Potato (Solanum tuberosum L.) Cultivars Interact with Wound Healing Period to Modulate Sprout Emergence, Crop Stand, and Productivity
Abstract
1. Introduction
2. Results
2.1. Evaluation of Wound Healing Ability
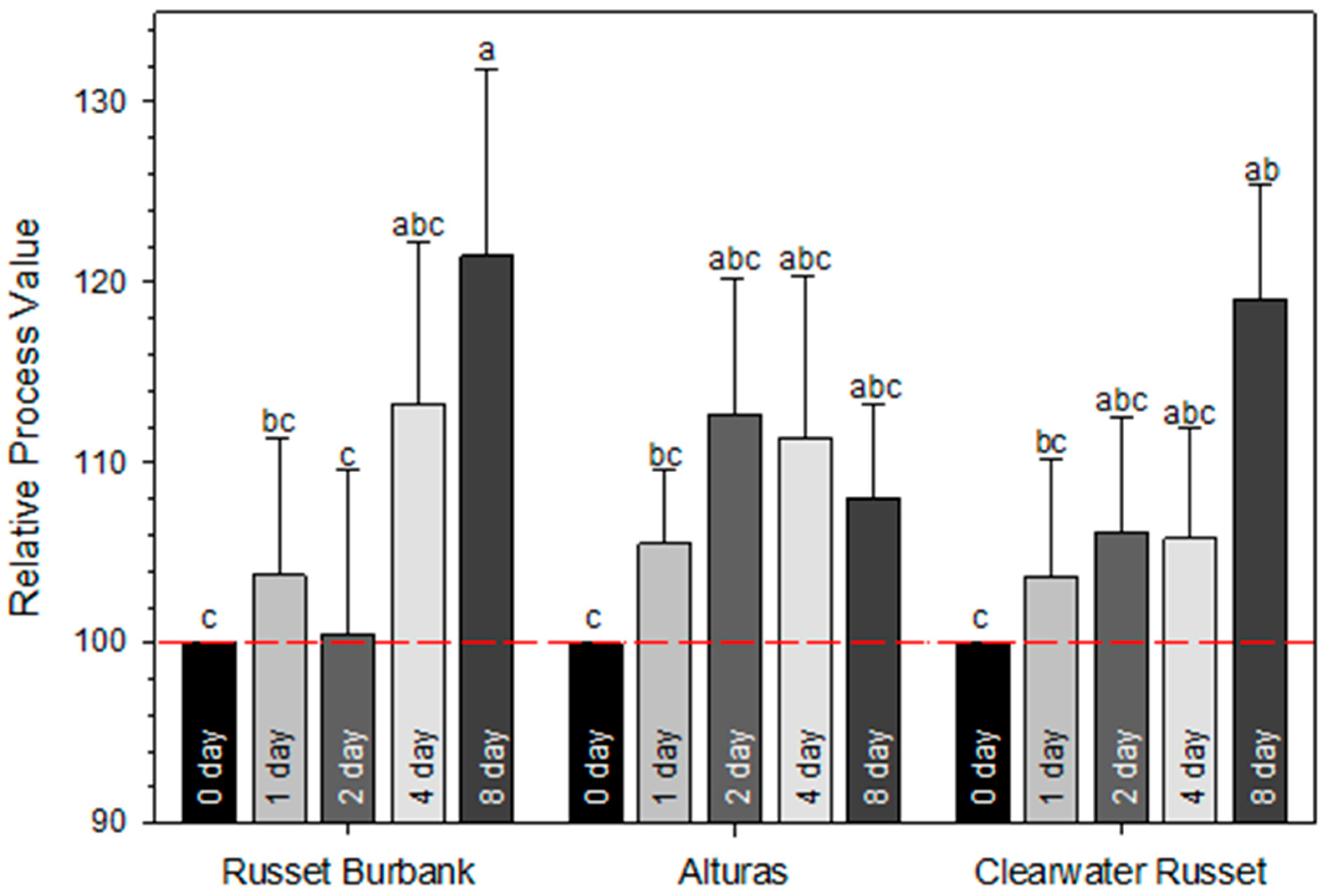
2.2. Expression of Feruloyl Transferase
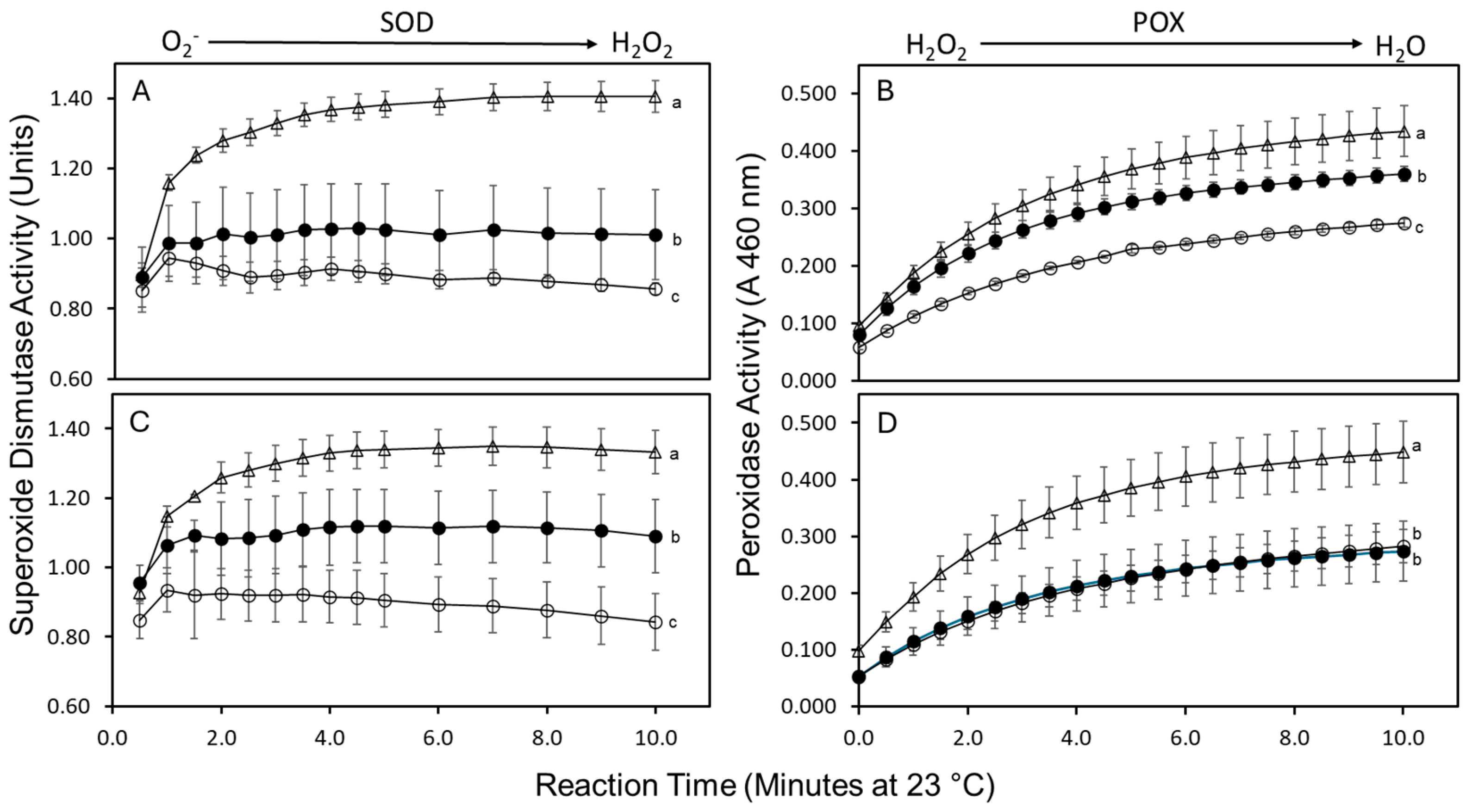
2.3. Superoxide Dismutase and Peroxidase Activities
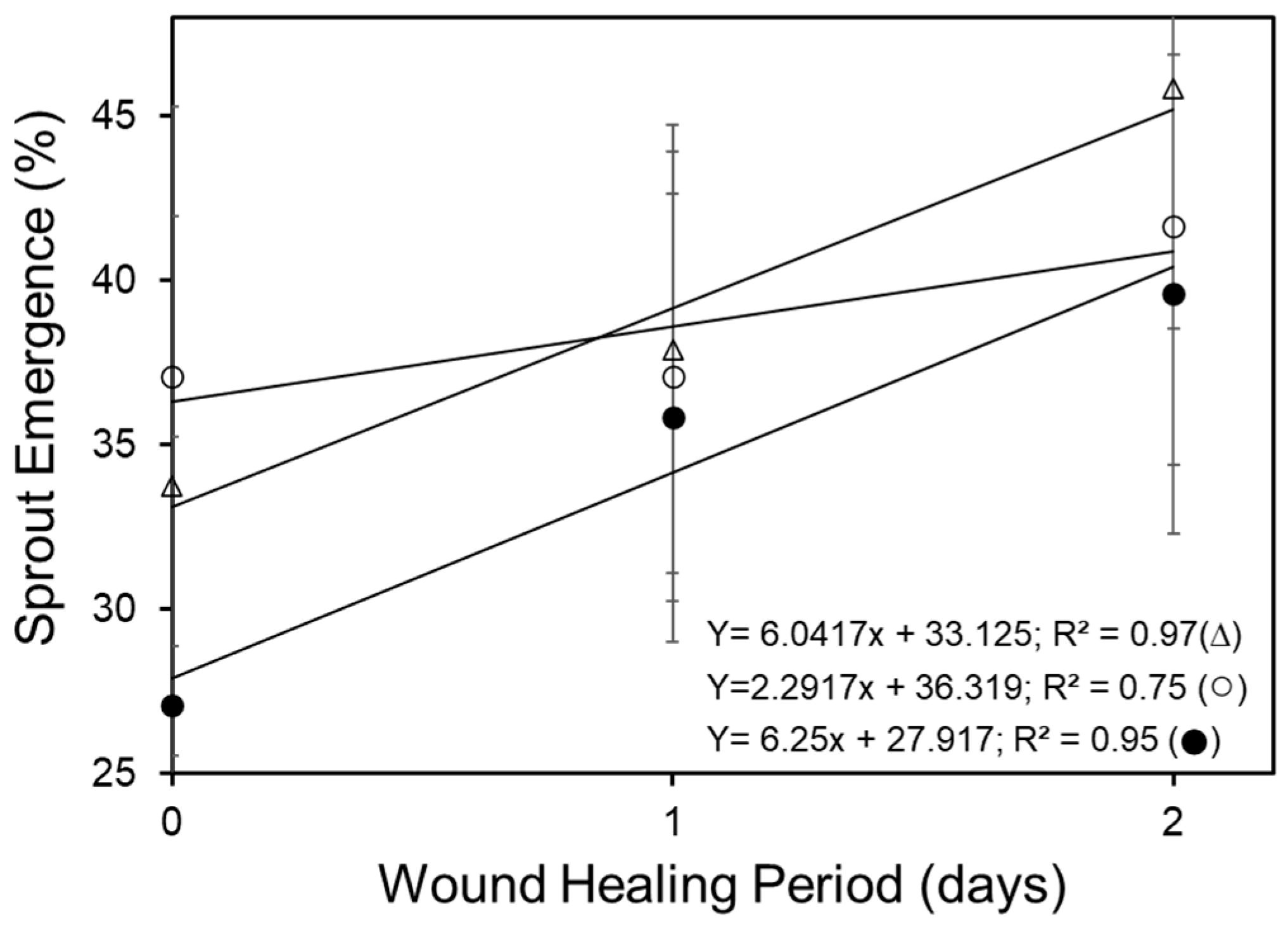
2.4. Sprout Emergence and Crop Stand
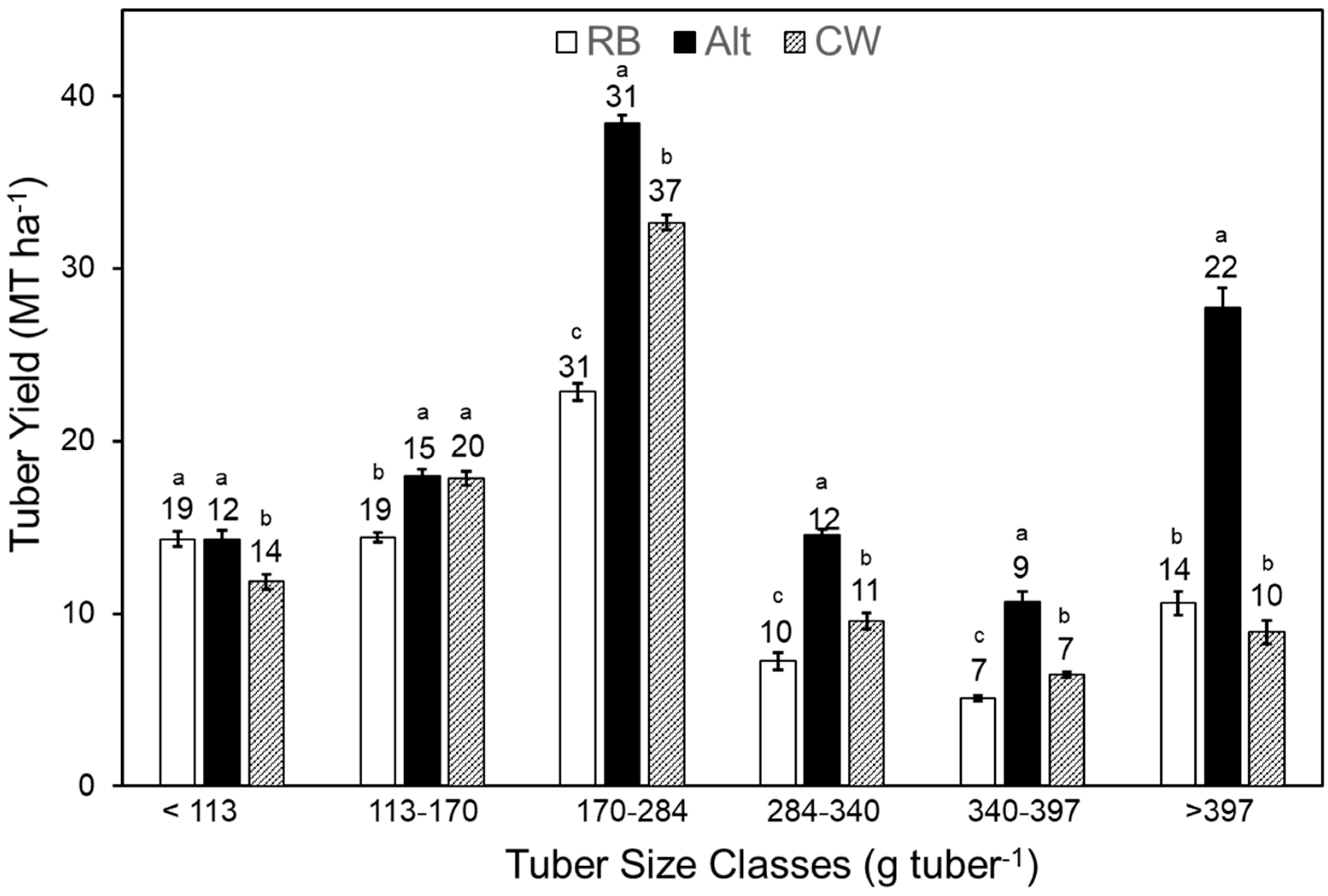
2.5. Total and Marketable Yield
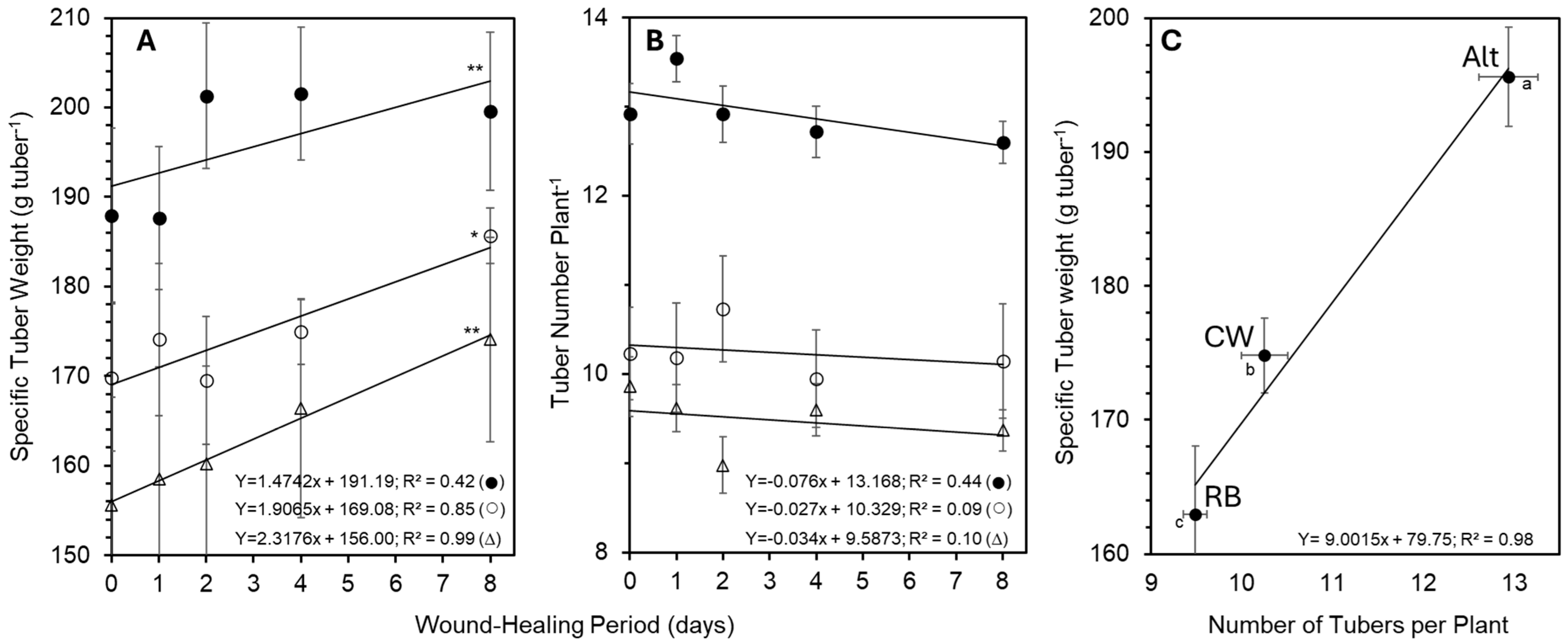
2.6. Tuber Size Distribution, Specific Tuber Weight, and Tuber Count
2.7. Estimation of Returns
3. Discussion
3.1. Cultivars Respond Differentially to Wounding
3.2. Wound Healing Period Affects Emergence and Crop Stand
3.3. Cultivars and Wound Healing Period Modulate Tuber Yield
3.4. Wound Healing Affects Returns
3.5. Cost of Wound Healing
4. Materials and Methods
4.1. Plant Material
4.2. Wound Healing and Field Planting
4.3. Data Acquisition
4.4. Financial Value
4.5. Assessment of Wound Healing Ability
4.6. Superoxide Dismutase and Peroxidase Activities
4.7. Protein Extraction and Immunodetection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alt | Alturas |
| CW | Clearwater Russet |
| RB | Russet Burbank |
| DAP | Days after planting |
| FHT | Feruloyl transferase |
| USA | United States of America |
| PNW | Pacific Northwest |
| ROS | Reactive oxygen species |
| NOX | NADPH Oxidase |
| SOD | Superoxide dismutase |
| POX | Peroxidases |
| SPP | Suberin polyphenolic domain |
| SPA | Suberin polyaliphatic domain |
| RH | Relative humidity |
| G3 | Generation 3 |
| USDA | United States Department of Agriculture |
| US No. 1 | United States Number 1 |
| US No. 2 | United States Number 2 |
| WA | Washington |
| MT | Metric tons |
| ha | Hectare(s) |
| USD | United States Dollars |
| NBT | Nitro blue tetrazolium |
| BCIP | 5-bromo-4-chloro-indolyl-phosphate |
| ANOVA | Analysis of variance |
| STW | Specific tuber weight |
References
- Chase, R.W.; Silva, G.H.; Kitchen, R.B. Pre-cutting of seed potatoes. Amer Potato J. 1989, 66, 723–729. [Google Scholar] [CrossRef]
- Lulai, E.C.; Corsini, D.L. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol. Mol. Plant Path. 1998, 53, 209–222. [Google Scholar] [CrossRef]
- Blauer, J.M. Personal communication. Washington State University: Pullman, WA, USA, 2021. [Google Scholar]
- Razem, F.A.; Bernards, M.A. Hydrogen peroxide is required for poly (phenolic) domain formation during wound-induced suberization. J. Agric. Food Chem. 2002, 50, 1009–1015. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Johnson, D.A. Potato Health Management, 2nd ed.; American Phytopathological Society: St. Paul, MN, USA, 2008. [Google Scholar]
- Serra, O.; Hohn, C.; Franke, R.; Prat, S.; Molinas, M.; Figueras, M. A feruloyl transferase involved in the biosynthesis of suberin and suberin-associated wax is required for maturation and sealing properties of potato periderm. Plant J. 2010, 62, 277–290. [Google Scholar] [CrossRef]
- Kashyap, A.; Jimenez-Jimenez, A.; Figueras, M.; Serra, O.; Valls, M.; Coll, N.S. The tomato feruloyl transferase FHT promoter is an accurate identifier of early development and stress-induced suberization. Plants 2023, 12, 1890. [Google Scholar] [CrossRef] [PubMed]
- Serra, O.; Chatterjee, S.; Figueras, M.; Molinas, M.; Stark, R.E. Deconstructing a plant macromolecular assembly: Chemical architecture, molecular flexibility, and mechanical performance of natural and engineered potato suberins. Biomacromolecules 2014, 15, 799–811. [Google Scholar] [CrossRef]
- Bernards, M.A. Demystifying suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Järvinen, R.; Rauhal, H.; Holopainen, U.; Kallio, H. Differences in suberin content and composition between two varieties of potatoes (Solanum tuberosum L.) and effect of post-harvest storage to the composition. Food. Sci. Technol. 2011, 44, 1355–1361. [Google Scholar] [CrossRef]
- Soliday, C.L.; Kolattukudy, P.E.; Davis, R.W. Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.). Planta 1979, 146, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Lendzian, K.J. Survival strategies of plants during secondary growth: Barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J. Expt. Bot. 2006, 57, 2535–2546. [Google Scholar] [CrossRef]
- Andersen, T.G.; Barberon, M.; Geldner, N. Suberization—The second life of an endodermal cell. Curr. Opin. Plant Biol. 2015, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef]
- Tanios, S.; Thangavel, T.; Eyles, A.; Tegg, R.S.; Nichols, D.S.; Corkrey, R.; Wilson, C.R. Suberin deposition in potato periderm: A novel resistance mechanism against tuber greening. New Phytol. 2020, 225, 1273–1284. [Google Scholar] [CrossRef]
- Franke, R.; Schreiber, L. Suberin-a bio polyester forming apoplastic plant interfaces. Curr. Opin. Plant Biol. 2007, 10, 252–259. [Google Scholar] [CrossRef]
- Woolfson, K.N.; Esfandiari, M.; Bernards, M.A. Suberin biosynthesis, assembly, and regulation. Plants 2022, 11, 555. [Google Scholar] [CrossRef]
- Serra, O.; Soler, M.; Hohn, C.; Sauveplane, V.; Pinot, F.; Franke, R.; Schreiber, L.; Prat, S.; Molinas, M.; Figueras, M. CYP86A33-targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae, and impairs the periderm’s water barrier function. Plant Physiol. 2009, 149, 1050–1060. [Google Scholar] [CrossRef]
- Boher, P.; Serra, O.; Soler, M.; Molinas, M.; Figueras, M. The potato suberin feruloyl transferase (FHT) which accumulates in the phellogen is induced by wounding and regulated by abscisic and salicylic acids. J. Exp. Bot. 2013, 64, 3225–3236. [Google Scholar] [CrossRef]
- Razem, F.A.; Bernards, M.A. Reactive oxygen species production in association with suberization: Evidence for an NADPH-dependent oxidase. J. Expt. Bot. 2003, 54, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.N.M.; Knowles, N.R. Wound-induced superoxide production and PAL activity decline with potato tuber age and wound-healing ability. Physiol. Plant. 2003, 117, 108–117. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Iyer, S.; Knowles, N.R. Strboh A homologue of NADPH oxidase regulates wound-induced oxidative burst and facilitates wound-healing in potato tubers. Planta 2007, 227, 25–36. [Google Scholar] [CrossRef]
- Koda, Y. Effects of storage temperature and wounding on cytokinin levels in potato tubers. Plant Cell Physiol. 1982, 23, 851–857. [Google Scholar] [CrossRef]
- Lulai, E.C.; Suttle, J.C.; Olson, L.L.; Neubauer, J.D.; Campbell, L.G.; Campbell, M.A. Wounding induces changes in cytokinin and auxin content in potato tuber but does not induce formation of gibberellins. J. Plant Physiol. 2016, 191, 22–28. [Google Scholar] [CrossRef]
- Bizuayehu, D.; Amare, K.G. The role of cytokinin and gibberellin in potato tuber sprouting. Innovare J. Agric. Sci. 2024, 12, 1–9. [Google Scholar] [CrossRef]
- Anonymous. Commercial Potato Production in North America. In The Potato Association of America Handbook; Bohl, W.H., Johnson, S.B., Eds.; Potato Association of America: Madison, WI, USA; Available online: https://vric.ucdavis.edu/pdf/POTATOES/Commercial%20Potato%20Production%20in%20North%20America%202010.pdf (accessed on 3 September 2024).
- United States Department of Agriculture. United States Standards for Grades of Potatoes (Effective 3 June 2011). Available online: https://www.ams.usda.gov/sites/default/files/media/Potato_Standard[1].pdf (accessed on 16 December 2024).
- Pavek, M.J.; Shelton, S.; Holden, Z.J.; Weddell, B.J. Impact of canopy destruction from simulated hail on potato yield and economic return. Am. J. Potato Res. 2018, 95, 33–44. [Google Scholar] [CrossRef]
- Blauer, J.M.; Mattinson, D.S. Effects of in-row spacing on yield, tuber size profiles, bruise damage, and crop values for cultivars Alturas, Clearwater Russet and Ranger Russet in the Columbia Basin. Amer. J. Potato Res. 2024, 101, 468–480. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Lulai, E.C.; Suttle, J.C.; Knowles, N.R. Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA. Planta 2010, 232, 1433–1445. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Robinson, D.S.; Bretherick, M.R.; Donnelly, J.K. The heat stability and isoenzyme composition of peroxidases in Ohane grapes. Int. J. Food Sci. Tech. 1989, 24, 613–618. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Knowles, L.O.; Knowles, N.R. Zebra chip disease enhances respiration and oxidative stress of potato tubers (Solanum tuberosum L.). Planta 2017, 246, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Knowles, N.R. Oxidative stress results in increased sinks for metabolic energy during aging and sprouting of potato seed-tubers. Plant Physiol. 1996, 112, 1301–1313. [Google Scholar] [CrossRef]

| Cultivar | WHP (days) | Percent Emergence a | Two-Year Average for Yield of Size Classes and Total Tuber Yield (MT ha−1) | Tuber Grade b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early | Late | <113 g | 113–170 g | 170–284 g | 284–340 g | 340–397 g | >397 g | Total | US #1 | US #2 | ||
| RB | 0 | 34 | 97 | 15.9 | 14.7 | 22.4 | 6.1 | 5.1 | 9.9 | 74.2 | 43.6 | 14.7 |
| 1 | 38 | 95 | 14.7 | 15.0 | 20.9 | 7.7 | 5.6 | 10.1 | 74.0 | 45.9 | 13.4 | |
| 2 | 46 | 97 | 13.6 | 13.2 | 23.0 | 6.7 | 4.5 | 8.5 | 69.5 | 41.8 | 14.1 | |
| 4 | 48 | 98 | 14.2 | 14.8 | 23.8 | 6.6 | 4.9 | 12.2 | 76.5 | 46.1 | 16.2 | |
| 8 | 57 | 100 | 13.1 | 14.2 | 24.2 | 9.1 | 5.3 | 12.5 | 78.4 | 48.8 | 16.5 | |
| Alt | 0 | 27 | 87 | 14.8 | 19.2 | 36.8 | 14.2 | 9.2 | 24.6 | 118.8 | 76.3 | 27.7 |
| 1 | 36 | 95 | 16.3 | 18.9 | 39.8 | 13.9 | 9.2 | 25.1 | 123.3 | 80.3 | 26.7 | |
| 2 | 40 | 93 | 13.1 | 18.0 | 39.1 | 16.2 | 11.6 | 28.9 | 126.9 | 84.7 | 29.1 | |
| 4 | 39 | 95 | 13.8 | 16.7 | 38.4 | 14.4 | 11.1 | 31.2 | 125.6 | 79.9 | 31.9 | |
| 8 | 33 | 93 | 13.5 | 17.0 | 37.9 | 14.0 | 12.4 | 28.9 | 123.7 | 84.6 | 25.6 | |
| CW | 0 | 37 | 94 | 12.8 | 17.2 | 31.9 | 8.0 | 6.2 | 8.6 | 84.7 | 70.1 | 1.8 |
| 1 | 37 | 97 | 11.8 | 18.3 | 31.7 | 9.9 | 5.9 | 8.2 | 85.9 | 72.4 | 1.7 | |
| 2 | 42 | 99 | 13.0 | 19.4 | 32.7 | 9.6 | 6.7 | 7.9 | 89.3 | 73.8 | 2.5 | |
| 4 | 42 | 100 | 11.5 | 17.1 | 32.4 | 9.2 | 6.7 | 8.0 | 85.0 | 71.0 | 2.5 | |
| 8 | 45 | 98 | 10.2 | 17.1 | 34.6 | 11.1 | 6.7 | 12.0 | 91.7 | 79.9 | 1.6 | |
| CV * WHP LSD0.05 c | 15.4 | 5.2 | 3.0 | 3.0 | 4.8 | 2.4 | 2.6 | 7.4 | 10.5 | 10.3 | 8.5 | |
| CV LSD0.05 | 6.9 | 2.3 | 1.3 | 1.3 | 2.1 | 1.1 | 1.5 | 3.3 | 4.7 | 4.6 | 3.8 | |
| WHP LSD0.05 | 8.9 | 3.0 | 1.7 | 1.7 | 2.7 | 1.4 | 1.1 | 4.3 | 6.1 | 6.1 | 4.9 | |
| CV d | 0.05 e | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| CVAlt/RB × CW | 0.05 | 0.001 | 0.05 | 0.01 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| CVRB/CW | ns | ns | 0.001 | 0.001 | 0.001 | 0.001 | 0.05 | ns | 0.001 | 0.001 | 0.001 | |
| WHP | 0.05 | 0.05 | 0.1 | ns | ns | 0.1 | ns | ns | ns | ns | ns | |
| WHPLT | 0.01 | 0.01 | 0.01 | ns | ns | 0.05 | 0.05 | 0.1 | 0.1 | 0.05 | ns | |
| WHPQT | ns | 0.1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| WHPCT | ns | ns | ns | ns | ns | 0.1 | ns | ns | ns | ns | ns | |
| Alt/RBCW × WHPLT | ns | ns | ns | ns | ns | ns | 0.05 | ns | ns | ns | ns | |
| Alt/RBCW × WHPQT | ns | 0.1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Alt/RBCW × WHPCT | ns | ns | ns | ns | ns | 0.1 | ns | ns | ns | ns | ns | |
| RB/CW × WHPQT | ns | 0.1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, C.L.; Lloyd, K.B.; Kumar, M.G.N.; Blauer, J.M. Potato (Solanum tuberosum L.) Cultivars Interact with Wound Healing Period to Modulate Sprout Emergence, Crop Stand, and Productivity. Plants 2025, 14, 1830. https://doi.org/10.3390/plants14121830
Buckley CL, Lloyd KB, Kumar MGN, Blauer JM. Potato (Solanum tuberosum L.) Cultivars Interact with Wound Healing Period to Modulate Sprout Emergence, Crop Stand, and Productivity. Plants. 2025; 14(12):1830. https://doi.org/10.3390/plants14121830
Chicago/Turabian StyleBuckley, Connor L., Keegan B. Lloyd, Mohan G. N. Kumar, and Jacob M. Blauer. 2025. "Potato (Solanum tuberosum L.) Cultivars Interact with Wound Healing Period to Modulate Sprout Emergence, Crop Stand, and Productivity" Plants 14, no. 12: 1830. https://doi.org/10.3390/plants14121830
APA StyleBuckley, C. L., Lloyd, K. B., Kumar, M. G. N., & Blauer, J. M. (2025). Potato (Solanum tuberosum L.) Cultivars Interact with Wound Healing Period to Modulate Sprout Emergence, Crop Stand, and Productivity. Plants, 14(12), 1830. https://doi.org/10.3390/plants14121830






