The Molecular Breeding of Different Ecotype Japonica Varieties Resistant to Rice Blast with High Genome Collinearity
Abstract
1. Introduction
2. Results
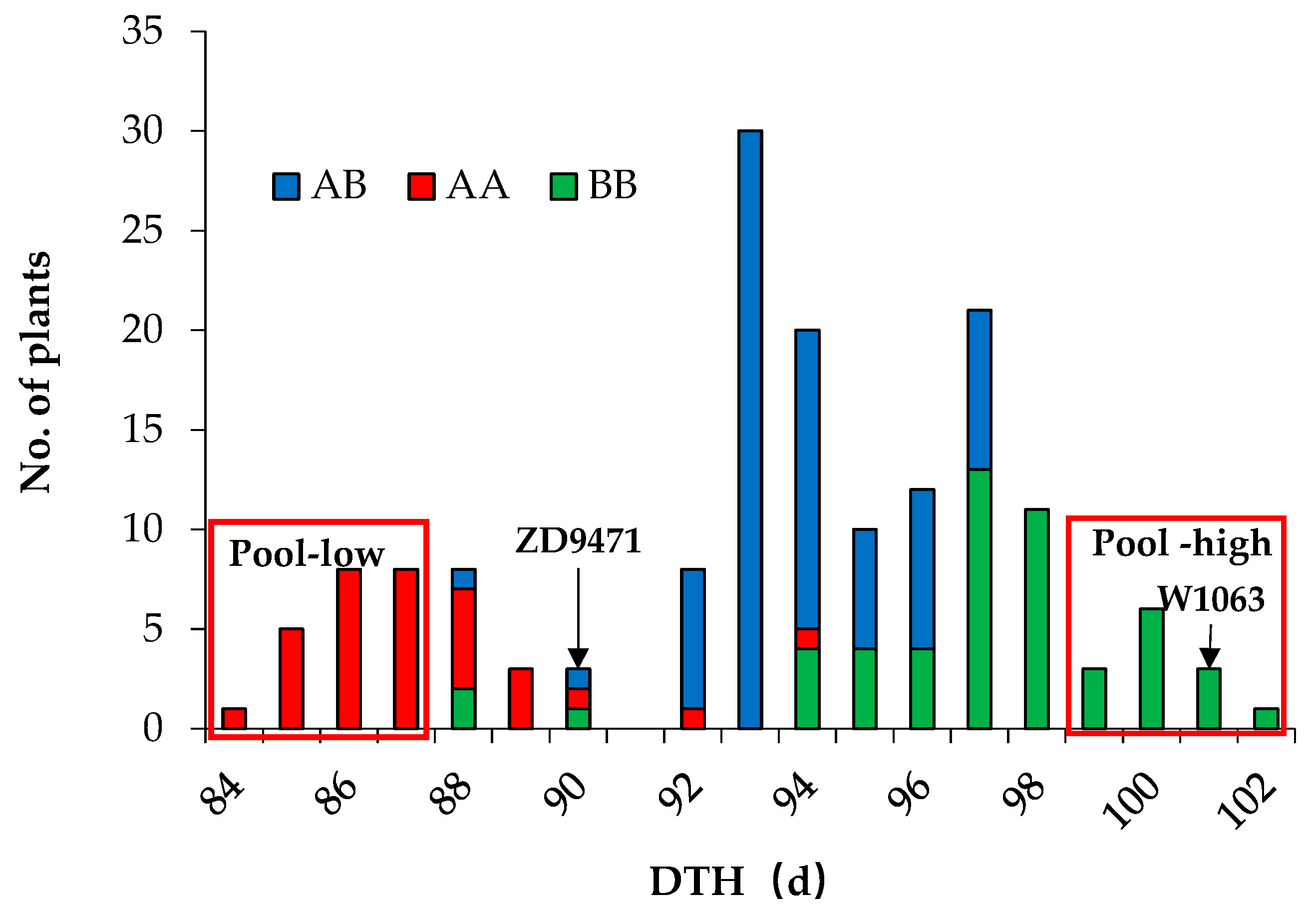
2.1. Hd1 Leads to Significant Differences in DTH
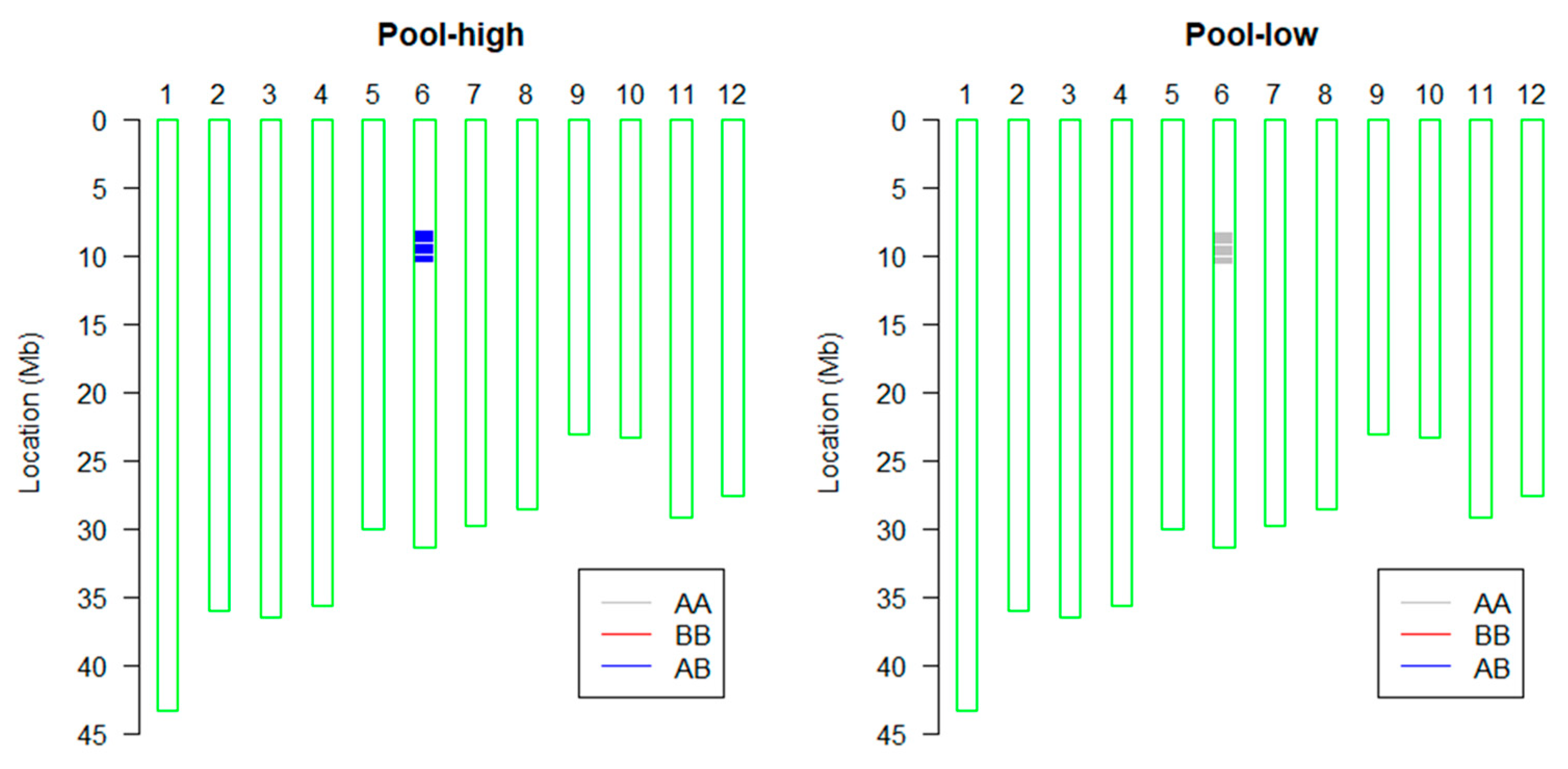
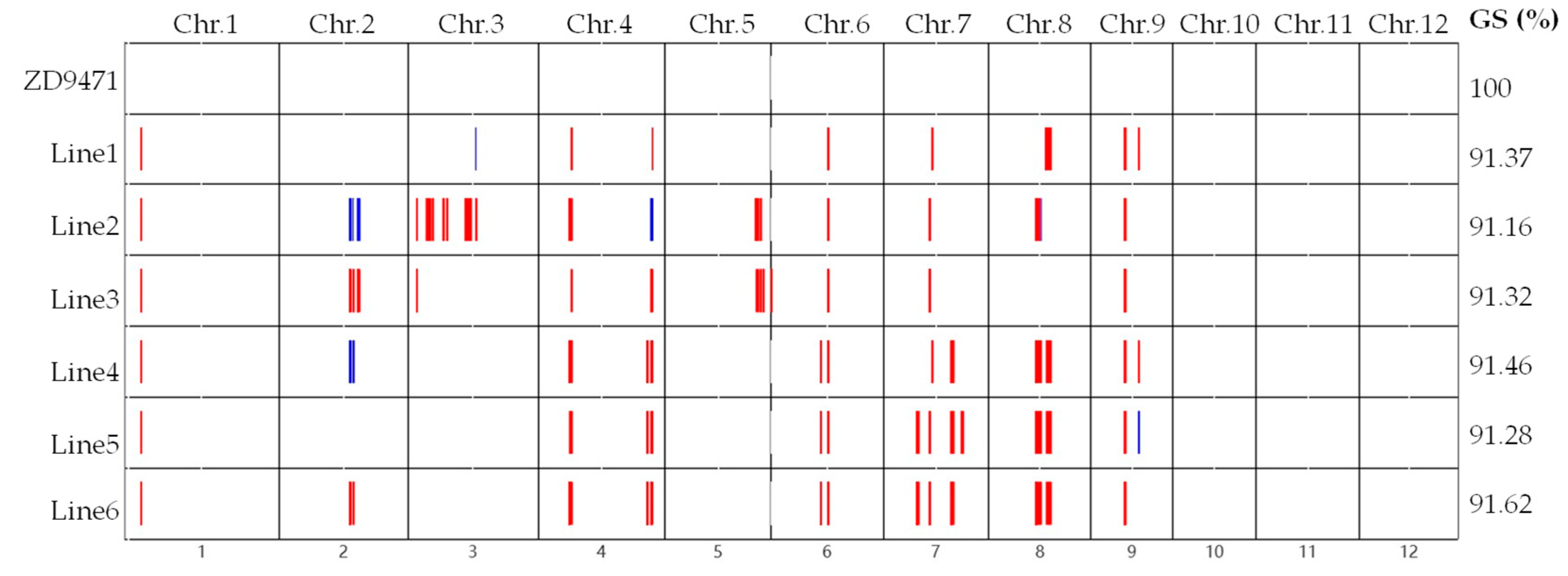
2.2. The Obtaining of Introgression Lines
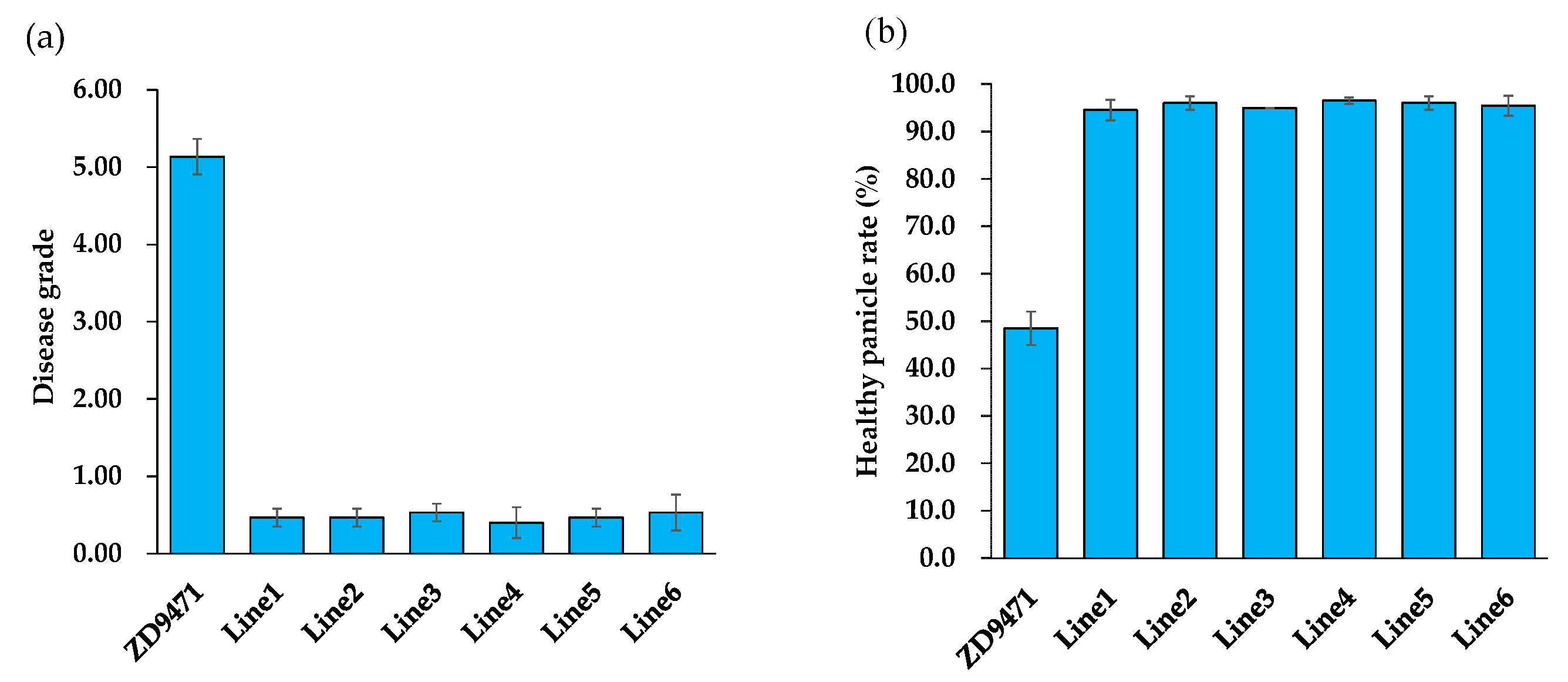
2.3. Pigm Significantly Enhances Panicle Blast Resistance
2.4. Agronomic and Quality Traits of Introgression Lines
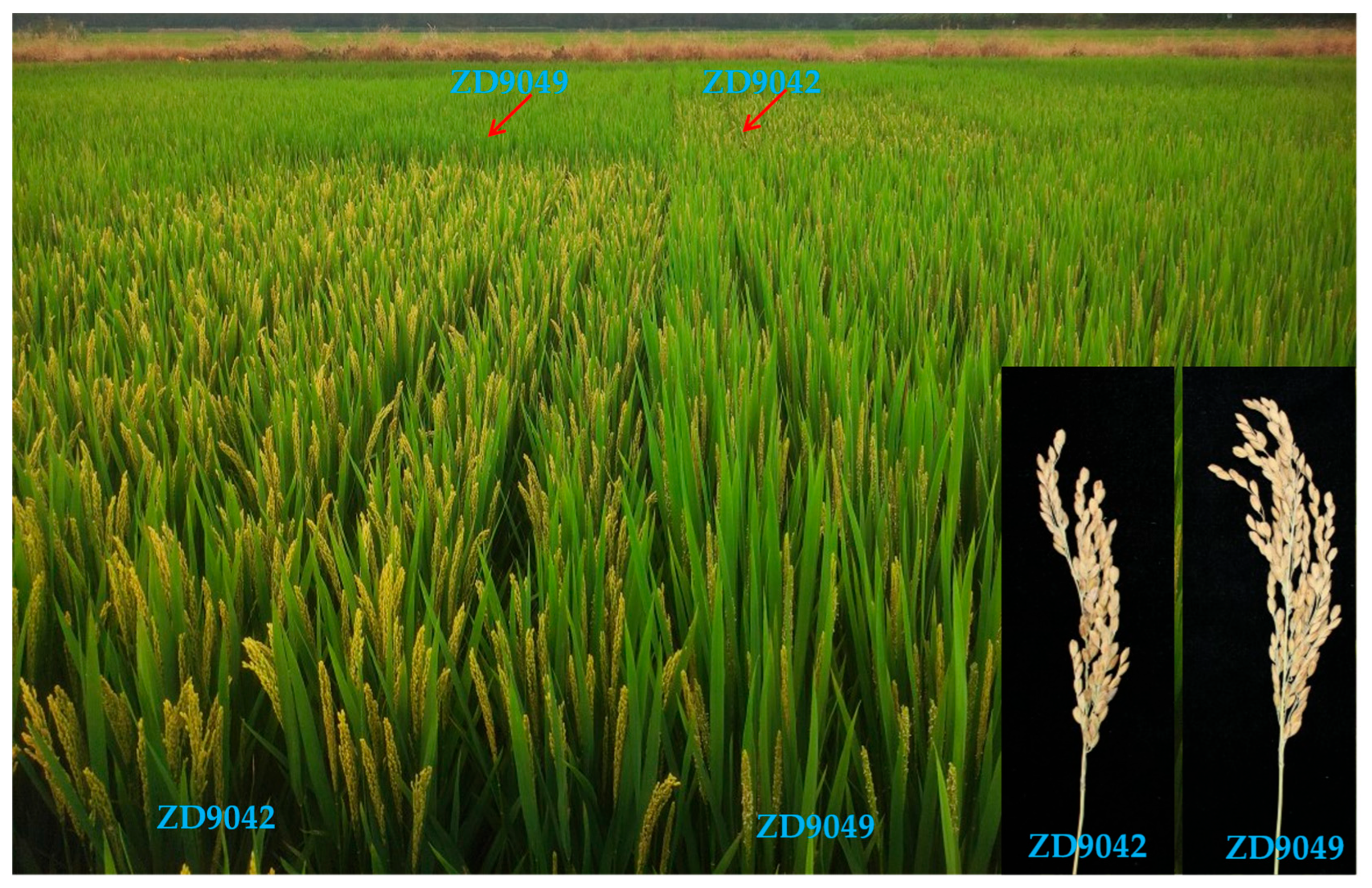
2.5. Two Representative Lines with High Yield, Good Quality, and Rice Blast Resistance Were Officially Approved
3. Discussion
4. Materials and Methods
4.1. Plant Materials and the Breeding Process
4.2. Identification of the DTH Gene
4.3. Sequence Analysis
4.4. Foreground Selection
4.5. Background Screening and Analysis
4.6. The Evaluation of Blast Resistance
4.6.1. Artificial Inoculation
4.6.2. Natural Field Nursey
4.7. Measurement of Yield and Main Agronomic Traits
4.8. Measurement of Main Quality Traits
4.8.1. Processing Quality
4.8.2. Appearance Quality
4.8.3. Physicochemical Properties
4.8.4. Rice Taste Value
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hua, J.; Zhou, N.; Zhang, H.; Huo, Z.; Xu, K.; Wei, H.; Gao, H.; Guo, B.; Dai, Q.; Zhou, P.; et al. Situation and strategies of Japonica rice production and development in southern China. China Rice 2014, 20, 5–11, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, D.; Ma, Z.; Chen, X.; Zhang, M.; Zhang, C.; Liu, G.; Wei, H.; Zhang, H. Differences in eating quality attributes between japonica rice from the northeast region and semi-glutinous japonica rice from the Yangtze river delta of China. Foods 2021, 10, 2770. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, K.; Zhou, F.; Zhang, L.; Hu, Z.; Cao, L.; Wu, S. Identification and resistance evaluation of rice blast resistance genes in japonica rice germplasm in Yangtze river delta. J. Nucl. Agri. Sci. 2022, 36, 0014–0023, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Qi, Z.; Du, Y.; Yu, J.; Zhang, R.; Yu, M.; Cao, H.; Song, T.; Pan, X.; Liang, D.; Liu, Y. Molecular detection and analysis of blast resistance genes in rice main varieties in Jiangsu province, China. Agronomy 2023, 13, 157. [Google Scholar] [CrossRef]
- Xu, M. Application status, problems and development strategies of high quality rice varieties in Jiangsu Province. Chin. Rice 2020, 26, 57–60, (In Chinese with English Abstract). [Google Scholar]
- Qi, Z.; Yu, J.; Zhang, R.; Yu, M.; Du, Y.; Cao, H.; Song, T.; Pan, X.; Liang, D.; Liu, Y. Identification and evaluation of resistance of new rice cultivars (lines) and main cultivars to rice blast in Jiangsu province from 2016 to 2020. Jiangsu Agric. Sci. 2022, 50, 91–96, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xiao, N.; Wu, Y.; Li, A. Strategy for use of rice blast resistance genes in rice molecular breeding. Rice Sci. 2020, 27, 263–277. [Google Scholar] [CrossRef]
- Li, W.; Chern, M.; Yin, J.; Wang, J.; Chen, X. Recent advances in broad-spectrum resistance to the rice blast disease. Curr. Opin. Plant. Biol. 2019, 50, 114–120. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Jin, Y.; He, N.; Cheng, Z.; Lin, S.; Huang, F.; Wang, W.; Li, Q.; Yang, D. Resistance spectrum analysis and breeding utilization of rice blast resistance gene Pigm-1. Plants 2025, 14, 535. [Google Scholar] [CrossRef]
- Yu, M.; Dai, Z.; Pan, C.; Chen, X.; Yu, L.; Zhang, X.; Li, Y.; Xiao, N.; Gong, H.; Sheng, S.; et al. Resistance spectrum difference between two broad-spectrum blast resistance genes, Pigm and Pi2, and their interaction effect on Pi1. Aata Agron. Sin. 2013, 39, 1927–1934, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, N.; Yu, L.; Pan, C.; Li, Y.; Zhang, X.; Liu, G.; Dai, Z.; Pan, X.; Li, A. Combination patterns of major R genes determine the level of resistance to the M. oryzae in rice (Oryza sativa L.). PLoS ONE 2015, 10, e0126130. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, N.; Chen, Y.; Yu, L.; Pan, C.; Li, Y.; Zhang, X.; Huang, N.; Ji, H.; Dai, Z.; et al. Comprehensive evaluation of resistance effects of pyramiding lines with different broad-spectrum resistance genes against Magnaporthe oryzae in rice (Oryza sativa L.). Rice 2019, 12, 11. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Xu, Z.; Gao, P.; Wu, Y.; Wu, K.; Zhao, J.; Wang, X.; Wang, J.; Li, M.; et al. Development of rice variety with durable and broad-spectrum resistance to blast disease through marker-assisted introduction of Pigm gene. Front. Plant Sci. 2022, 13, 937767. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, M.; Wang, X.; Xu, Z.; Wu, K.; Sun, Q.; Du, H.; Younas, M.; Zhang, Y.; Feng, Z.; et al. Identification of elite R-gene combinations against blast disease in japonica rice varieties. Int. J. Mol. Sci. 2023, 24, 3984. [Google Scholar] [CrossRef]
- Sun, C.; Chen, D.; Fang, J.; Wang, P.; Deng, X.; Chu, C. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways. Protein Cell 2014, 5, 889–898. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, S.; Cui, S.; Hou, H.; Wu, H.; Hao, B.; Cai, L.; Xu, Z.; Liu, L.; Jiang, L.; et al. Transcriptional and post-transcriptional regulation of heading date in rice. New Phytol. 2021, 230, 943–956. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.C.; Yan, W.H.; Zhang, Z.Y.; Lu, L.; Han, Z.M.; Zhao, H.; Liu, H.Y.; Song, P.; Hu, Y.; et al. Combinations of the Ghd7, Ghd8 and Hd1 genes largely define the ecogeographical adaptation and yield potential of cultivated rice. New Phytol. 2015, 208, 1056–1066. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, Y.J.; Wang, S.L.; Fan, Y.Y.; Zhuang, J.Y. Genetic interaction of Hd1 with Ghd7, DTH8 and Hd2 largely determines Eco-geographical adaption of rice varieties in Southern China. Rice Sci. 2021, 28, 114–118. [Google Scholar] [CrossRef]
- Leng, Y.; Gao, Y.; Chen, L.; Yang, Y.; Huang, L.; Dai, L.; Ren, D.; Xu, Q.; Zhang, Y.; Ponce, K.; et al. Heading date 1 preponderant alleles from indica cultivars to breed high-yield, high-quality japonica rice varieties for cultivation in south China. Plant Biotechnol. J. 2020, 18, 119–128. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, L.; Xu, J.; Liu, X.; Liu, S.; Zhai, H.; Wan, J. The distribution of japonica rice cultivars in the lower region of the Yangtze river valley is determined by its photoperiod-sensitivity and heading date genotypes. J. Integr. Plant Biol. 2009, 51, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhu, Y.; Chen, Z.; Zhang, H. Yield characteristics of japonica/indica hybrids rice in the middle and lower reaches of the Yangtze river in China. J. Integr. Agric. 2020, 19, 2394–2406. [Google Scholar] [CrossRef]
- Xiao, N.; Pan, C.; Li, Y.; Wu, Y.; Cai, Y.; Lu, Y.; Wang, R.; Yu, L.; Shi, W.; Kang, H.; et al. Genomic insight into balancing high yield, good quality, and blast resistance of japonica rice. Genome Biol. 2021, 22, 283. [Google Scholar] [CrossRef]
- Qian, Q.; Guo, L.; Smith, S.; Li, J. Breeding high-yield superior quality hybrid super rice by rational design. Natl. Sci. Rev. 2016, 39, 283–294. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, W.; Wang, G. Balancing immunity and yield in crop plants. Trends Plant Sci. 2017, 22, 1069–1079. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2483. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhou, C.; Zhao, Y.; Zhou, S.L.; Wang, W.T.; Zhou, D.X. The rice enhancer of zeste [E(z)] genes SDG711 and SDG718 are respectively involved in long day and short day signaling to mediate the accurate photoperiod control of flowering time. Front. Plant Sci. 2014, 5, 591. [Google Scholar] [CrossRef]
- Peng, P.; Jiang, H.; Luo, L.; Ye, C.; Xiao, Y. Pyramiding of multiple genes to improve rice blast resistance of photo-thermo sensitive male sterile line, without yield penalty in hybrid rice production. Plants 2023, 12, 1389. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.E.; Yang, L.Y.; Zhang, Z.G. One stone for multiple birds: PigmR integrates multiple defense pathways for high and broad-spectrum blast resistance in rice. Stress Biol. 2025, 5, 22. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Chen, Y.; Pan, C.H.; Xiao, N.; Yu, L.; Li, Y.H.; Zhang, X.X.; Pan, X.B.A.; Chen, X.J.; Liang, C.Z.; et al. Development and evaluation of near-isogenic lines with different blast resistance alleles at the Piz locus in japonica rice from the lower region of the Yangtze River, China. Plant Dis. 2017, 101, 1283–1291. [Google Scholar] [CrossRef]
- Wang, K.; Fu, C.J.; Fu, X.X.; Qin, P.; Hu, X.C.; Zhang, X.W.; Deng, Z.; Yan, T.Z.; Jiang, N.; Li, Y.F.; et al. Enhancing the blast resistance of an elite thermo-sensitive genic male sterile line (TGMS) Longke638S and its derived hybrid varieties by incorporating Pigm gene. Mol. Breed. 2025, 45, 35. [Google Scholar] [CrossRef] [PubMed]
- Monna, L.; Lin, H.; Kojima, S.; Sasaki, T.; Yano, M. Genetic dissection of a genomic region for a quantitative trait locus, Hd3, into two loci, Hd3a and Hd3b, controlling heading date in rice. Theor. Appl. Genet. 2002, 104, 772–778. [Google Scholar] [CrossRef]
- Matsubara, K.; Ogiso-Tanaka, E.; Hori, K.; Ebana, K.; Ando, T.; Yano, M. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant. Cell. Physiol. 2012, 53, 709–716. [Google Scholar] [CrossRef]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef]
- Gao, Z.; Zeng, D.; Cheng, F.; Tian, Z.; Guo, L.; Su, Y.; Yan, M.; Jiang, H.; Dong, G.; Huang, Y.; et al. ALK, the key gene for gelatinization temperature, is a modifier gene for gel consistency in rice. J. Integr. Plant Biol. 2011, 53, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, X.; Cheng, K.; Du, H.; Ouyang, Y.; Chen, J.; Qiu, S.; Huang, J.; Jiang, Y.; Jiang, L.; et al. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 2012, 337, 1336–1340. [Google Scholar] [CrossRef]
- NY/T 1433-2014; Ministry of Agriculture of the People’s Republic of China. Protocol for Identification of Rice Varieties—SSR Marker Method. China Standards Press: Beijing, China, 2014; pp. 1–17. (In Chinese)
- Lu, J.; Jiang, Z.; Chen, J.; Xie, M.; Huang, W.; Li, J.; Zhuang, C.; Liu, Z.; Zheng, S. SET DOMAIN GROUP 711-mediated H3K27me3 methylation of cytokinin metabolism genes regulates organ size in rice. Plant Physiol. 2023, 194, 2069–2085. [Google Scholar] [CrossRef]
- Zeng, S.; Li, C.; Du, C.; Sun, L.; Jing, D.; Lin, T.; Yu, B.; Qian, H.; Yao, W.; Zhou, Y.; et al. Development of specific markers for Pigm in marker-assisted breeding of panicle blast resistant japonica rice. Chin. J. Rice Sci. 2018, 32, 453–461, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- DB32/T1123-2007; Examining Rules for Identification Method and Evaluating Standard of Resistance of Rice Cultivars (Lines) to Rice Blast. Jiangsu Provincial Bureau of Quality and Technical Supervision: Nanjing, China, 2007; pp. 1–8. (In Chinese)
- NY/T 2646-2014; Ministry of Agriculture of the People’s Republic of China. Technical Specification for Identification and Evaluation of Blast Resistance in Rice Variety Regional Test. China Standards Press: Beijing, China, 2014; pp. 1–10. (In Chinese)
- NY/T 2639-2014; Ministry of Agriculture of the People’s Republic of China. Determination of Amylose Content in Rice-Spectrophotometry Method. China Standards Press: Beijing, China, 2014; pp. 1–4. (In Chinese)
- Yang, Y.; Guo, M.; Sun, S.; Zou, Y.; Yin, S.; Liu, Y.; Tang, S.; Gu, M.; Yang, Z.; Yan, C. Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat. Commun. 2019, 10, 1949. [Google Scholar] [CrossRef]


| Line | Genotype | Main Agronomic Traits in 2019 Hainan | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DTH (d) | PH (cm) | PN | PL (cm) | GNP | SSR (%) | TGW (g) | YPP (g) | ||
| ZD9471 | Hd1H8 | 83.67 ± 0.58 a | 83.60 ± 0.40 a | 7.20 ± 0.17 a | 14.56 ± 0.20 a | 114.64 ± 5.10 b | 87.68 ± 2.19 a | 25.32 ± 0.23 a | 23.63 ± 0.72 b |
| Line1 | Pigm/Hd1H8 | 84.67 ± 0.58 a | 84.20 ± 0.82 a | 8.20 ± 1.32 a | 14.78 ± 0.30 a | 138.43 ± 7.09 a | 87.35 ± 1.43 a | 25.46 ± 0.38 a | 26.71 ± 0.45 a |
| Line2 | Pigm/Hd1H8 | 85.00 ± 1.00 a | 83.73 ± 0.50 a | 7.80 ± 0.95 a | 14.45 ± 0.41 a | 129.79 ± 7.04 ab | 87.94 ± 1.84 a | 25.45 ± 0.36 a | 26.72 ± 0.89 a |
| Line3 | Pigm/Hd1H8 | 85.67 ± 0.58 a | 83.10 ± 0.95 a | 8.20 ± 1.01 a | 14.54 ± 0.43 a | 132.32 ± 6.47 a | 87.25 ± 0.54 a | 25.41 ± 0.33 a | 26.68 ± 0.57 a |
| Line4 | Pigm/Hd1H13 | 85.67 ± 0.58 a | 83.40 ± 0.72 a | 8.00 ± 0.62 a | 15.07 ± 0.27 a | 129.62 ± 5.25 ab | 87.55 ± 2.01 a | 25.28 ± 0.33 a | 26.90 ± 0.33 a |
| Line5 | Pigm/Hd1H13 | 85.67 ± 0.58 a | 83.20 ± 0.36 a | 7.80 ± 0.60 a | 14.86 ± 0.18 a | 131.66 ± 7.35 ab | 87.09 ± 0.96 a | 25.26 ± 0.26 a | 26.47 ± 0.82 a |
| Line6 | Pigm/Hd1H13 | 85.00 ± 1.00 a | 83.37 ± 0.70 a | 7.60 ± 1.08 a | 14.83 ± 0.32 a | 130.98 ± 5.03 ab | 87.48 ± 1.17 a | 25.32 ± 0.49 a | 26.76 ± 0.28 a |
| Genotype | Main Agronomic Traits in 2020 Jiangsu | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DTH (d) | PH (cm) | PN | PL (cm) | GNP | SSR (%) | TGW (g) | YPP (g) | ||
| ZD9471 | Hd1H8 | 85.67 ± 0.58 b | 83.60 ± 0.40 b | 8.00 ± 0.95 a | 16.72 ± 0.20 b | 147.02 ± 6.51 b | 91.18 ± 1.64 a | 24.38 ± 0.27 a | 28.22 ± 0.40 c |
| Line1 | Pigm/Hd1H8 | 85.67 ± 0.58 b | 83.07 ± 0.25 b | 8.27 ± 0.95 a | 17.06 ± 0.20 b | 165.24 ± 6.21 b | 91.65 ± 1.51 a | 24.45 ± 0.21 a | 33.04 ± 2.31 c |
| Line2 | Pigm/Hd1H8 | 86.00 ± 1.00 b | 83.33 ± 0.42 b | 8.27 ± 1.36 a | 16.92 ± 0.10 b | 159.77 ± 5.11 b | 91.20 ± 1.22 a | 24.51 ± 0.17 a | 32.42 ± 2.42 c |
| Line3 | Pigm/Hd1H8 | 86.67 ± 0.58 b | 83.07 ± 0.55 b | 7.93 ± 0.96 a | 17.06 ± 0.41 b | 164.93 ± 6.73 b | 91.23 ± 0.31 a | 24.52 ± 0.20 a | 34.82 ± 4.66 bc |
| Line4 | Pigm/Hd1H13 | 95.33 ± 0.58 a | 95.20 ± 0.36 a | 8.00 ± 0.85 a | 17.90 ± 0.12 a | 194.74 ± 5.19 a | 91.88 ± 1.09 a | 24.18 ± 0.08 a | 43.77 ± 4.88 a |
| Line5 | Pigm/Hd1H13 | 95.67 ± 0.58 a | 95.27 ± 0.64 a | 8.07 ± 1.03 a | 17.91 ± 0.32 a | 198.73 ± 7.75 a | 91.93 ± 1.63 a | 24.18 ± 0.13 a | 43.87 ± 1.59 a |
| Line6 | Pigm/Hd1H13 | 96.33 ± 0.58 a | 94.77 ± 0.35 a | 7.83 ± 0.84 a | 17.88 ± 0.22 a | 198.63 ± 8.39 a | 91.41 ± 1.46 a | 24.21 ± 0.30 a | 42.60 ± 2.53 ab |
| Line | Genotype | Main Quality Characteristics in 2020 Jiangsu | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRR (%) | HRR (%) | CGP (%) | CD (%) | AC (%) | PC (%) | AS | TS | CTV | ||
| ZD9471 | Hd1H8 | 81.95 ± 0.28 ab | 69.39 ± 0.22 a | 18.42 ± 0.42 a | 5.23 ± 0.15 a | 14.92 ± 0.12 a | 10.50 ± 0.30 a | 7.73 ± 0.06 b | 8.13 ± 0.06 b | 73.87 ± 0.06 b |
| Line1 | Pigm/Hd1H8 | 81.97 ± 0.40 ab | 69.15 ± 0.39 ab | 16.25 ± 0.16 b | 4.61 ± 0.32 b | 14.84 ± 0.15 a | 10.35 ± 0.17 a | 7.67 ± 0.06 b | 8.20 ± 0.06 b | 74.07 ± 0.06 b |
| Line2 | Pigm/Hd1H8 | 82.02 ± 0.41 a | 69.20 ± 0.40 ab | 16.43 ± 0.18 b | 4.72 ± 0.28 ab | 14.77 ± 0.08 a | 10.31 ± 0.19 a | 7.67 ± 0.06 b | 8.17 ± 0.06 b | 74.00 ± 0.10 b |
| Line3 | Pigm/Hd1H8 | 81.94 ± 0.53 ab | 69.16 ± 0.91 ab | 16.44 ± 0.13 b | 4.69 ± 0.25 ab | 14.80 ± 0.11 a | 10.23 ± 0.13 a | 7.67 ± 0.06 b | 8.20 ± 0.00 b | 74.03 ± 0.12 b |
| Line4 | Pigm/Hd1H13 | 80.93 ± 0.46 b | 67.46 ± 0.26 c | 7.75 ± 0.26 c | 2.23 ± 0.13 c | 13.96 ± 0.06 b | 8.94 ± 0.05 b | 9.33 ± 0.06 a | 9.47 ± 0.06 a | 80.40 ± 0.20 a |
| Line5 | Pigm/Hd1H13 | 80.90 ± 0.25 b | 67.73 ± 0.36 c | 7.12 ± 0.32 cd | 1.98 ± 0.08 c | 13.95 ± 0.09 b | 8.92 ± 0.05 b | 9.37 ± 0.06 a | 9.43 ± 0.06 a | 80.40 ± 0.10 a |
| Line6 | Pigm/Hd1H13 | 81.01 ± 0.29 ab | 67.97 ± 0.47 bc | 6.59 ± 0.34 d | 1.75 ± 0.20 c | 13.95 ± 0.06 b | 8.90 ± 0.06 b | 9.37 ± 0.06 a | 9.43 ± 0.06 a | 80.47 ± 0.12 a |
| Traits | ZD9471 | Zhendao 9042 | Zhendao 9049 | ||
|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | ||
| Growth period (d) | 151.6 | 148.5 | 150.1 | 155.9 | 157.1 |
| Plant height (cm) | 93.5 | 94.6 | 90.4 | 98.1 | 92.6 |
| Panicles number (M/ha) | 2.9 | 3.2 | 3.0 | 2.6 | 2.9 |
| Grain number per panicle | 127.4 | 143.4 | 150.9 | 196.8 | 155.8 |
| Seed setting rate (%) | 88.6 | 87.2 | 88.5 | 92.3 | 94.5 |
| 1000-grain weight (g) | 25.3 | 26.0 | 25.9 | 25.6 | 25.8 |
| Yield (T/ha) | 9.9 | 10.1 | 9.7 | 11.0 | 10.5 |
| Brown rice rate (%) | 82.2 | 83.8 | 83.3 | 84.4 | 83.0 |
| Head rice rate (%) | 71.1 | 74.2 | 73.3 | 75.2 | 72.9 |
| Chalky grain rate (%) | 17 | 8 | 3 | 17 | 3 |
| Chalkiness degree (%) | 2.4 | 0.5 | 0.3 | 2.3 | 0.3 |
| Amylose content (%) | 15.7 | 16.0 | 17.8 | 14.2 | 18.3 |
| Gel consistency (mm) | 75 | 84 | 75 | 70 | 76 |
| Alkali spreading value | 6 | 7 | 7 | 7 | 7 |
| Quality grade | 2 | 1 | 1 | 2 | 2 |
| Leaf blast resistant index | 0 | 0 | 0 | 0 | 0 |
| Panicle blast resistant index | 5 | 3 | 3 | 1 | 1 |
| Resistance composite index | 4.30 | 4.75 | 3.25 | 1.75 | 2.00 |
| Grading incidence | MS | MS | MR | R | R |
| Marker | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Physical Location (bp) |
|---|---|---|---|
| Pigm-2 | TCTGAATTATTGTGGTCGTG | CCGTTCACATCAGTTTTTCT | 10,366,681–10,366,821 |
| Pigm-4 | ATGCTCGATTCGTTACATTT | CGTCCCACACTTTCTTTTT | 10,436,075–10,436,229 |
| Hd1-SNP | FAM-F1: GAAGGTGACAAGTTCATGCTGGCGAGCGGGTTCGCGGAGTA | TGCTCGCGCACGCCCACACGCTCCGG | 9,336,752–9,336,854 |
| HEX-F2: GAAGGTCGGAGTCAACGGATTGGCGAGCGGGTTCGCGGAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Du, C.; Yang, Y.; Hu, Q.; Li, C.; Feng, F.; Guo, M.; Jing, D.; Lin, T.; Gong, H.; et al. The Molecular Breeding of Different Ecotype Japonica Varieties Resistant to Rice Blast with High Genome Collinearity. Plants 2025, 14, 1836. https://doi.org/10.3390/plants14121836
Zeng S, Du C, Yang Y, Hu Q, Li C, Feng F, Guo M, Jing D, Lin T, Gong H, et al. The Molecular Breeding of Different Ecotype Japonica Varieties Resistant to Rice Blast with High Genome Collinearity. Plants. 2025; 14(12):1836. https://doi.org/10.3390/plants14121836
Chicago/Turabian StyleZeng, Shengyuan, Cancan Du, Yihao Yang, Qingfeng Hu, Chuang Li, Fang Feng, Min Guo, Dedao Jing, Tianzi Lin, Hongbing Gong, and et al. 2025. "The Molecular Breeding of Different Ecotype Japonica Varieties Resistant to Rice Blast with High Genome Collinearity" Plants 14, no. 12: 1836. https://doi.org/10.3390/plants14121836
APA StyleZeng, S., Du, C., Yang, Y., Hu, Q., Li, C., Feng, F., Guo, M., Jing, D., Lin, T., Gong, H., & Yan, C. (2025). The Molecular Breeding of Different Ecotype Japonica Varieties Resistant to Rice Blast with High Genome Collinearity. Plants, 14(12), 1836. https://doi.org/10.3390/plants14121836







