Brassinosteroids: Biosynthesis, Signaling, and Hormonal Crosstalk as Related to Fruit Yield and Quality
Abstract
1. Introduction
2. Chemical Structure of Brassinosteroids
3. Biosynthesis, Transport, Signaling, and Homeostasis of Brassinosteroids
3.1. Biosynthesis
3.2. Transport
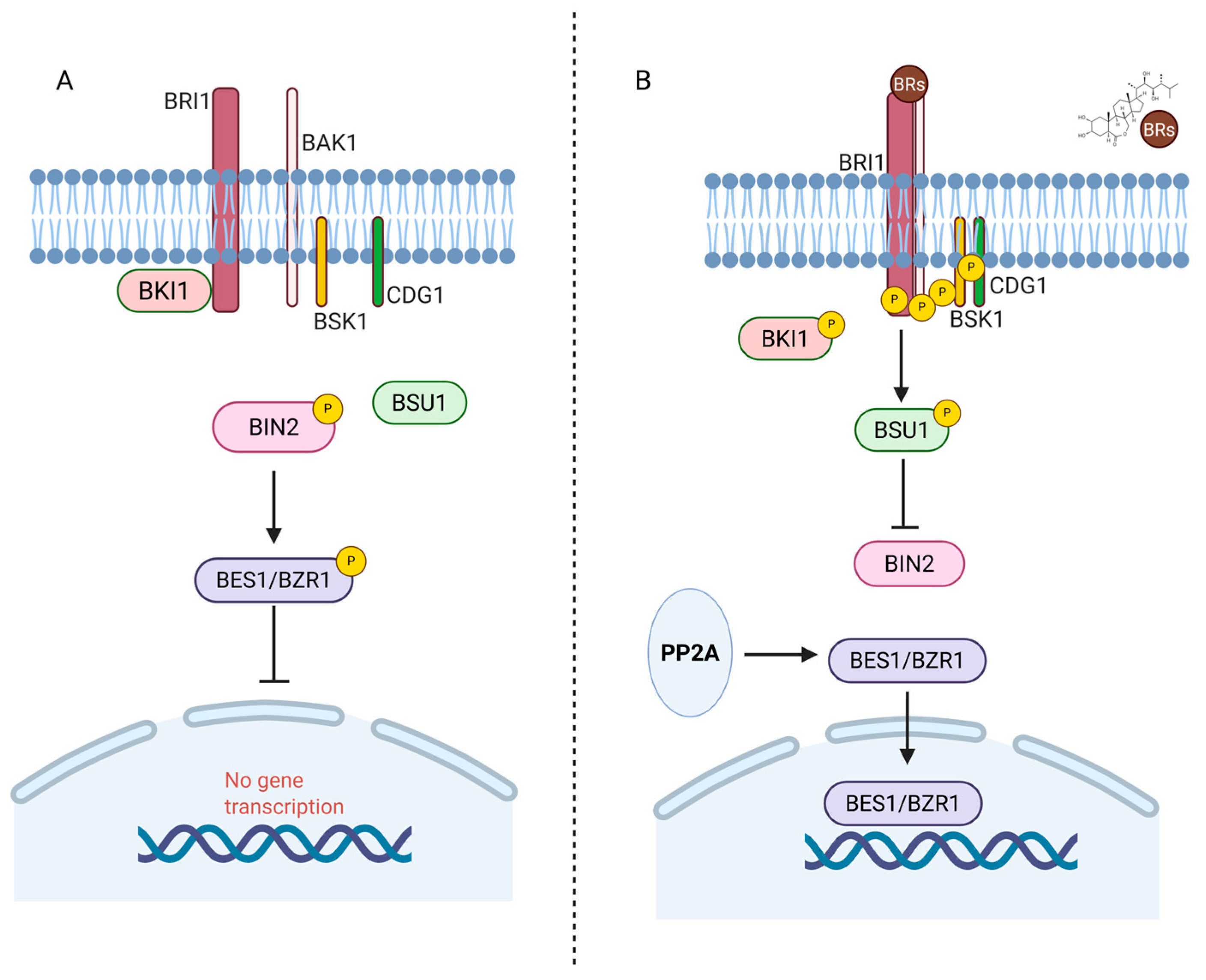
3.3. Signal Transduction Pathways and BR Homeostasis
4. Effects of Brassinosteroids on Fruit Crop Development
4.1. Flowering, Fruit Set, and Maturation
4.2. Fruit Size
4.3. Fruit Firmness
4.4. Sugar Accumulation
4.5. Color Development
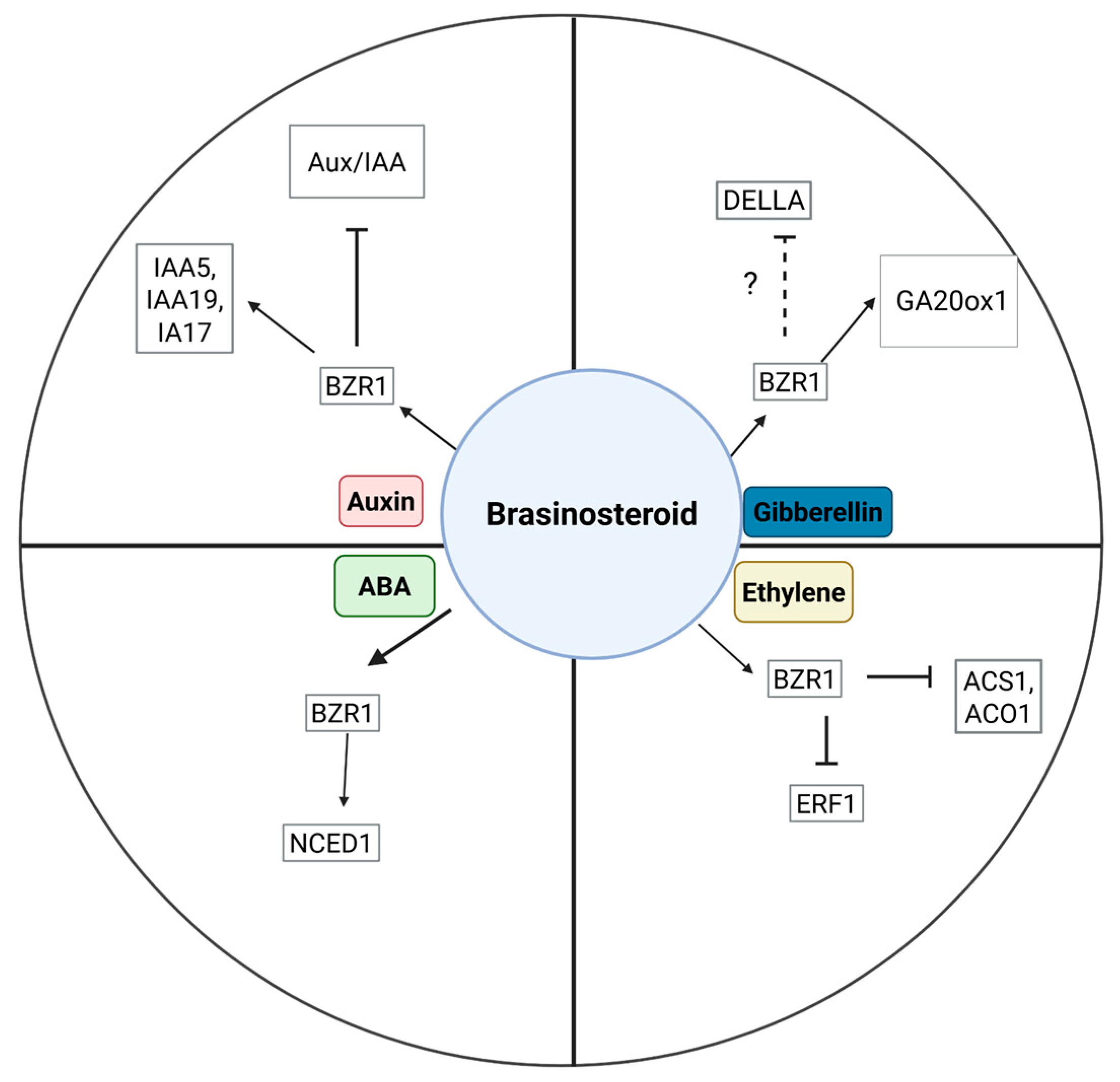
5. BRs Interaction with Other Plant Hormones During Fruit Development, Ripening, and Maturation
5.1. Auxin
5.2. Gibberellin
5.3. Cytokinin
5.4. Ethylene
5.5. Abscisic Acid
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; David Warthen, J., Jr.; Steffens, G.L.; Flippen-Anderson, J.L.; Carter Cook, J., Jr. Brassinolide, a Plant Growth-Promoting Steroid Isolated from Brassica napus Pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In Plant Hormones: Physiology, Biochemistry and Molecular Biology; Springer: Dordrecht, The Netherlands, 1995; pp. 1–12. [Google Scholar]
- Bari, R.; Jones, J.D.G. Role of Plant Hormones in Plant Defence Responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Alferez, F.; Zacarias, L. Interaction Between Ethylene and Abscisic Acid in the Regulation of Citrus Fruit Maturation. In Biology and Biotechnology of the Plant Hormone Ethylene II; Springer: Dordrecht, The Netherlands, 1999; pp. 183–184. [Google Scholar]
- Alferez, F.; de Carvalho, D.U.; Boakye, D. Interplay between Abscisic Acid and Gibberellins, as Related to Ethylene and Sugars, in Regulating Maturation of Non-Climacteric Fruit. Int. J. Mol. Sci. 2021, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, G.; Jaramillo, A.; Blázquez, M.A. Integral Control of Plant Gravitropism through the Interplay of Hormone Signaling and Gene Regulation. Biophys. J. 2011, 101, 757–763. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins—A New Family of Plant Hormones from Rape Pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef]
- Takao, Y.; Masahiro, A.; Nobutaka, T. Castasterone, a new phytosterol with plant-hormone potency, from chestnut insect gall. Tetrahedron Lett. 1982, 23, 1275–1982. [Google Scholar]
- Clouse, S.D.; Langford, M.; Mcmorris, T.C. A Brassinosteroid-Insensitive Mutant in Arabidopsis Thaliana Exhibits Multiple Defects in Growth and Development. Plant Phy. 1996, 111, 671–678. [Google Scholar] [CrossRef]
- Bajguz, A.; Tretyn, A. The Chemical Characteristic and Distribution of Brassinosteroids in Plants. Phy. Chem. 2002, 62, 1027–1046. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A.; Alférez, F. Homobrassinolide Delays Huanglongbing Progression in Newly Planted Citrus (Citrus Sinensis) Trees. Plants 2024, 13, 1229. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid Signal Transduction from Cell-Surface Receptor Kinases to Nuclear Transcription Factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.M.; Ross, J.J.; Jager, C.E.; Reid, J.B. Brassinosteroid Transport. J. Exp. Bot. 2008, 59, 17–24. [Google Scholar] [CrossRef]
- Fujioka, S.; Sakurai, A. Brassinosteroids. Nat. Prod. Rep. 1997, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D.; Sasse, J.M. BRASSINOSTEROIDS: Essential Regulators of Plant Growth and Development. Anal. Rev. Plant Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive Overview of the Brassinosteroid Biosynthesis Pathways: Substrates, Products, Inhibitors, and Connections. Front. Plant Sci. 2020, 11, 1034. [Google Scholar] [CrossRef]
- Yu, L.; Cai, W.J.; Ye, T.; Feng, Y.Q. A New Boronic Acid Reagent for the Simultaneous Determination of C27-, C28-, and C29-Brassinosteroids in Plant Tissues by Chemical Labeling-Assisted Liquid Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 1623–1632. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of Brassinosteroids on the Plant Responses to Environmental Stresses. Plant Phy. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Perez-Sancho, J.; Platre, M.P.; Callebaut, B.; Smokvarska, M.; Ferrer, K.; Luo, Y.; Nolan, T.M.; Sato, T.; Busch, W.; et al. Plasmodesmata Mediate Cell-to-Cell Transport of Brassinosteroid Hormones. Nat. Chem. Biol. 2023, 19, 1331–1341. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and Metabolism of Brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-Localized BZR1 Mediates Brassinosteroid-Induced Growth and Feedback Suppression of Brassinosteroid Biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Han, Z.; Kim, T.W.; Wang, J.; Cheng, W.; Chang, J.; Shi, S.; Wang, J.; Yang, M.; Wang, Z.Y.; et al. Structural Insight into Brassinosteroid Perception by BRI1. Nature 2011, 474, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Wu, Y.; Yu, Y.; Li, J.; Mu, X.; Xu, L.; Wang, X.; Qi, G.; Tang, J.; Wang, D.; et al. Copine Proteins Are Required for Brassinosteroid Signaling in Maize and Arabidopsis. Nat. Commun. 2024, 15, 2028. [Google Scholar] [CrossRef] [PubMed]
- He, J.X.; Gendron, J.M.; Sun, Y.; Gampala, S.S.; Gendron, N.; Sun, C.Q.; Wang, Z.Y. BZR1 Is a Transcriptional Repressor with Dual Roles in Brassinosteroid and Growth Responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef]
- Zhang, Y.; Berman, A.; Shani, E. Plant Hormone Transport and Localization: Signaling Molecules on the Move. Annu. Rev. Plant Biol. 2025, 139, 51. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J. Regulation of Brassinosteroid Biosynthesis and Inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef]
- Baghel, M.; Nagaraja, A.; Srivastav, M.; Meena, N.K.; Senthil Kumar, M.; Kumar, A.; Sharma, R.R. Pleiotropic Influences of Brassinosteroids on Fruit Crops: A Review. Plant Growth Regul. 2019, 87, 375–388. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, C.; Zhao, C.; Zhang, L.; Han, M.; An, N.; Ren, X. Effects of Brassinosteroid Associated with Auxin and Gibberellin on Apple Tree Growth and Gene Expression Patterns. Hortic. Plant J. 2019, 5, 93–108. [Google Scholar] [CrossRef]
- Zhu, T.; Tan, W.R.; Deng, X.G.; Zheng, T.; Zhang, D.W.; Lin, H.H. Effects of Brassinosteroids on Quality Attributes and Ethylene Synthesis in Postharvest Tomato Fruit. Postharvest Biol. Technol. 2015, 100, 196–204. [Google Scholar] [CrossRef]
- Wang, X.; Chory, J. Brassinosteroids Regulate Dissociation of BKI1, a Negative Regulator of BRI1, from the Plasma Membrane. Science 2006, 313, 1115–1118. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Schomburg, F.M.; Amasino, R.M.; Vierstra, R.D.; Nagy, F.; Davis, S.J. Attenuation of Brassinosteroid Signaling Enhances FLC Expression and Delays Flowering. Development 2007, 134, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Luo, B.; Hu, M.; Fu, S.; Liu, J.; Jiang, M.; Zhao, Y.; Huang, S.; Wang, S.; Wang, X. Brassinosteroid Signaling Downstream Suppressor BIN2 Interacts with SLFRIGIDA-LIKE to Induce Early Flowering in Tomato. Int. J. Mol. Sci. 2022, 23, 11264. [Google Scholar] [CrossRef]
- Zullo, M.A.T.; Adam, G. Brassinosteroid Phytohormones-Structure, Bioactivity and Applications. Braz. J. Plant Phy 2002, 14, 143–181. [Google Scholar] [CrossRef]
- Ramraj, V.M.; Vyas, B.N.; Godrej, N.B.; Mistry, K.B.; Swami, B.N.; Singh, N. Effects of 28-Homobrassinolide on Yields of Wheat, Rice, Groundnut, Mustard, Potato and Cotton. J. Agric. Sci. 1997, 128, 405–413. [Google Scholar] [CrossRef]
- Peng, J.; Tang, X.; Feng, H. Effects of Brassinolide on the Physiological Properties of Litchi Pericarp (Litchi Chinensis Cv. Nuomoci). Sci. Hortic. 2004, 101, 407–416. [Google Scholar] [CrossRef]
- Gomes, M.d.M.A.; Campostrini, E.; Leal, N.R.; Viana, A.P.; Ferraz, T.M.; Siqueira, L.d.N.; Rosa, R.C.C.; Netto, A.T.; Nuñez-Vázquez, M.; Zullo, M.A.T. Brassinosteroid Analogue Effects on the Yield of Yellow Passion Fruit Plants (Passiflora Edulis f. Flavicarpa). Sci. Hortic. 2006, 110, 235–240. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kuraishi, S. Stimulation of Fruit Set of ‘Morita’ navel Orange with Brassinolide. Int. Soc. Hortic. Sci. 1988, 239, 345–348. [Google Scholar] [CrossRef]
- Iwahori, S.; Tominaga, S.; Higuchi, S. Retardation of Abscission of Citrus Leaf and Fruitlet Explants by Brassinolide. Plant Growth Reg. 1990, 9, 119–125. [Google Scholar] [CrossRef]
- Habibi, F.; Serrano, M.; Zacarías, L.; Valero, D.; Guillén, F. Postharvest Application of 24-Epibrassinolide Reduces Chilling Injury Symptoms and Enhances Bioactive Compounds Content and Antioxidant Activity of Blood Orange Fruit. Front. Plant Sci. 2021, 12, 629733. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Al-Obeed, R.S.; Makhasha, E.; Mostafa, L.Y.; Abdelzaher, R.A.E.; Rihan, H.Z. Improving Mangoes’ Productivity and Crop Water Productivity by 24-Epibrassinosteroids and Hydrogen Peroxide under Deficit Irrigation. Agric. Water Manag. 2024, 298, 108860. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Sas-Paszt, L.; Awad, R.M.; Mosa, W.F.A. Apricot (Prunus Armeniaca) Performance under Foliar Application of Humic Acid, Brassinosteroids, and Seaweed Extract. Horticulturae 2023, 9, 519. [Google Scholar] [CrossRef]
- Chai, Y.M.; Zhang, Q.; Tian, L.; Li, C.L.; Xing, Y.; Qin, L.; Shen, Y.Y. Brassinosteroid Is Involved in Strawberry Fruit Ripening. Plant Growth Regul. 2013, 69, 63–69. [Google Scholar] [CrossRef]
- Zahedipour-Sheshglani, P.; Asghari, M. Impact of Foliar Spray with 24-Epibrassinolide on Yield, Quality, Ripening Physiology and Productivity of the Strawberry. Sci. Hortic. 2020, 268, 109376. [Google Scholar] [CrossRef]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on Steroids. Brassinosteroids Are Involved in Grape Berry Ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.E.; Díaz, K.; Carvajal, R.; Espinoza, L.; Alcalde, J.A.; Pérez-Donoso, A.G. Exogenous Applications of Brassinosteroids Improve Color of Red Table Grape (Vitis vinifera L. Cv. “Redglobe”) Berries. Front. Plant Sci. 2018, 9, 363. [Google Scholar] [CrossRef]
- Shan, W.; Guo, Y.F.; Wei, W.; Chen, J.Y.; Lu, W.J.; Yuan, D.B.; Su, X.G.; Kuang, J.F. Banana MaBZR1/2 Associate with MaMPK14 to Modulate Cell Wall Modifying Genes during Fruit Ripening. Plant Cell Rep. 2020, 39, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; Cruz-Izquierdo, S.; García-Zavala, J.J.; Chávez-Servia, J.L.; Hernández-Leal, E.; Bonilla-Barrientos, O. Fruit Size QTLs Affect in a Major Proportion the Yield in Tomato. Chil. J. Agric. Res. 2015, 75, 402. [Google Scholar] [CrossRef]
- Gillaspy, G.; Hilla, B.-D.; Gruissem, W. Fruits: A Developmental Perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef]
- Zhang, C.; Tanabe, K.; Wang, S.; Tamura, F.; Yoshida, A.; Matsumoto, K. The Impact of Cell Division and Cell Enlargement on the Evolution of Fruit Size in Pyrus Pyrifolia. Ann. Bot. 2006, 98, 537–543. [Google Scholar] [CrossRef]
- Okello, R.C.O.; Heuvelink, E.; De Visser, P.H.B.; Struik, P.C.; Marcelis, L.F.M. What Drives Fruit Growth? Funct. Plant Biol. 2015, 42, 817–827. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Aror, N.K.; Bedi, S. Brassinosteroids Improve Quality of Table Grapes (Vitis Vinifera L.) Cv. Flame Seedless. Trop. Agri. Res. 2015, 26, 368–379. [Google Scholar] [CrossRef]
- Roghabadi, M.A.; Pakkish, Z. Role of Brassinosteroid on Yield, Fruit Quality and Postharvest Storage of “Tak Danehe Mashhad” Sweet Cherry (Prunus avium L.). Agric. Commun. 2014, 2, 49–56. [Google Scholar]
- Ramos, Â.P.; Zanardi, A.M.; Do Amarante, C.V.T.; Steffens, C.A.; Pereira-Netto, A.B. Effects of an Auxin and a Brassinosteroid on Physical, Chemical and Biochemical Attributes of ‘Galaxy’ Apples. Ciencia Rural. 2019, 49, e20180311. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Ban, Q.; Han, S.; Rao, J. Role of Brassinosteroids in Persimmon (Diospyros Kaki L.) Fruit Ripening. J. Agric. Food Chem. 2018, 66, 2637–2644. [Google Scholar] [CrossRef]
- Mumtaz, M.A.; Li, F.; Zhang, X.; Tao, J.; Ge, P.; Wang, Y.; Wang, Y.; Gai, W.; Dong, H.; Zhang, Y. Altered Brassinolide Sensitivity1 Regulates Fruit Size in Association with Phytohormones Modulation in Tomato. Horticulturae 2022, 8, 1008. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z.; Symons, G.M.; Reid, J.B. Role of Brassinosteroids, Ethylene, Abscisic Acid, and Indole-3-Acetic Acid in Mango Fruit Ripening. J. Plant Growth Regul. 2012, 31, 363–372. [Google Scholar] [CrossRef]
- Li, J.; Guo, T.; Guo, M.; Dai, X.; Xu, X.; Li, Y.; Song, Z.; Liang, M. Exogenous BR Delayed Peach Fruit Softening by Inhibiting Pectin Degradation Enzyme Genes. Front. Plant Sci. 2023, 14, 1226921. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.; Niu, C.; Chen, H.; Zhu, F.; Farouk, A.; Lu, J.; Chen, C.; Ban, Z.; Huang, J. Brassinolide Alleviates Chilling Injury of Sweet Cherry (Prunus avium L. Cv. Tieton) during Cold Storage. Horticulturae 2024, 10, 675. [Google Scholar] [CrossRef]
- Ayub, R.A.; Reis, L.; Bosetto, L.; Lopes, P.Z.; Galvão, C.W.; Etto, R.M. Brassinosteroid Plays a Role on Pink Stage for Receptor and Transcription Factors Involved in Strawberry Fruit Ripening. Plant Growth Regul. 2018, 84, 159–167. [Google Scholar] [CrossRef]
- Yu, J.Q.; Huang, L.F.; Hu, W.H.; Zhou, Y.H.; Mao, W.H.; Ye, S.F.; Nogués, S. A Role for Brassinosteroids in the Regulation of Photosynthesis in Cucumis Sativus. J. Exp. Bot. 2004, 55, 1135–1143. [Google Scholar] [CrossRef]
- Xu, F.; Gao, X.; Xi, Z.M.; Zhang, H.; Peng, X.Q.; Wang, Z.Z.; Wang, T.M.; Meng, Y. Application of Exogenous 24-Epibrassinolide Enhances Proanthocyanidin Biosynthesis in Vitis Vinifera ‘Cabernet Sauvignon’ Berry Skin. Plant Growth Regul. 2015, 75, 741–750. [Google Scholar] [CrossRef]
- Symons, G.M.; Reid, J.B. Brassinosteroids Do Not Undergo Long-Distance Transport in Pea. Implications for the Regulation of Endogenous Brassinosteroid Levels. Plant Physiol. 2004, 135, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D. The Brassinosteroid Signaling Pathway-New Key Players and Interconnections with Other Signaling Networks Crucial for Plant Development and Stress Tolerance. Int. J. Mol. Sci. 2013, 14, 8740–8774. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A Dynamic Interplay between Phytohormones Is Required for Fruit Development, Maturation, and Ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Sun, Y.; Asghari, M.; Zahedipour-Sheshgelani, P. Foliar Spray with 24-Epibrassinolide Enhanced Strawberry Fruit Quality, Phytochemical Content, and Postharvest Life. J. Plant Growth Regul. 2020, 39, 920–929. [Google Scholar] [CrossRef]
- Li, A.; Wu, X.; Huang, Y.; Pan, X.; Yao, K.; Liu, Z.; Wang, C.; Liao, W. The Involvement of Brassinolides in Fruit Ripening: Crosstalk with Plant Growth Regulators and Transcription Factors. Food Qual. Saf. 2024, 8, fyad071. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the Sixth Class of Phytohormones: A Molecular View from the Discovery to Hormonal Interactions in Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Pattison, R.J.; Csukasi, F.; Catalá, C. Mechanisms Regulating Auxin Action during Fruit Development. Physiol. Plant 2014, 151, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in Fruit Development and Maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Kumar, R.; Tyagi, A.K.; Sharma, A.K. Genome-Wide Analysis of Auxin Response Factor (ARF) Gene Family from Tomato and Analysis of Their Role in Flower and Fruit Development. Mol. Gen. Genom. 2011, 285, 245–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Chen, C.; Li, C.; Xia, R.; Li, J. Genome-Wide Characterization of the Auxin Response Factor (ARF) Gene Family of Litchi (Litchi Chinensis Sonn.): Phylogenetic Analysis, MiRNA Regulation and Expression Changes during Fruit Abscission. PeerJ 2019, 7, e6677. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ge, M.; Yu, A.; Song, W.; Fang, J.; Leng, X. Effects of Ethylene on Berry Ripening and Anthocyanin Accumulation of ‘Fujiminori’ Grape in Protected Cultivation. J. Sci. Food Agric. 2022, 102, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Wang, N.; Xu, H.F.; Jiang, S.H.; Fang, H.C.; Su, M.Y.; Zhang, Z.Y.; Zhang, T.L.; Chen, X.S. Auxin Regulates Anthocyanin Biosynthesis through the Aux/IAA–ARF Signaling Pathway in Apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Mockler, T.C.; Chory, J. Interdependency of Brassinosteroid and Auxin Signaling in Arabidopsis. PLoS Biol. 2004, 2, e258. [Google Scholar] [CrossRef]
- Bao, F.; Shen, J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z. Brassinosteroids Interact with Auxin to Promote Lateral Root Development in Arabidopsis. Plant Physiol. 2004, 134, 1624–1631. [Google Scholar] [CrossRef]
- Kim, H.; Park, P.-J.; Hwang, H.-J.; Lee, S.-Y.; Oh, M.-H.; Kim, S.-G. Brassinosteroid Signals Control Expression of the AXR3/IAA17 Gene in the Cross-Talk Point with Auxin in Root Development. Biosci. Biotechnol. Biochem. 2006, 70, 768–773. [Google Scholar] [CrossRef]
- Gregory Vert; Walcher, C.L.; Chory, J.; Nemhauser, J.L. Integration of Auxin and Brassinosteroid Pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 2008, 105, 9829–9834. [Google Scholar] [CrossRef]
- Unterholzner, S.J.; Rozhon, W.; Papacek, M.; Ciomas, J.; Lange, T.; Kugler, K.G.; Mayer, K.F.; Sieberer, T.; Poppenberger, B. Brassinosteroids Are Master Regulators of Gibberellin Biosynthesis in Arabidopsis. Plant Cell 2015, 27, 2261–2272. [Google Scholar] [CrossRef]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Zhong, R.; Tang, J.; Pervaiz, T.; Zhou, S.; Liu, J.; Wang, B.; Jia, H. Brassinolide and Gibberellin Promote Grape Fruit Development and Quality. Sci. Hortic. 2024, 338, 113619. [Google Scholar] [CrossRef]
- Singh Thapliyal, V.; Rai, P.N.; Bora, L. Influence of Pre-Harvest. Application of Gibberellin and Brassinosteroid on Fruit. Growth and Quality Characteristics of Pear (Pyrus Pyrifolia (Burm.) Nakai) Cv. Gola. J. App. Nat. Sci. 2016, 8, 2305–2310. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Qi, Q.; Li, H.; Li, Z.; Chen, S.; Ding, Q.; Wang, Q.; Yan, Z.; Gai, Y.; et al. Gibberellins Play a Role in Regulating Tomato Fruit Ripening. Plant Cell Physiol. 2019, 60, 1619–1629. [Google Scholar] [CrossRef]
- Wu, M.; Liu, K.; Li, H.; Li, Y.; Zhu, Y.; Su, D.; Zhang, Y.; Deng, H.; Wang, Y.; Liu, M. Gibberellins Involved in Fruit Ripening and Softening by Mediating Multiple Hormonal Signals in Tomato. Hortic. Res. 2024, 11, uhad275. [Google Scholar] [CrossRef] [PubMed]
- Barro-Trastoy, D.; Carrera, E.; Baños, J.; Palau-Rodríguez, J.; Ruiz-Rivero, O.; Tornero, P.; Alonso, J.M.; López-Díaz, I.; Gómez, M.D.; Pérez-Amador, M.A. Regulation of Ovule Initiation by Gibberellins and Brassinosteroids in Tomato and Arabidopsis: Two Plant Species, Two Molecular Mechanisms. Plant J. 2020, 102, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Burbidge, C.A.; Boss, P.K.; Davies, C. Changes in Transcription of Cytokinin Metabolism and Signalling Genes in Grape (Vitis Vinifera L.) Berries Are Associated with the Ripening-Related Increase in Isopentenyladenine. BMC Plant Biol. 2015, 15, 223. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The Regulation of Ethylene Biosynthesis: A Complex Multilevel Control Circuitry. New Phy. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Ji, Y.; Qu, Y.; Jiang, Z.; Yan, J.; Chu, J.; Xu, M.; Su, X.; Yuan, H.; Wang, A. The Mechanism for Brassinosteroids Suppressing Climacteric Fruit Ripening. Plant Physiol. 2021, 185, 1875–1893. [Google Scholar] [CrossRef]
- Guo, Y.F.; Shan, W.; Liang, S.M.; Wu, C.J.; Wei, W.; Chen, J.Y.; Lu, W.J.; Kuang, J.F. MaBZR1/2 Act as Transcriptional Repressors of Ethylene Biosynthetic Genes in Banana Fruit. Physiol. Plant 2019, 165, 555–568. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.; Qin, G.; Tian, S. Effects of Brassinosteroids on Postharvest Disease and Senescence of Jujube Fruit in Storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Trainotti, L.; Pavanello, A.; Casadoro, G. Different Ethylene Receptors Show an Increased Expression during the Ripening of Strawberries: Does Such an Increment Imply a Role for Ethylene in the Ripening of These Non-Climacteric Fruits? J. Exp. Bot. 2005, 56, 2037–2046. [Google Scholar] [CrossRef]
- Iannetta, P.P.M.; Laarhoven, L.J.; Medina-Escobar, N.; James, E.K.; McManus, M.T.; Davies, H.V.; Harren, F.J.M. Ethylene and Carbon Dioxide Production by Developing Strawberries Show a Correlative Pattern That Is Indicative of Ripening Climacteric Fruit. Physiol. Plant 2006, 127, 247–259. [Google Scholar] [CrossRef]
- Sun, J.H.; Luo, J.J.; Tian, L.; Li, C.L.; Xing, Y.; Shen, Y.Y. New Evidence for the Role of Ethylene in Strawberry Fruit Ripening. J. Plant Growth Regul. 2013, 32, 461–470. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Shang, Y.; Song, M.; Ma, H. Identification and Characterization of Auxin Response Factor (ARF) Family Members Involved in Fig (Ficus carica L.) Fruit Development. PeerJ 2022, 10, e13798. [Google Scholar] [CrossRef] [PubMed]
- Alferez, F.; Christopher, V.; Vashisth, T. Update on brassinosteroids for HLB Management. Citrus Ind. 2019, 19, 16–18. [Google Scholar]
- Bai, Q.; Huang, Y.; Shen, Y. The Physiological and Molecular Mechanism of Abscisic Acid in Regulation of Fleshy Fruit Ripening. Front. Plant Sci. 2021, 11, 619953. [Google Scholar] [CrossRef]
- Chen, P.; Sun, Y.F.; Kai, W.B.; Liang, B.; Zhang, Y.S.; Zhai, X.W.; Jiang, L.; Du, Y.W.; Leng, P. Interactions of ABA Signaling Core Components (SlPYLs, SlPP2Cs, and SlSnRK2s) in Tomato (Solanum Lycopersicon). J. Plant Physiol. 2016, 205, 67–74. [Google Scholar] [CrossRef]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid Signaling Positively Regulates Abscisic Acid Biosynthesis in Response to Chilling Stress in Tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T.; Rodrigo, M.J. A Sweet Orange Mutant Impaired in Carotenoid Biosynthesis and Reduced ABA Levels Results in Altered Molecular Responses along Peel Ripening. Sci. Rep. 2019, 9, 9813. [Google Scholar] [CrossRef]

| Crop | Effect of BR Application | Time of Application | Reference |
|---|---|---|---|
| Climacteric | |||
| Passion fruit | Increase in number of fruits, increase in soluble solids content | At flower anthesis | [38] |
| Mango | Increase in fruit set and quality in drought stress conditions Fruit softening, increase in sugars | Flower bud induction and differentiation, full bloom, and beginning of fruit set Hard mature fruit stage | [42,58] |
| Apricot | Increase in fruit set, yield, total soluble sugars, and vitamin C | At swelling bud stage, at the balloon stage, just after fruit set, and one month before harvest | [43] |
| Persimmon | Fruit softening, increase in sugars | At preclimacteric stage | [56] |
| Galaxy apple | Increase in soluble solids, color, and antioxidant activities | Every 15–21 days, starting 40 days after full bloom | [55] |
| Peach | Delay of fruit softening | After harvest | [59] |
| Non-climacteric | |||
| Litchi | Fruit cracking reduction | Before flower anthesis | [37] |
| Navel oranges | Increase in fruit set, delay in organ abscission | At flower anthesis and 25 days afterward | [39] |
| Blood oranges | Increase in anthocyanin content, delay in sugar decrease during storage | After harvest | [41] |
| Strawberry | Accelerate maturation Increase in sugars, growth acceleration | Big-green stage 15 days after anthesis (fast fruit growing stage) and at the end of maturation | [44,45] |
| Grape berries | Accelerate maturation. Sugar accumulation | Onset of color change | [46] |
| Sweet cherries | Reduction in chilling injury, fruit firmness is maintained Increase in fruit size and anthocyanin content | After harvest Swollen bud stage | [54,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryal, D.; Alferez, F. Brassinosteroids: Biosynthesis, Signaling, and Hormonal Crosstalk as Related to Fruit Yield and Quality. Plants 2025, 14, 1865. https://doi.org/10.3390/plants14121865
Aryal D, Alferez F. Brassinosteroids: Biosynthesis, Signaling, and Hormonal Crosstalk as Related to Fruit Yield and Quality. Plants. 2025; 14(12):1865. https://doi.org/10.3390/plants14121865
Chicago/Turabian StyleAryal, Divya, and Fernando Alferez. 2025. "Brassinosteroids: Biosynthesis, Signaling, and Hormonal Crosstalk as Related to Fruit Yield and Quality" Plants 14, no. 12: 1865. https://doi.org/10.3390/plants14121865
APA StyleAryal, D., & Alferez, F. (2025). Brassinosteroids: Biosynthesis, Signaling, and Hormonal Crosstalk as Related to Fruit Yield and Quality. Plants, 14(12), 1865. https://doi.org/10.3390/plants14121865





