The Antibacterial and Anti-Inflammatory Potential of Cinnamomum camphora chvar. Borneol Essential Oil In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of BEO
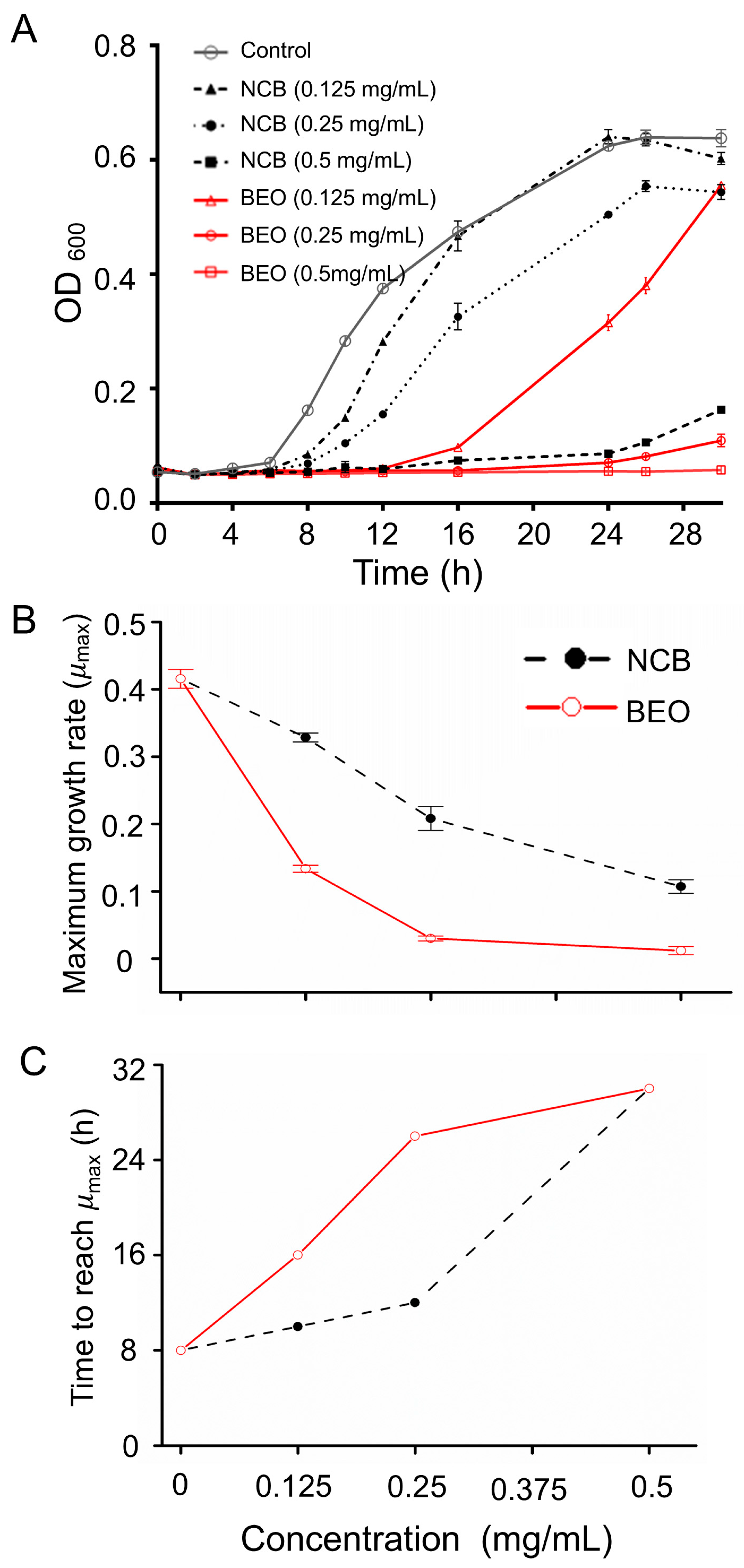
2.2. Inhibitory Effect of BEO and NCB on S. epidermidis
2.2.1. Minimum Inhibitory Concentration
2.2.2. Inhibition of Microbial Growth
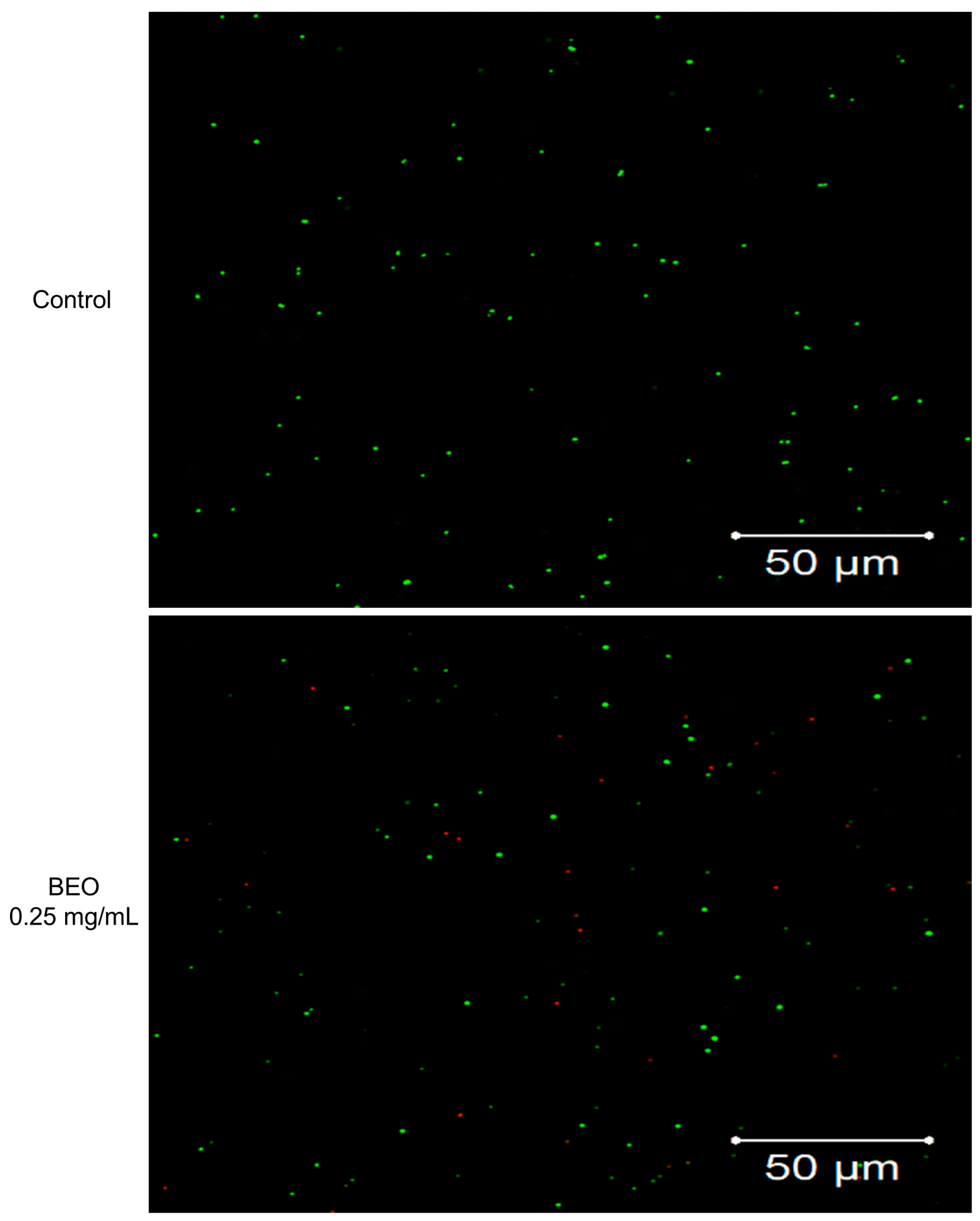
2.2.3. Cell Membrane Integrity Determination by SYTO9/PI Staining/Laser Confocal Microscopy
2.3. Effect of BEO on Cell Membrane Integrity
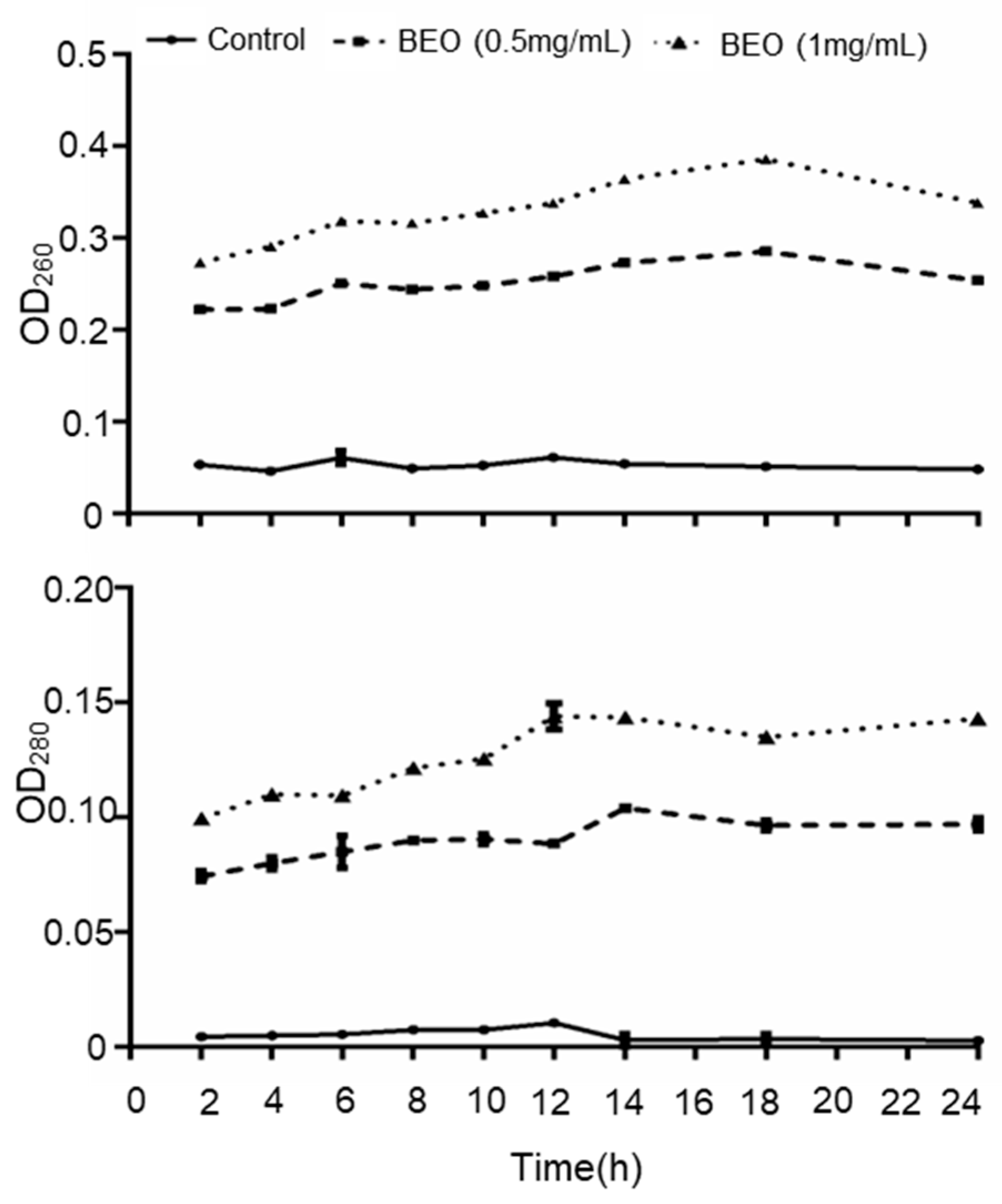
2.3.1. Leakage of UV-Absorbing Substances
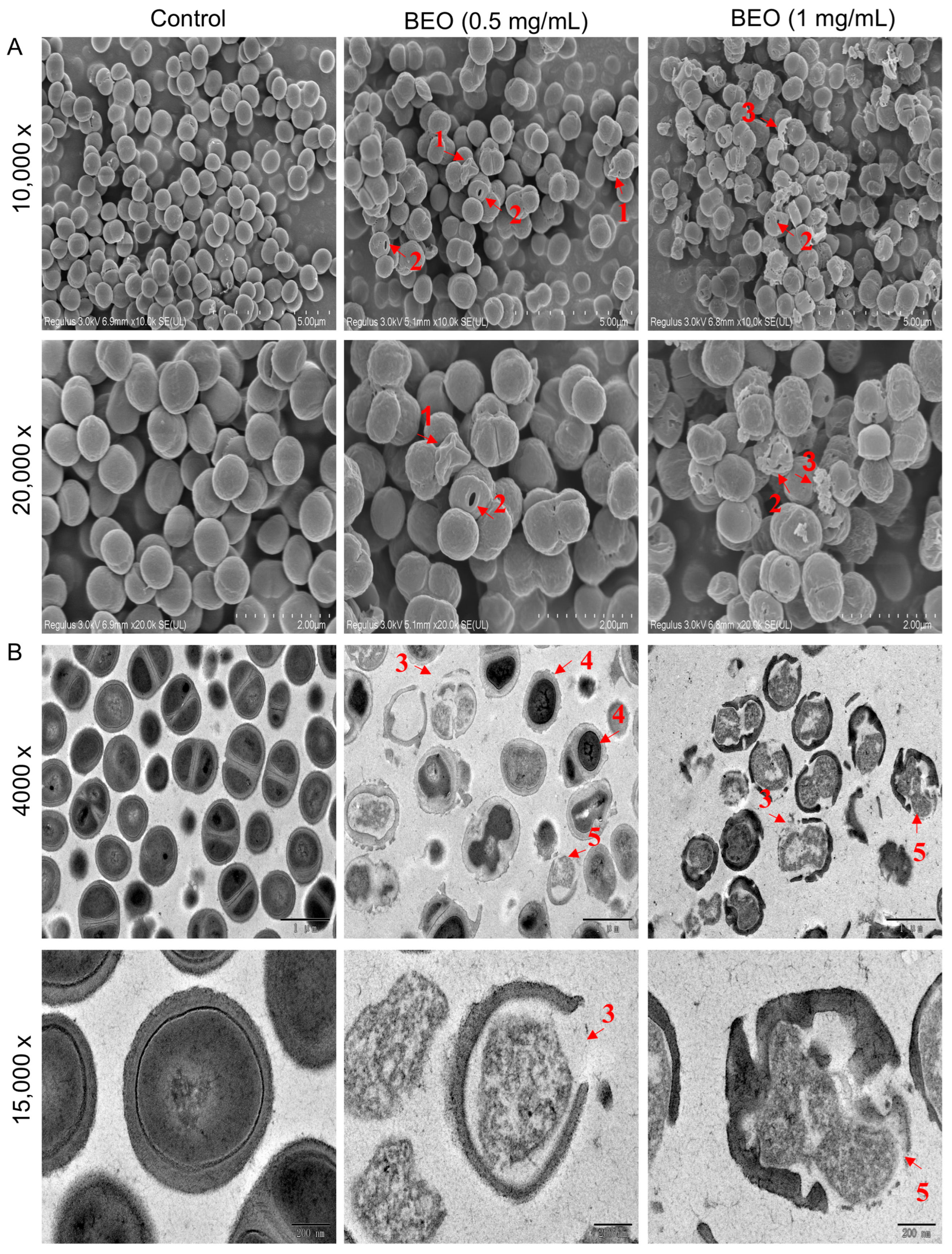
2.3.2. Observation of BEO-Induced Cell Damage by Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.4. Effect of BEO on LPS-Induced Inflammation in RAW 264.7 Mouse Macrophage Cells
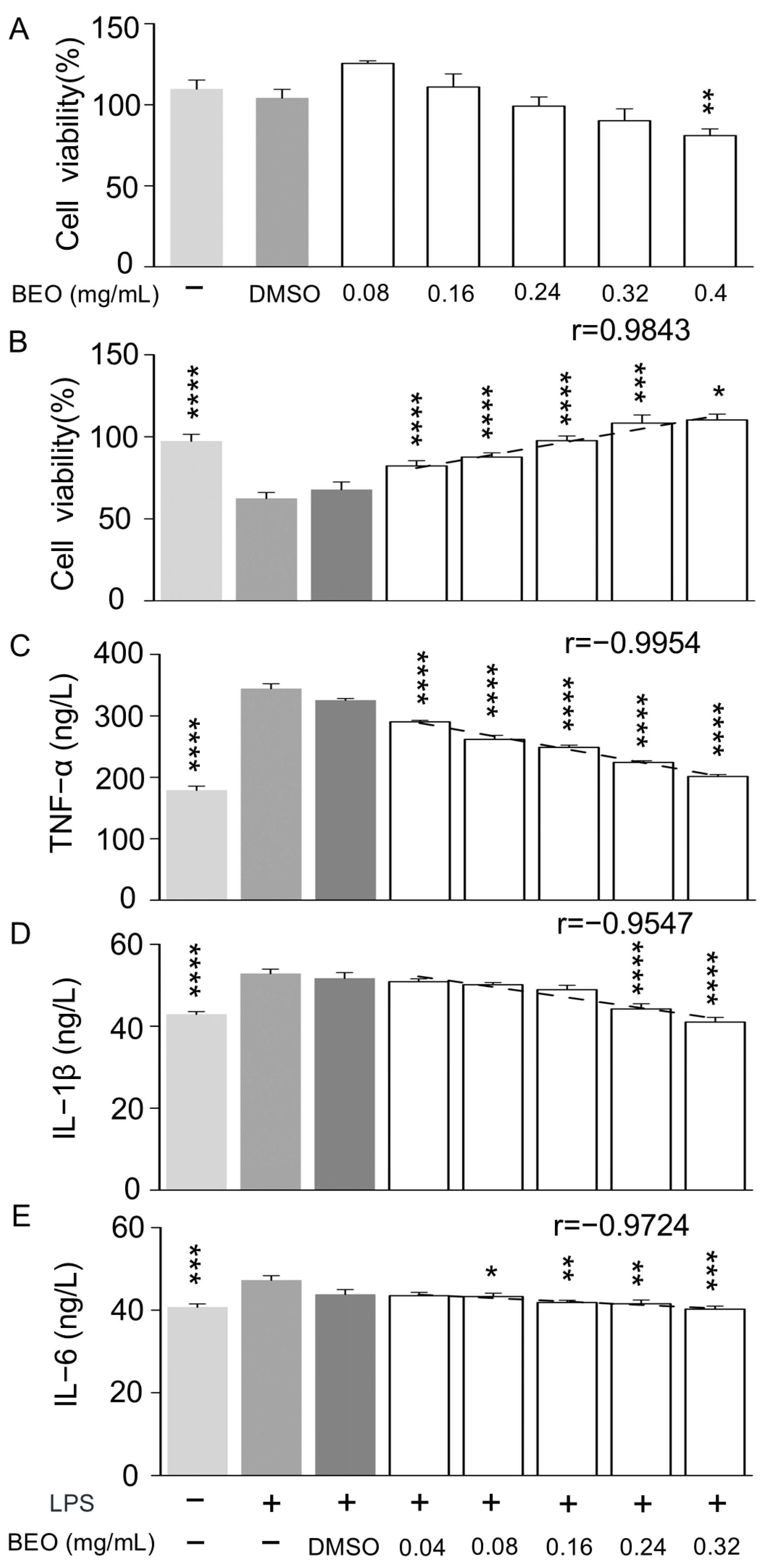
2.4.1. Effect of BEO on RAW 264.7 Cell Viability
2.4.2. Effect of BEO on LPS-Induced Inflammation in RAW 264.7 Cells
2.5. Network Pharmacology Analysis
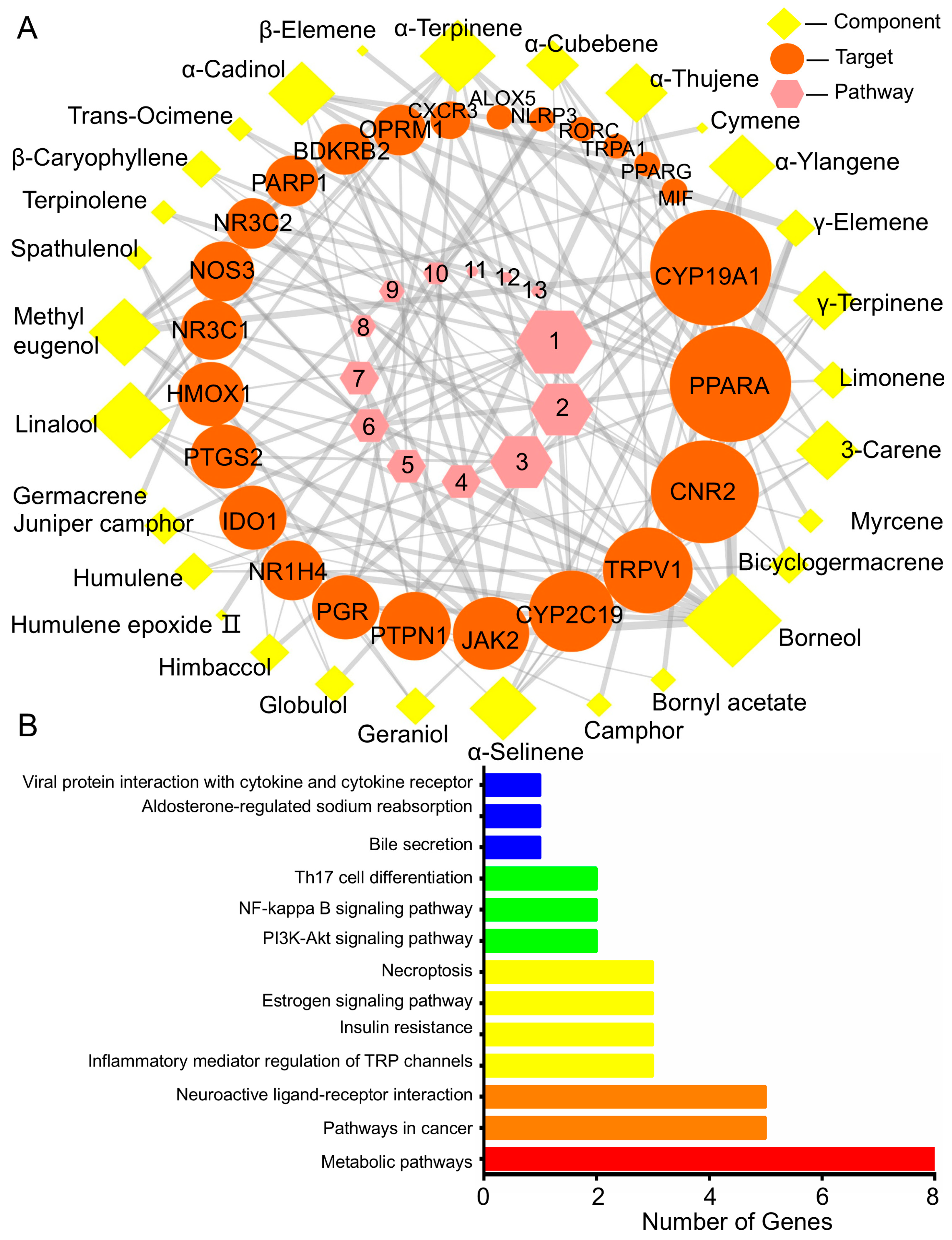
2.5.1. BEO Constituents and Their Therapeutic Targets
2.5.2. Target- Pathway Cascade Analysis in Network Pharmacology
3. Materials and Methods
3.1. Materials and Reagents
3.2. Chemical Compositional Analysis of BEO
3.3. Bacterial Strains and Culturing
3.4. Antibacterial Effect of BEO on S. epidermidis
3.4.1. Determination of the MIC of BEO and NCB
3.4.2. Measurement of the Growth Curve
3.4.3. Effect of BEO and NCB Treatment on Cell Membrane Integrity
3.4.4. Determination of Bacterial Cell Membrane Integrity
3.4.5. Scanning Electron Microscopy (SEM)
3.4.6. Transmission Electron Microscopy (TEM)
3.5. Effect of BEO In Vitro on LPS-Induced Inflammation in Murine Macrophages (RAW 264.7)
3.5.1. Cell Culture
3.5.2. Cell Viability Assay After LPS Treatment
3.5.3. Cytokine Measurement in RAW 264.7 Cells After LPS Treatment
3.6. Network Pharmacology Analysis
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEO | Cinnamomum camphora chvar. Borneol essential oil |
| NCB | Natural crystalline borneol |
| MIC | Minimum inhibitory concentration |
| LD | Linear dichroism |
| μmax | Maximum growth rate |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| LPS | Lipopolysaccharide |
| DMSO | Dimethyl sulfoxide |
| TNF-α | Tumor necrosis factor-α |
| (IL)-1β | Interleukin-1β |
| ELISA | Enzyme-linked immunosorbent assay |
| CFU | Colony Forming Units |
| CLSM | Confocal laser scanning microscopy |
| FBS | Fetal bovine serum |
| IC50 | Half maximal inhibitory concentration |
| CYP | Cytochrome P450 |
| SEM | Standard error of mean |
| PPAR-α | Peroxisome proliferator-activated receptor-α |
| CNR2 | Cannabinoid receptor 2 |
| TRP | Transient receptor potential cation channel V1 |
| CTW | Component target weight |
| CPW | Component pathway weight |
References
- Xiao, S.; Yu, H.; Xie, Y.; Guo, Y.; Fan, J.; Yao, W. The anti-inflammatory potential of Cinnamomum camphora (L.) J.Presl essential oil in vitro and in vivo. J. Ethnopharmacol. 2021, 267, 113516. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhan, C.; Huang, X.; Hong, L.; Fang, L.; Wang, W.; Su, J. Durable Antibacterial Cotton Fabrics Based on Natural Borneol-Derived Anti-MRSA Agents. Adv. Healthc. Mater. 2020, 9, e2000186. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.; Junior, R.G.; Quintans, J.D.; Barreto, R.D.; Bonjardim, L.R.; Cavalcanti, S.C.; Quintans, L.J., Jr. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 2013, 808460. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Shi, J.; Jiang, W.; Huang, W.; Chen, K.; Wang, X.; Zhang, G.; Li, Y.; Cao, C.; et al. Edaravone Dexborneol Alleviates Ischemic Injury and Neuroinflammation by Modulating Microglial and Astrocyte Polarization While Inhibiting Leukocyte Infiltration. Int. Immunopharmacol. 2024, 130, 111700. [Google Scholar] [CrossRef]
- Deng, J.; Wang, K.; Yang, J.; Wang, A.; Chen, G.; Ye, M.; Chen, Q.; Lin, D. β-Caryophyllene Promotes the Survival of Random Skin Flaps by Upregulating the PI3K/AKT Signaling Pathway. Phytomedicine 2024, 130, 155726. [Google Scholar] [CrossRef]
- Kathem, S.H.; Nasrawi, Y.S.; Mutlag, S.H.; Nauli, S.M. Limonene Exerts Anti-Inflammatory Effect on LPS-Induced Jejunal Injury in Mice by Inhibiting NF-κB/AP-1 Pathway. Biomolecules 2024, 14, 334. [Google Scholar] [CrossRef]
- Noroozi, F.; Asle-Rousta, M.; Amini, R.; Sahraeian, Z. Alpha-Pinene Ameliorates Liver Fibrosis by Suppressing Oxidative Stress, Inflammation, and the TGF-β/Smad3 Signaling Pathway. Iran. J. Basic Med. Sci. 2025, 28, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimabadi, A.H.; Mazoochi, A.; Kashi, F.J.; Djafari-Bidgoli, Z.; Batooli, H. Essential oil composition and antioxidant and antimicrobial properties of the aerial parts of Salvia eremophila Boiss. from Iran. Food Chem. Toxicol. 2010, 48, 1371–1376. [Google Scholar] [CrossRef]
- Kleinschmidt, S.; Huygens, F.; Faoagali, J.; Rathnayake, I.U.; Hafner, L.M. Staphylococcus epidermidis as a cause of bacteremia. Future Microbiol. 2015, 10, 1859–1879. [Google Scholar] [CrossRef]
- Polatoglu, K.; Demirci, F.; Demirci, B.; Goren, N.; Can Baser, K.H. Antimicrobial activity and essential oil composition of a new T. argyrophyllum (C. Koch) Tvzel var. argyrophyllum chemotype. J. Oleo Sci. 2010, 59, 307–313. [Google Scholar] [CrossRef]
- Orchard, A.; Vuuren, V. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid.-Based Compl. Alt. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion-In Vitro and In Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Mahomoodally, M.F. Essential oils from 9 exotic and endemic medicinal plants from Mauritius shows in vitro antibacterial and antibiotic potentiating activities. S. Afr. J. Bot. 2020, 132, 355–362. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Li, W.; Zhang, J.M.; Wang, D.; Fu, J.; Wang, P. Comparison of Chemical Profiles, Anti-Inflammatory Activity, and UPLC-Q-TOF/MS-Based Metabolomics in Endotoxic Fever Rats between Synthetic Borneol and Natural Borneol. Molecules 2017, 22, 1446. [Google Scholar] [CrossRef]
- Yu, H.; Ren, X.N.; Liu, Y.L.; Xie, Y.F.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Extraction of Cinnamomum camphora chvar. Borneol essential oil using neutral cellulase assisted-steam distillation: Optimization of extraction, and analysis of chemical constituents. Ind. Crop. Prod. 2019, 141, 111794. [Google Scholar] [CrossRef]
- Oliva, M.d.L.; Carezzano, M.E.; Giuliano, M.; Daghero, J.; Zygadlo, J.; Bogino, P.; Giordano, W.; Demo, M. Anti-microbial activity of essential oils of Thymus vulgaris and Origanum vulgare on phytopathogenic strains isolated from soybean. Plant Biol. 2015, 17, 758–765. [Google Scholar] [CrossRef]
- Kim, S.S.; Baik, J.S.; Oh, T.H.; Yoon, W.J.; Lee, N.H.; Hyun, C.G. Biological activities of Korean Citrus obovoides and Citrus natsudaidai essential oils against acne-inducing bacteria. Biosci. Biotechnol. Biochem. 2008, 72, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, A.; Zeng, T.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Essential Oil Composition and Antimicrobial Activity of Liquidambar formosana Oleoresin. Plants 2020, 9, 822. [Google Scholar] [CrossRef]
- Chi, X.; Ding, J.; Zhang, Y.; Chen, Y.; Han, Y.; Lin, Y.; Jiang, J. Berberine Protects against Dysentery by Targeting Both Shigella Filamentous Temperature Sensitive Protein Z and Host Pyroptosis: Resolving in Vitro-Vivo Effect Discrepancy. Phytomedicine 2025, 139, 156517. [Google Scholar] [CrossRef]
- Liu, G.; Song, Z.Q.; Yang, X.L.; Gao, Y.K.; Wang, C.T.; Sun, B.G. Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control. 2016, 62, 309–316. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Mahajan, P.; Pandiya, S.; Bajaj, A.; Verma, S.K.; Yadav, P.; Kharat, A.S.; Khan, A.U.; Dua, M.; Johri, A.K. Antibiotic Resistance: A Global Crisis, Problems and Solutions. Crit. Rev. Microbiol. 2024, 50, 896–921. [Google Scholar] [CrossRef]
- Xu, C.; Li, J.; Yang, L.; Shi, F.; Yang, L.; Ye, M. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control. 2017, 73, 1445–1451. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.Y.; Wang, Y.F.; Jiang, P.P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. African Flora Has the Potential to Fight Multidrug Resistance of Cancer. Biomed. Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- Augustus, A.R.; Jana, S.; Samsudeen, M.B.; Nagaiah, H.P.; Shunmugiah, K.P. In Vitro and in Vivo Evaluation of the Anti-Infective Potential of the Essential Oil Extracted from the Leaves of Plectranthus amboinicus (Lour.) Spreng against Klebsiella pneumoniae and Elucidation of Its Mechanism of Action through Proteomics Approach. J. Ethnopharmacol. 2024, 330, 118202. [Google Scholar] [CrossRef]
- Li, D.; Li, G.; Chen, Y.; Li, Y.; Zhang, J.; Gao, D.; Sun, L.; Liu, B. Astragaloside IV protects ATDC5 cells from lipopolysac-charide-caused damage through regulating miR-203/MyD88. Pharm. Biol. 2020, 58, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Cui, Y.; Yu, Q.; Xie, X.; Liu, Y.; Wei, M.; Ci, X.; Peng, L. Modulation of LPS-stimulated pulmonary inflammation by Borneol in murine acute lung injury model. Inflammation 2014, 37, 1148–1157. [Google Scholar] [CrossRef]
- Abidi, A.H.; Alghamdi, S.S.; Dabbous, M.K.; Tipton, D.A.; Mustafa, S.M.; Moore, B.M. Cannabinoid type-2 receptor agonist, inverse agonist, and anandamide regulation of inflammatory responses in IL-1beta stimulated primary human periodontal ligament fibroblasts. J. Periodontal Res. 2020, 55, 762–783. [Google Scholar] [CrossRef]
- Nesterkina, M.; Kravchenko, I. Synthesis and Pharmacological Properties of Novel Esters Based on Monoterpenoids and Glycine. Pharmaceuticals 2017, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Kim, M.J.; Son, H.J.; Kweon, H.J.; Kim, J.T.; Kim, Y.; Shim, J.; Suh, B.C.; Rhyu, M.R. Five hTRPA1 Agonists Found in Indigenous Korean Mint, Agastache rugosa. PLoS ONE 2015, 10, e0127060. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N.; Rao, A.A. Gene expression profile in obesity and type 2 diabetes mellitus. Lipids Health Dis. 2007, 6, 35. [Google Scholar] [CrossRef]
- Idrovo, J.P.; Boe, D.M.; Kaahui, S.; Yang, W.L.; Kovacs, E.J. Hepatic inflammation after burn injury is associated with necroptotic cell death signaling. J. Trauma Acute Care Surg. 2020, 89, 768–774. [Google Scholar] [CrossRef]
- Qu, S.L.; Chen, L.; Wen, X.S.; Zuo, J.P.; Wang, X.Y.; Lu, Z.J.; Yang, Y.F. Suppression of Th17 cell differentiation via sphingosine-1-phosphate receptor 2 by cinnamaldehyde can ameliorate ulcerative colitis. Biomed. Pharmacother. 2020, 134, 111116. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, W.; Hu, J.; Mi, X.; Han, Y.; Ren, S.; Jiang, S.; Wang, Y.; Li, X.; Li, W. Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-kappaB and PI3K/Akt Signal Pathways. Antioxidants 2019, 8, 395. [Google Scholar] [CrossRef]
- Bajer, T.; Janda, V.; Bajerova, P.; Kremr, D.; Eisner, A.; Ventura, K. Chemical composition of essential oils from plantago lanceolata L. leaves extracted by hydrodistillation. J. Food Sci. Technol. 2016, 53, 1576–1584. [Google Scholar] [CrossRef]
- Diao, M.M.; Qi, D.P.; Xu, M.M.; Lu, Z.X.; Lv, F.X.; Bie, X.M.; Zhang, C.; Zhao, H.Z. Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control. 2018, 85, 339–344. [Google Scholar] [CrossRef]
- Wang, L.H.; Zeng, X.A.; Wang, M.S.; Brennan, C.S.; Gong, D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. Biochim. Biophys. Acta Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Y.; Wang, Y.; Zhang, Z.; Wang, T.; Zhou, Q.; Wang, X. Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions. Biomed. Pharmacother. 2017, 90, 244–252. [Google Scholar] [CrossRef] [PubMed]
| Gentamicin | BEO | NCB | T. argyrophyllum Essential Oil | S. eremophila Essential Oil | |
|---|---|---|---|---|---|
| MIC against S. epidermidis | 0.5 | 0.5 | 0.5 | 0.5 | 0.125 |
| Main compounds (content %) | |||||
| Borneol | 16.4 | 98.4 | 15 | 21.8 | |
| β-Caryophyllene | 10.7 | - | 0.3 | 4.5 | |
| Camphor | 10.6 | 0.8 | 26.6 | - | |
| Limonene | 8.2 | - | 0.3 | 2.7 | |
| α-Pinene | 7.5 | - | 2.4 | 18.8 | |
| Myrcene | 6.2 | - | - | 0.6 | |
| Camphene | 4.4 | - | 4.5 | 5.5 | |
| γ-Terpinene | 3.7 | - | 0.5 | 0.6 | |
| Bicyclogermacrene | 2.8 | - | - | 1.5 | |
| Terpinolene | 1.6 | - | 0.5 | 0.3 | |
| β-Pinene | 1.4 | - | 1.3 | 0.9 | |
| Spathulenol | 0.9 | - | 0.9 | 1.2 | |
| Sabenene | 0.9 | - | 0.3 | - | |
| Caryophyllene oxide | 0.8 | - | 0.4 | - | |
| Linalool | 0.5 | - | - | 1.5 | |
| α-Terpinene | 0.4 | - | 0.2 | 0.3 | |
| Bornyl acetate | 0.2 | - | 3.3 | 18.7 | |
| Globulol | 0.1 | - | - | 2.9 | |
| Aromadendrene | 0.1 | - | - | 1.7 | |
| References | Ebrahimabadi et al., 2010 [8] | Xiao et al., 2020 [1] | Polatoglu, 2010 [10] | Ebrahimabadi et al., 2010 [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W. The Antibacterial and Anti-Inflammatory Potential of Cinnamomum camphora chvar. Borneol Essential Oil In Vitro. Plants 2025, 14, 1880. https://doi.org/10.3390/plants14121880
Xiao S, Yu H, Guo Y, Cheng Y, Yao W. The Antibacterial and Anti-Inflammatory Potential of Cinnamomum camphora chvar. Borneol Essential Oil In Vitro. Plants. 2025; 14(12):1880. https://doi.org/10.3390/plants14121880
Chicago/Turabian StyleXiao, Shanshan, Hang Yu, Yahui Guo, Yuliang Cheng, and Weirong Yao. 2025. "The Antibacterial and Anti-Inflammatory Potential of Cinnamomum camphora chvar. Borneol Essential Oil In Vitro" Plants 14, no. 12: 1880. https://doi.org/10.3390/plants14121880
APA StyleXiao, S., Yu, H., Guo, Y., Cheng, Y., & Yao, W. (2025). The Antibacterial and Anti-Inflammatory Potential of Cinnamomum camphora chvar. Borneol Essential Oil In Vitro. Plants, 14(12), 1880. https://doi.org/10.3390/plants14121880








