxicity in aquatic environments. This comparison is particularly significant when evaluating the acute toxicity thresholds reported for citronellal or citronellol relative to those for emamectin benzoate. For model organisms of fish, algae, and invertebrates, the LC
50/EC
50 values for emamectin benzoate are markedly lower than those of citronellal and citronellol. This indicates that emamectin benzoate can exhibit substantially higher acute toxicity across these groups. Such findings emphasize the potential ecological risks associated with the use of emamectin benzoate, particularly when it is released into aquatic environments. Conversely, the predicted removal of emamectin benzoate through wastewater treatment processes appears to be significantly more effective compared to that of citronellal or citronellol. This discrepancy is likely attributable to the distinct physicochemical properties of emamectin benzoate, particularly its higher molecular weight and probable greater affinity or favorable adsorption onto soil or sludge matrices. In contrast, the lower molecular weight and reduced adsorption affinity of citronellal and citronellol may limit their retention in solid fractions, thereby reducing the overall efficiency of their removal. In fact, citronellal is approved for use in a wide range of products, including air fresheners, cleaning agents, and floor polishes [
39]. The International Fragrance Association (IFRA) classifies citronellal as non-persistent, non-bioaccumulative, and non-toxic, signifying that it degrades readily in the environment, does not accumulate in living organisms, and poses minimal ecological toxicity [
40]. Its compliance with IFRA Environmental Standards reflects comprehensive testing, confirming its safety for inclusion in fragrance formulations.
From a human health safety perspective, citronellal and citronellol demonstrate significantly higher acute toxicity thresholds compared to emamectin benzoate based on data from both oral and dermal exposure routes. According to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), emamectin benzoate is categorized as a Class 3 toxicant. This designation applies to substances with oral LD
50 values ranging from 50 to 300 mg/kg or dermal LD
50 values between 200 and 1000 mg/kg, reflecting a moderate level of acute toxicity. In contrast, citronellal and citronellol are classified as Class 5 molecules, which include compounds with oral or dermal LD
50 values exceeding 2000 mg/kg [
41]. This classification represents the lowest acute toxicity category under the GHS framework, indicating a significantly reduced risk of adverse effects upon single-dose exposure. The substantial difference in toxicity levels highlights the comparatively safer toxicological profile of citronellal and citronellol. This distinction in toxicity also extends to the predicted toxicity endpoints for these compounds. Citronellal and citronellol, despite their lower acute toxicity (Class 5 toxicants under the GHS), may still exert mild adverse effects on external parameters, such as skin sensitization or eye irritation. These effects, while noteworthy, are generally localized and reversible, and are consistent with their widespread use in cosmetics, fragrances, and other consumer products where such risks are considered manageable through proper formulation and labeling [
42]. In contrast, emamectin benzoate, classified as a Class 3 toxicant, is associated with a broader spectrum of potential systemic toxicity endpoints. These include genotoxicity, drug-induced liver injury, drug-induced neurotoxicity, and ototoxicity. Genotoxicity refers to the compound’s potential to damage genetic material, raising concerns about long-term carcinogenic risks [
43]. Drug-induced liver injury suggests hepatotoxic effects, which could result in metabolic disturbances or hepatic dysfunction with prolonged or high-dose exposure. Neurotoxicity and ototoxicity further highlight emamectin benzoate’s potential to disrupt the central nervous system or auditory function, respectively, underscoring its comparatively higher risk profile [
44]. The divergence in predicted toxicity endpoints reflects fundamental differences in the chemical properties, mechanisms of action, and biological interactions of these compounds. While citronellal and citronellol may require attention to mitigate minor irritative effects in specific applications, their lack of systemic toxicities such as genotoxicity or organ-specific damage makes them safer candidates for human exposure. Conversely, emamectin benzoate’s more severe toxicity endpoints necessitate stricter regulatory controls, risk assessments, and limitations on its usage to minimize human health risks. These findings reinforce the potential of citronellal and citronellol as safer alternatives in contexts requiring low-toxicity agents, particularly when compared to substances like emamectin benzoate.
Overall, the ecotoxicological characteristics of the main compounds of C. citriodora EO emphasize its relevance as a valid candidate for safer pest management strategies. Compared to emamectin benzoate, it can exhibit lower environmental persistence, a reduced bioaccumulation potential, and a greater tendency to volatilize, collectively supporting a diminished risk to non-target ecosystems. The toxicological data further indicate substantially higher acute exposure thresholds and a potential absence of systemic toxic effects, such as genotoxicity or neurotoxicity, thereby supporting a more favorable safety profile for human exposure. Notably, certain constituents have exhibited very strong nematicidal activity, underscoring the potential of C. citriodora EO and its main components as effective, environmentally benign alternatives to conventional synthetic pesticides.
Interestingly, the in vitro culture of
C. citriodora shoots yielded volatile profiles dominated by citronellal (64%), with a lower chemical complexity compared to the EO extracted from the mature plant. The distinctive lemon-like scent of
C. citriodora was immediately notable in the cultured explants; this is likely attributable to the high density of trichomes, suggesting a resemblance to the juvenile leaf stage. In eucalypt species, pronounced leaf dimorphism is evident between juvenile and mature leaves. Juvenile leaves, observed in seedlings, are oval to round, occasionally sessile, glaucous, and densely covered with soft trichomes. In contrast, the leaves of mature trees are alternate, entire, petiolate, and lanceolate, with a thick, stiff, highly cutinized, and coriaceous structure, reflecting adaptations to reduce water loss and withstand environmental stressors [
45]. Remarkably, the EO obtained from
C. citriodora in vitro shoots showed stronger activity against the PWN than the in vivo
C. citriodora EO or the main pure compounds tested. This suggests that at their respective proportions in the EO, its main compounds may interact synergistically against the PWN. These types of interaction have previously been seen for monoterpenes, such as geraniol, against plant parasitic nematodes and must definitely exist between other monoterpenes and sesquiterpenes [
28,
46]. The in vitro system demonstrated the potential for rapid and scalable production of nematicidal volatiles, with an 8-fold increase in biomass after 6 weeks in culture. In other studies involving
C. citriodora, in vitro shoot culture has been identified as an effective method for the rapid propagation of this economically significant species [
47]. While in vitro shoot culture remains a relatively costly technique and may not be suitable to produce low-cost compounds, it offers notable advantages. These include consistent, year-round production—an essential feature for industrial applications—as well as the potential for optimization to meet specific objectives and enhance targeted traits. Nevertheless, the use of EOs as biopesticides in agricultural applications still presents several limitations. Mainly, they exhibit rapid degradation when exposed to environmental factors such as sunlight, air, and moisture, resulting in short residual activity and the need for frequent reapplication. Additionally, some EOs can be phytotoxic at higher concentrations, potentially harming the crops they are intended to protect. Furthermore, the lack of standardized regulatory frameworks can hinder their integration into pest management programs.
Even though our study showcases the promising nematicidal potential and favorable environmental and toxicological profile of C. citriodora EO and its main components, key gaps can be highlighted. For instance, the molecular mechanisms responsible for nematicidal activity or the interactions among EO constituents are not fully understood and warrant further investigation using, for example, transcriptomic and/or metabolomic approaches. Future studies must aim for field-level assessments, necessary to validate laboratory efficacy and assess real-world environmental fate, phytotoxicity, and non-target effects. Also, establishing standardized formulations and exploring encapsulation or slow-release technologies may help overcome the current limitations in EO stability. These approaches could greatly facilitate the practical integration of C. citriodora EO into sustainable pest management frameworks.