Abstract
The incorporation of Bacillus thuringiensis (Bt) rice straw into fields may influence the growth of subsequent crops, but its ecological risks for winter vegetables remain largely unreported. Investigating the effects of Bt rice straw extracts on the seed germination and plant growth of pakchoi (Brassica campestris L. ssp. Chinensis Makino var. communis Tsen et Lee) can provide a theoretical foundation for ecological risk assessments. In this study, straw extracts from non-Bt rice (Tianyouhuazhan), homozygous Bt rice (T775), and heterozygous Bt rice (F1 of T775 hybrid) were used as experimental materials at concentrations of 10, 20, and 40 g·L−1. Results showed that, compared to non-Bt extract, 40 g·L−1 homozygous Bt extract increased seedling height and leaf peroxidase (POD) activity but inhibited catalase (CAT) and root superoxide dismutase (SOD) activities. The 20 g·L−1 extract boosted root CAT activity yet suppressed leaf CAT and POD activities. The 10 g·L−1 extract enhanced root length but reduced leaf CAT and POD activities. The 40 g·L−1 heterozygous Bt extract increased leaf and root POD activity but inhibited germination rate and leaf SOD activity. The 10 g·L−1 extract promoted root length and seedling POD activity but suppressed leaf POD activity. In plant growth assessments, the 10 g·L−1 homozygous Bt extract reduced underground dry weight, and the 10 g·L−1 heterozygous Bt extract inhibited both above and underground dry weight, while the 20 g·L−1 heterozygous Bt extract increased aboveground dry weight. In conclusion, the effects of homozygous and heterozygous Bt rice straw extracts on pakchoi varied with concentration and physiological indices, showing no clear pattern. Optimizing straw return concentrations based on Bt rice variety differences is essential to mitigate ecological risks.
1. Introduction
Rice (Oryza sativa L.) serves as a principal staple crop, sustaining more than half of the global population, with its production being critically linked to worldwide food security. Statistical data indicate that annual global rice production is approximately 740 million metric tons, of which nearly 90% originates from Asian countries. This predominant cultivation in Asia underscores the region’s pivotal role in maintaining global rice supply chains and food security frameworks [1]. In China, rice is the main grain crop and plays a vital role in the country’s grain production sector. Improving rice varieties is key to ensuring the sustainable development of the seed industry and national food security [2]. However, lepidopterans and other pests are an important factor restricting rice production, and the pests such as borers and rice planthoppers have caused a 20–40% loss of global rice production, seriously threatening agricultural production [3].
The advent of transgenic technology has offered a groundbreaking approach to address the persistent challenge of developing insect-resistant rice varieties, revolutionizing modern crop improvement strategies. From 1996 to 2023, over 40 billion mu of GM crops have been planted globally [4]. Insecticidal proteins derived from Bt have been extensively utilized in transgenic crops to control lepidopteran pests [5]. Studies have reported that, in pesticide-free environments, transgenic Bt rice exhibits high resistance to pests and offers a significant yield advantage over non-Bt rice, with an increase of 9.1% to 38% [6]. Bt transgenic rice is the primary GM rice variety resistant to lepidopteran pests, with its insecticidal protein activating insecticidal activity by binding to specific receptors in target pests, leading to pest mortality while being harmless to humans [7,8,9,10,11]. However, despite the economic benefits associated with Bt rice cultivation, its potential ecological risks have attracted significant public concerns [2,12]. Despite demonstrating significant pest control efficacy in experimental trials, Bt rice has not yet been approved for commercial cultivation in the majority of countries, including China. Studies have shown that Bt proteins may enter the soil through root exudates or straw returning to the field, potentially affecting the structure and function of the soil microbial community [13]. The expression of Bt proteins may potentially affect ecosystems by modifying plant phenotypic traits (e.g., root exudate composition), regulating plant–organism interactions, and inducing trophic cascade effects. These potential ecological impacts require systematic evaluation through whole-plant experiments within environmental risk assessment (ERA) frameworks [14].
China is the world’s largest producer of agricultural straw, generating approximately 230 million tons annually [15]. In the Yangtze River rice-growing regions, the “rice–winter vegetable” rotation system predominates, where mechanized straw incorporation coupled with vegetable cultivation has become a key strategy for enhancing soil fertility. The direct return of rice straw to the field after harvest not only averts the loss of vital nutrients, including nitrogen, phosphorus, and potassium, during the decomposition process but also promotes the recycling of organic matter. Additionally, this practice alleviates pollution stemming from straw burning and improves soil fertility [16,17,18,19]. Notably, the October–November straw incorporation period overlaps with winter vegetable sowing, allowing early-stage straw leachates to directly interact with vegetable seedlings through soil solutions [20].
However, Bt rice straw contains Bt proteins and various bioactive allelopathic compounds, which may influence the growth of subsequent vegetable crops through the release of straw extracts [21,22,23]. Current research on the ecological risk assessment of Bt rice primarily focuses on its impact on non-target organisms in aboveground ecosystems [24,25,26,27,28,29], and the effects of Bt rice planting and straw incorporation on cropping remain relatively understudied [30,31,32,33]. While straw incorporation enhances soil fertility, it may also have potential implications for vegetable crop rotations. However, current risk assessments mainly focus on the direct effects of Bt crop cultivation, and there remains a lack of systematic studies on the long-term impacts of Bt rice straw incorporation on winter vegetable production.
Straw decomposition is a complex process influenced by multiple factors. In the early stages, straw initially undergoes a leaching phase, which gradually transitions into decomposition over time [34]. The extracts produced during straw decomposition can affect crop seed germination, induce physiological and biochemical responses in seedlings, and enhance their resistance to external stress. In laboratory experiments investigating the effects of straw incorporation on crop seedlings, the leaching method is widely used [35]. This method involves extracting straw-derived compounds and mixing them with natural soil to create a straw decomposition solution, simulating the decomposition process in the field and providing an experimental environment that closely mirrors real agricultural conditions.
Extensive studies have demonstrated that straw decomposition solutions regulate seed germination and influence seedling growth in various crops [36]. For instance, rice straw extract has been shown to promote the germination of sweet corn seeds, maintain the stability and integrity of plasma membranes, and enhance seedling growth and development. When rice straw is used as mulch in edamame fields, weed emergence rates can be reduced by approximately 20%, and weed seedling growth is significantly suppressed [37,38]. Yang et al. found that the water extract of thickened plum leaves exhibited an inhibitory effect on the seed germination of radish, pakchoi, and white birch [39]. Tang et al. reported that basil extract had a dual effect on little white seedlings, promoting seedling height, fresh weight, and dry weight at low concentrations while inhibiting them at higher concentrations [40]. Additionally, Tang et al. found that low concentrations of straw decomposition solution significantly reduced superoxide SOD and POD activities in buckwheat seedlings [36]. Li et al. observed that high concentrations of rice stem extract increased SOD and CAT activities in germinated wheat seeds 48 h after sowing [41]. Similarly, Xie et al. found that high concentrations of Torreya sinensis extract significantly elevated malondialdehyde (MDA) levels and the activities of antioxidant enzymes (SOD, CAT, and POD) in radish, mustard, and cabbage seedlings [42].
However, the impact of Bt rice straw extract on seed germination, protective enzyme activity, and the growth of pakchoi remains unclear. In this study, both non-Bt and Bt rice straw were used as experimental materials, and Bt rice straw extracts of varying concentrations and from different varieties were prepared. By comparing the effects of different Bt rice straw extracts, this study aims to provide a theoretical foundation for selecting Bt rice varieties that have minimal impact on pakchoi growth or even exhibit potential growth-promoting effects. Ultimately, the findings will contribute to a more comprehensive and scientifically sound basis for the rational and efficient utilization of Bt rice straw in agricultural production.
2. Results
2.1. Effects of Different Rice Straw Extracts on Seed Germination Rate and Seedling Growth of Pakchoi
Different rice straw extracts exhibited varying effects on the seed germination rate and seedling growth of pakchoi (Table 1). The seed germination rate of pakchoi exhibited a concentration-dependent decline, showing gradual decrease with increasing extract concentrations. Compared to non-Bt rice straw extracts, the 10 g·L−1 homozygous Bt rice straw extract significantly promoted root elongation in pakchoi seedlings, while the 40 g·L−1 homozygous Bt rice straw extract notably increased seedling height. Additionally, the 10 g·L−1 heterozygous Bt rice straw extract significantly enhanced root length, whereas the 40 g·L−1 heterozygous Bt rice straw extract significantly reduced the seed germination rate of pakchoi.

Table 1.
Effects of Bt rice straw extract on germination rate and seedling growth of pakchoi.
2.2. Effects of Different Rice Straw Extracts on Protective Enzyme Activities of Pakchoi Seedlings
2.2.1. Effects on SOD Activity of Pakchoi Seedlings
Different rice straw extracts had varying effects on SOD activity in the leaves and roots of pakchoi seedlings. As shown in Figure 1, compared to the non-Bt rice straw extract, the homozygous Bt rice straw extract at concentrations of 10, 20, and 40 g·L−1 showed no significant effect on SOD activity in pakchoi leaves. However, the 40 g·L−1 heterozygous Bt rice straw extract significantly inhibited SOD activity in the leaves, with an inhibition rate of 16.03%.
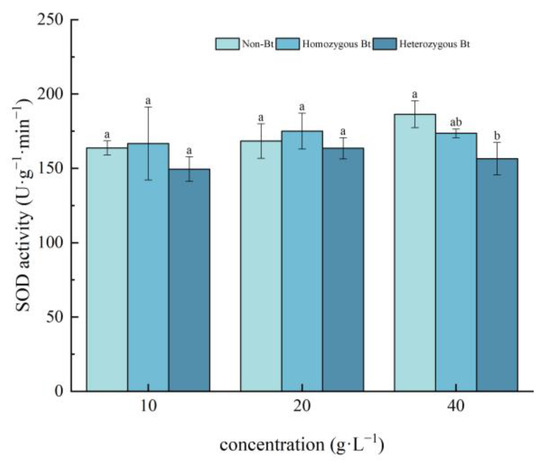
Figure 1.
The effect of Bt rice straw extracts on SOD activity in the leaves of pakchoi seedlings. Values within the same column sharing the same letter indicate no significant difference (p > 0.05), whereas different letters denote a significant difference (p < 0.05). “Non-Bt” refers to the straw of non-Bt rice; “Homozygous Bt” refers to the straw of homozygous Bt rice; “Heterozygous Bt” refers to the straw of heterozygous Bt rice.
As shown in Figure 2, compared to the non-Bt rice straw extract, the homozygous Bt rice straw extract at a concentration of 40 g·L−1 significantly suppressed SOD activity in the roots of pakchoi seedlings, with an inhibition rate of 23.13%.
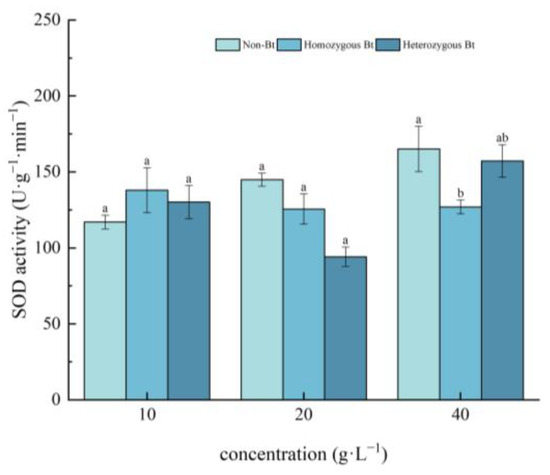
Figure 2.
The effect of Bt rice straw extracts on SOD activity in the roots of pakchoi seedlings. Values within the same column sharing the same letter indicate no significant difference (p > 0.05), whereas different letters denote a significant difference (p < 0.05). “Non-Bt” refers to the straw of non-Bt rice; “Homozygous Bt” refers to the straw of homozygous Bt rice; “Heterozygous Bt” refers to the straw of heterozygous Bt rice.
2.2.2. Effects on CAT Activity of Pakchoi Seedlings
As shown in Figure 3, CAT activity in the leaves of pakchoi seedlings decreased with increasing extract concentration. Compared to the non-Bt rice straw extract, the homozygous Bt rice straw extract at concentrations of 10, 20, and 40 g·L−1 significantly inhibited CAT activity in the leaves, with inhibition rates of 48.86%, 77.29%, and 75.61%, respectively. In contrast, the heterozygous Bt rice straw extract had no significant effect on CAT activity in the leaves of pakchoi seedlings.
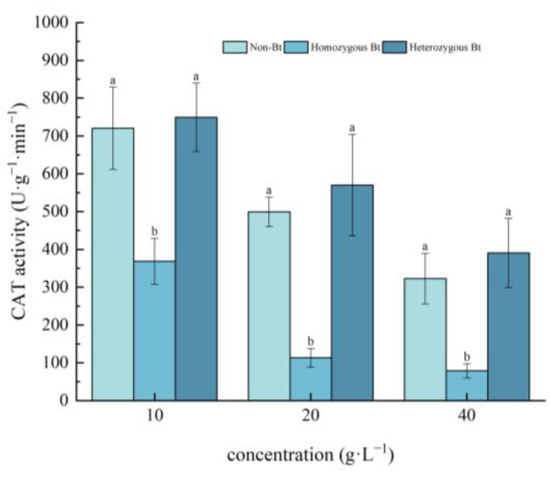
Figure 3.
The effect of Bt rice straw extracts on CAT activity in the leaves of pakchoi seedlings. Values within the same column sharing the same letter indicate no significant difference (p > 0.05), whereas different letters denote a significant difference (p < 0.05). “Non-Bt” refers to the straw of non-Bt rice; “Homozygous Bt” refers to the straw of homozygous Bt rice; “Heterozygous Bt” refers to the straw of heterozygous Bt rice.
Different rice straw extracts had varying effects on CAT activity in the roots of pakchoi seedlings (Figure 4). Compared to the non-Bt rice straw extract, the homozygous Bt rice straw extract at 20 g·L−1 significantly enhanced CAT activity in the roots, with an increase of 51.29%. However, the homozygous Bt rice straw extract at 40 g·L−1 significantly inhibited root CAT activity, with an inhibition rate of 80.18%. In contrast, the heterozygous Bt rice straw extract had no significant effect on CAT activity in the roots of pakchoi seedlings.
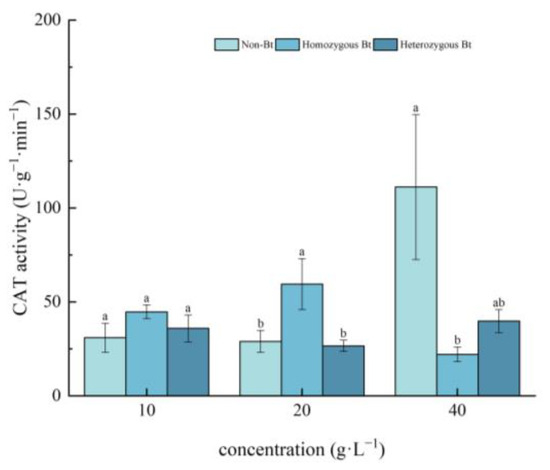
Figure 4.
The effect of Bt rice straw extracts on CAT activity of pakchoi seedling roots. Values within the same column sharing the same letter indicate no significant difference (p > 0.05), whereas different letters denote a significant difference (p < 0.05). “Non-Bt” refers to the straw of non-Bt rice; “Homozygous Bt” refers to the straw of homozygous Bt rice; “Heterozygous Bt” refers to the straw of heterozygous Bt rice.
2.2.3. Effects on POD Activity of Pakchoi Seedlings
Different rice straw extracts had varying effects on POD activity in the leaves and roots of pakchoi seedlings (Figure 5 and Figure 6). As shown in Figure 5, compared to the non-Bt rice straw extract, the homozygous Bt rice straw extracts at 10 g·L−1 and 20 g·L−1 significantly inhibited POD activity in the leaves of pakchoi seedlings, with inhibition rates of 31.16% and 54.48%, respectively. However, the homozygous Bt rice straw extract at 40 g·L−1 significantly enhanced POD activity in the leaves, with an increase of 46.56%. Similarly, the heterozygous Bt rice straw extracts at 10 g·L−1 and 20 g·L−1 significantly suppressed POD activity in the leaves, with inhibition rates of 65.90% and 75.56%, respectively. In contrast, the 40 g·L−1 heterozygous Bt rice straw extract significantly increased POD activity in the leaves, with a growth rate of 51.66%.
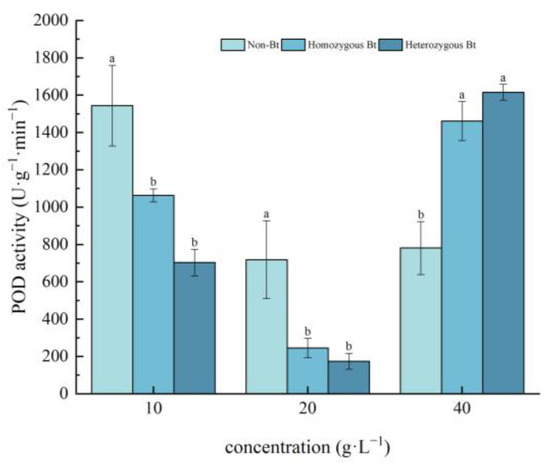
Figure 5.
The effect of Bt rice straw extracts on POD activity in the leaves of pakchoi seedlings. Values within the same column sharing the same letter indicate no significant difference (p > 0.05), whereas different letters denote a significant difference (p < 0.05). “Non-Bt” refers to the straw of non-Bt rice; “Homozygous Bt” refers to the straw of homozygous Bt rice; “Heterozygous Bt” refers to the straw of heterozygous Bt rice.
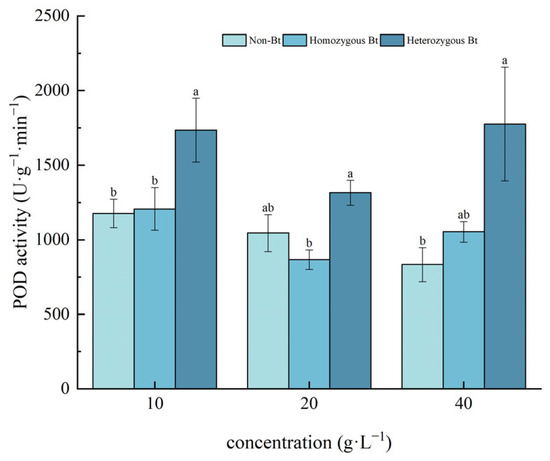
Figure 6.
The effect of Bt rice straw extracts on POD activity of the roots of pakchoi seedlings. Values within the same column sharing the same letter indicate no significant difference (p > 0.05), whereas different letters denote a significant difference (p < 0.05). “Non-Bt” refers to the straw of non-Bt rice; “Homozygous Bt” refers to the straw of homozygous Bt rice; “Heterozygous Bt” refers to the straw of heterozygous Bt rice.
As shown in Figure 6, compared to the non-Bt rice straw extract, the different concentrations of the homozygous Bt rice straw extract had no significant effect on POD activity in the roots of pakchoi seedlings. However, the heterozygous Bt rice straw extracts at 10 g·L−1 and 40 g·L−1 significantly increased POD activity in the roots, with growth rates of 32.21% and 53.07%, respectively.
2.3. Effects of Different Rice Straw Extracts on Growth of Pakchoi
The effects of the three types of rice straw extracts on the growth of pakchoi plants varied depending on the rice variety and extract concentration (Table 2). Compared to the non-Bt rice straw extract, different concentrations of both the homozygous and heterozygous Bt rice straw extracts had no significant effects on plant height or chlorophyll content in pakchoi. However, the homozygous Bt rice straw extract at 10 g·L−1 significantly inhibited the underground dry weight of pakchoi. Additionally, the heterozygous Bt rice straw extract at 20 g·L−1 significantly increased the aboveground dry weight, while the heterozygous Bt rice straw extract at 10 g·L−1 significantly inhibited both the aboveground and underground dry weights of pakchoi.

Table 2.
Effects of Bt rice straw extracts on growth of pakchoi.
3. Discussion
Currently, research on the effects of Bt rice straw extract is limited. This study explored the impacts of homozygous and heterozygous Bt rice straw extracts on seed germination, protective enzyme activity, and plant growth, providing a theoretical basis for further investigating Bt rice extract effect on winter vegetables. Returning straw to the field plays a crucial role in improving soil organic matter and structure. While numerous studies highlight its benefits, potential negative effects should not be ignored. Stress factors can disrupt active oxygen metabolism, ultimately affecting plant health [43]. Many studies have shown that seed germination and seedling growth are inhibited in a concentration-dependent manner as the concentration of straw extract increases [44,45,46,47]. For instance, water extracts from the roots, stems, leaves, and tassels of corn stalks significantly inhibited the seed germination of shepherd’s purse, and the germination rate and index decreased as extract concentration increased [48]. Similarly, wheat straw extracts strongly inhibit lettuce and cress root growth, with higher concentrations associated with greater inhibition [35]. The impact of rice straw extract on sweet corn seeds and wheat germination and seedling growth was generally characterized by mild promotion at low concentrations and strong inhibition at high concentrations [49,50]. Additionally, corn straw extract has been shown to inhibit wheat and cucumber seed germination, with the inhibitory effect intensifying at higher concentrations [51,52]. Furthermore, Zhang et al. found that the allelopathic compound benzoic acid inhibits Arabidopsis seedlings’ taproot elongation by reducing the size of the meristematic and elongation zones via auxin signaling [53].
In this study, pakchoi exhibited a concentration-dependent response to Bt rice straw extracts: seed germination and root length decreased with increasing extract concentrations across all treatments. Notably, high-concentration heterozygous Bt extracts significantly suppressed germination compared to non-Bt controls, whereas low concentrations of both homozygous and heterozygous Bt extracts promoted root elongation (Table 1), consistent with Liu et al. [54]. This biphasic effect may arise from subthreshold stimulation, where low-dose Bt components enhance root cell expansion without exceeding toxicity thresholds, combined with potential hormonal modulation (e.g., auxin/cytokinin rebalancing) that promotes root growth. Intriguingly, high-concentration homozygous Bt extract increased seedling height, likely through components stimulating shoot-specific cell elongation. Furthermore, while low concentrations may activate stress-primed defense responses to facilitate root development, growth inhibition at high concentrations is likely mediated by oxidative damage, as evidenced by suppressed CAT/SOD activities (Figure 1, Figure 2, Figure 3 and Figure 4).
The experimental results demonstrated that neither homozygous nor heterozygous Bt rice straw extracts significantly affected pakchoi plant height or chlorophyll content. This finding differs from the results of Elisante et al. and Jaballah et al., maybe due to the role of Bt protein in regulating metabolite release and influencing chlorophyll levels in plants [55,56]. Furthermore, a low concentration of homozygous Bt rice straw extract significantly inhibited underground dry weight, while a low concentration of heterozygous Bt rice straw extract inhibited both aboveground and underground dry weight in pakchoi (Table 2). Gong et al. reported that incorporating corn, wheat, and Jerusalem artichoke straw promoted cucumber growth to varying degrees, with effects differing based on straw variety and cucumber growth stage [57]. The differential responses observed in our study likely reflect fundamental differences in nutrient utilization strategies between cucurbitaceous (cucumber) and brassicaceous (pakchoi) species, particularly in their metabolic responsiveness to rice-derived secondary metabolites. Nutritional profiling revealed significant compositional differences between Bt and non-Bt rice straw (Table 3). Homozygous Bt straw exhibited significantly elevated phosphorus content coupled with reduced potassium levels compared to non-Bt counterparts, while heterozygous Bt materials demonstrated more pronounced potassium deficiency. The observed growth inhibition, particularly in belowground biomass, may be associated with potassium limitation, given its critical roles in osmoregulation and nutrient translocation. However, the precise mechanistic linkage between straw nutritional characteristics and seedling growth responses warrants further investigation, particularly concerning potential interactions between Bt proteins and mineral nutrient availability.

Table 3.
Physicochemical properties of rice straw extracts.
SOD serves as the first line of defense against oxidative stress in plants and is present in every cell. Its primary function is to convert or break down toxic superoxide anions (O2−) into hydrogen peroxide (H2O2) and molecular oxygen (O2) [58]. CAT, an essential heme enzyme, plays a crucial role in converting hydrogen peroxide into water and oxygen, contributing to plant metabolism and signal recognition [59]. POD is a key enzyme in lignin biosynthesis, facilitating the polymerization of lignin monomers to reinforce cell structure [60]. Zhao et al. suggested that changes in antioxidant enzyme activity may result from disruptions in intracellular reactive oxygen species (ROS) metabolism caused by endogenous inhibitory substances [61]. These disruptions lead to ROS accumulation and trigger the plant’s antioxidant enzyme stress response, which can directly inhibit CAT, SOD, and POD activities in certain recipient plants. The upregulation of SOD and POD activities helps eliminate excess ROS and protect plant cells from oxidative damage. Tang et al. reported that low concentrations of straw decomposition solution had minimal effects on the antioxidant enzyme activity of common buckwheat seedlings [36]. However, as the concentration increased, SOD and POD activities significantly declined, while malondialdehyde (MDA) levels rose sharply, indicating oxidative stress-induced damage and inhibited seedling growth. Similarly, Li et al. found that high concentrations of rice stem extract increased SOD and CAT activities in germinated wheat seeds 48 h after sowing [41]. Shen et al. observed that low concentrations of rice extract could enhance CAT and POD activities in sweet corn leaves [49].
The results of this study demonstrated that, compared with the liquid phase of non-Bt rice straw extract, a high concentration of homozygous Bt rice straw extract inhibited CAT and SOD activity in pakchoi leaves and CAT activity in the roots, while promoting POD activity in the leaves (Figure 2, Figure 3, Figure 4 and Figure 5). A low concentration of homozygous Bt rice straw extract inhibited CAT and POD activity in pakchoi leaves (Figure 3 and Figure 5). Additionally, a high concentration of heterozygous Bt rice straw extract suppressed SOD activity in pakchoi leaves but enhanced POD activity in both the leaves and roots (Figure 1, Figure 5 and Figure 6). In contrast, a low concentration of heterozygous Bt rice straw extract inhibited POD activity in the leaves while promoting it in the roots (Figure 5 and Figure 6). The effects of Bt rice straw extract on the activity of protective enzymes in the roots and leaves of pakchoi were complex and varied depending on concentration and measured parameters. Chen et al. found that the antioxidant enzyme activity of maize, soybean, and wheat treated with different concentrations of maize straw extract exhibited a dual effect, either inhibiting or promoting activity [62]. Furthermore, as concentration varied, the effects did not follow a linear pattern but rather displayed diverse responses, including promotion, inhibition, alternating promotion/inhibition, or no significant change. The protective enzyme system plays a critical role in plant stress responses and growth regulation. The disruption of these protective enzyme activities reflects an imbalance in plant reactive oxygen species metabolism, suggesting that, when exposed to Bt rice straw extracts, pakchoi cells are vulnerable to oxidative damage. As a primary producer in the ecosystem, alterations in pakchoi’s physiological state can cascade through the food chain, thereby threatening the biodiversity and ecological equilibrium of the entire farmland ecosystem. These irregular changes can be attributed to the complex composition of the extracts and the inherent differences in pakchoi’s physiological regulation mechanisms. This complexity exacerbates the challenge of conducting accurate ecological risk assessments. In real-world farmland ecosystems, the diverse growth stages of pakchoi, coupled with fluctuating environmental conditions, render the ecological impacts of Bt rice straw extracts even more unpredictable. This unpredictability poses significant uncertainties in maintaining ecosystem stability.
The findings of this study provide a theoretical basis for understanding the effects of Bt rice straw extract on pakchoi seed germination, protective enzyme activity, and plant growth. However, several limitations should be acknowledged. First, the conclusions drawn here are specific to the tested Bt rice lines (homozygous and heterozygous) and the experimental conditions applied; they should not be extrapolated to other Bt crop varieties or field environments without further validation. Second, while our experiments included biological replicates, the potential interactions between Bt protein and other allelopathic compounds in straw extracts require more targeted investigations (e.g., Bt protein quantification, genetic background controls) to establish causality. Third, the short-term laboratory observations may not fully reflect the complex dynamics of straw decomposition and plant–microbe interactions in natural agroecosystems. Future research should incorporate field trials to explore the long-term impact of Bt rice straw on vegetable growth and soil ecosystems. Such studies will offer more comprehensive insights and theoretical support for optimizing Bt rice cultivation and the sustainable utilization of straw in agricultural practices.
4. Materials and Methods
4.1. Test Materials
The straw varieties used in this experiment included homozygous Bt rice (T775), heterozygous Bt rice (F1 of T775), and non-Bt conventional rice (Tianyouhuazhan). We obtained all materials from Researcher Wang Feng (Rice Research Institute of Guangdong Academy of Agricultural Sciences, Guangzhou, China). The straw materials used in this study were all sourced from field cultivation within the same experimental station. The straw samples were randomly collected from each plot and then mixed. Both homozygous and heterozygous Bt rice lines in this study express the Cry1Ab insecticidal protein. The target organisms of this Bt protein are mainly Lepidoptera pests such as Chilo suppressalis (striped stem borer) and Tryporyza incertulas (yellow stem borer). After the rice plants matured, the stalks (including stems and leaves) of the three varieties were collected, dried, crushed, and thoroughly mixed for subsequent use. The tested vegetable variety was pakchoi, with seeds purchased from Guangzhou Xiangsheng Seed Co., Ltd. (Guangzhou, China).
4.2. Preparation of Extract
Straw samples of homozygous Bt rice, heterozygous Bt rice, and non-Bt rice were each prepared from a composite of stems and leaves randomly collected from 20 healthy field-grown plants per variety. After oven-drying at 65 °C (DHG-9140A drying oven, Shanghai Jinghong Experimental Equipment Co., Ltd., Shanghai, China) to constant weight, the materials were homogenized through a 40-mesh sieve. Precisely, 4.0 g of each processed straw sample was weighed into 100 mL conical flasks. Each flask was then filled with 100 mL of distilled water, sealed with film, and placed on a shaker (TS-2102 constant temperature oscillator, Shanghai Tiancheng Experimental Equipment Co., Ltd., Shanghai, China) at 25 °C with a rotation speed of 100 rpm for 24 h. After soaking, the mixture in each flask was transferred to a 50 mL centrifuge tube and centrifuged at 4000× g for 10 min at room temperature. The supernatant was collected to obtain the rice straw extract mother liquor at a concentration of 40 g·L−1. The physicochemical properties of the tested rice straw extracts are shown in Table 3. Finally, the mother liquor was diluted with distilled water to prepare solutions at concentrations of 10 g·L−1, 20 g·L−1, and 40 g·L−1, which were then stored at 4 °C for further use. Total nitrogen was determined by the Kjeldahl method, total phosphorus by the molybdenum–antimony anti-spectrophotometric method, total potassium by flame photometry, and Bt protein by double-antibody sandwich ELISA (EnviroLogix Cry1Ab/1Ac Plate Kit, EnviroLogix Inc., Portland, ME, USA).
4.3. Seed Germination Test
Using the Petri dish filter paper germination method, surface-sterilized pakchoi seeds (0.5% H2O2 for 15 min, followed by 2–3 rinses with deionized water) were evenly distributed on qualitative filter paper in sterile 9 cm Petri dishes (25 seeds/dish). Each treatment consisted of four replicate dishes (co-cultivation), with 5 mL of rice straw extract at specified concentrations (10, 20, or 40 g·L−1). The samples were incubated in an artificial climate chamber (light/dark cycle: 12/12 h, temperature: 25 °C, humidity: 70%) for one week. The number of germinated seeds was recorded, and four representative seedlings were randomly selected from each treatment group to measure root length, seedling height, and dry weight. Additionally, leaf and root samples were collected to determine the activities of SOD, POD, and CAT in the leaves and roots of pakchoi. All treatment groups were established with four independent biological replicates. For detailed sample sizes and replication strategies, refer to Supplementary Table S1.
4.4. Plant Growth Test
Pakchoi seedlings with uniform growth were selected and transplanted into pots containing 600 g of soil (dimensions: upper diameter × lower diameter × height = 12 cm × 9 cm × 12 cm) in a completely randomized design. Three concentrations of rice straw extract were applied, i.e., (1) 10 g·L−1, (2) 20 g·L−1, and (3) 40 g·L−1. The experiment included three rice varieties, i.e., homozygous Bt rice (T775), heterozygous Bt rice (F1 generation of the T775 hybrid), and non-Bt ordinary rice (Tianyouhuazhan), and four replicates were set up for each treatment. After the pakchoi seedlings reached a stable growth state, the straw extract solution was added once every two days, a 5 mL volume each time (injected into the soil around the roots). Plant height, chlorophyll content, and the dry weight of both aboveground and belowground parts were measured in the sixth week after treatment.
4.5. Measurement Items
The germination rate was calculated using the following formula: germination rate (%) = (number of germinated seeds ÷ total test seeds) × 100%. Seedling height and root length were measured using a vernier caliper, while the height of pakchoi plants was measured with a measuring tape. Chlorophyll content was determined and calculated following the method of Wang Xuekui [63]. The aboveground and belowground parts of pakchoi were dried in an oven at 105 °C for 30 min, then further dried at 60 °C until a constant weight was achieved, and the dry weight was recorded. SOD activity was measured using the NBT photochemical reduction method following the protocol of Gao Junfeng [64]. CAT activity was determined using the hydrogen peroxide decomposition method according to Li Hesheng [65]. POD activity was assessed using the guaiacol method, following the method described by Gao Junfeng [64].
4.6. Data Analysis
The data were organized and charts were created using Excel 2010. Statistical analyses were performed using SPSS 25.0 software, with one-way ANOVA conducted to assess variations among treatments. Duncan’s multiple range test was used for pairwise comparisons and significance analysis of differences.
5. Conclusions
There were significant differences in the effects of homozygous and heterozygous Bt rice straw extracts on seed germination, protective enzyme activity, and plant growth in pakchoi. A low concentration of homozygous Bt rice straw extract significantly promoted root elongation in pakchoi seedlings but inhibited CAT and POD activities in the leaves and reduced underground dry weight. At a high concentration, the homozygous Bt rice straw extract significantly increased seedling height while inhibiting CAT and SOD activities in the leaves. However, it also significantly enhanced POD activity in the leaves. Similarly, a low concentration of heterozygous Bt rice straw extract significantly increased root length and POD activity in pakchoi seedlings but inhibited POD activity in the leaves and reduced both aboveground and underground dry weight. At a high concentration, heterozygous Bt rice straw extract significantly suppressed seed germination and SOD activity in the leaves, while significantly enhancing POD activity in both the leaves and roots. Additionally, a 20 g·L−1 concentration of heterozygous Bt rice straw extract significantly promoted aboveground dry weight. In conclusion, compared to the non-Bt rice straw extract, both Bt rice straw extracts exhibited varying degrees of inhibitory and promotive effects on seed germination, protective enzyme activity, and plant growth in pakchoi, without following a clear or consistent pattern.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14121797/s1, Table S1: Experimental treatment group design and replication details.
Author Contributions
Conceptualization, Y.F. and W.S.; software, C.Z.; validation, C.Z., W.S., and Y.P.; formal analysis, C.Z. and Y.P.; data curation, W.S.; writing—original draft preparation, C.Z. and W.S.; writing—review and editing, Y.F.; visualization, C.Z., W.S., and Y.P.; supervision, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (31370543, 41101279).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Feng Wang (Rice Research Institute of Guangdong Academy of Agricultural Sciences, China) for providing us with rice materials.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rayee, R.; Anh, L.H.; Khanh, T.D.; Xuan, T.D. Potential Momilactones in Rice Stress Tolerance and Health Advantages. Agronomy 2024, 14, 405. [Google Scholar] [CrossRef]
- Liu, Z.H.; Tian, Y.; Chen, H.N.; Zhou, Z.H.; Zheng, J.; Yang, X.H. Research Progress and Application Status of Transgenic Breeding in Rice. China Seed Ind. 2023, 11–17. [Google Scholar] [CrossRef]
- Liu, H.J. Screening of Endogenous miRNAs in Chilo suppressalis and Development of Transgenic Insect-Resistant Rice. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2022. [Google Scholar]
- Agbio Investor. Global GM Crop Area Review. Available online: https://gm.agbioinvestor.com (accessed on 26 February 2025).
- Hu, Y.T.; Tian, C.B.; Feng, Y.J.; Ma, W.D.; Zhang, Y.J.; Yang, Q.; Zhang, X.R. Transgenic early japonica rice: Integration and expression characterization of stem borer resistance Bt gene. Gene 2024, 927, 148753. [Google Scholar] [CrossRef]
- Wang, K.X.; Zhang, K.R.; Cao, C.G.; Jiang, Y. Effect of Bt traits on transgenic rice’s growth and weed competitiveness. J. Integr. Agric. 2023, 22, 2346–2358. [Google Scholar] [CrossRef]
- Song, Z.Y.; Lin, X.F.; Yan, Y.Z.; Jin, Y.M. Overview of Research on Insect-Resistant Transgenic Rice and Its Safety. Agric. Technol. 2020, 40, 15–16. [Google Scholar]
- Li, C.M.; Han, G.J.; Liu, Q.; Qi, J.H.; Xu, J. Cultivation Characteristics and Insecticidal Activity Analysis of Bacillus thuringiensis Bt-8. J. South. Agric. 2016, 47, 2072–2077. [Google Scholar]
- Sujayanand, G.K.; Pandey, S.; Jagadeeswaran, R.; Chandra, A.; Kumar, V.; Dubey, S.; Dubey, J. Characterization of entomotoxic and nematotoxic genes from indigenous Bacillus thuringiensis strains and their biocontrol potential. Egypt. J. Biol. Pest Control 2023, 33, 88. [Google Scholar]
- Wang, Y.Y.; Dai, P.L.; Chen, X.P.; Romeis, J.; Shi, J.R.; Peng, Y.F.; Li, Y.H. Ingestion of Bt rice pollen does not reduce the survival or hypopharyngeal gland development of Apis mellifera adults. Environ. Toxicol. Chem. 2017, 36, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, L.H.; Knauth, S.; Eickhorst, T. Degradation of transgenic Bt-rice straw incorporated with two different paddy soils. J. Environ. Manag. 2019, 244, 415–421. [Google Scholar] [CrossRef]
- Yaqoob, A.; Shahid, A.A.; Samiullah, T.R.; Rao, A.Q.; Khan, M.A.U.; Thair, S.; Mirza, S.A.; Husnain, T. Risk assessment of Bt crops on the non-target plant-associated insects and soil organisms. J. Sci. Food Agric. 2016, 96, 2613–2619. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, C.; Ge, L.; Hu, C.; Wu, G.G.; Sun, Y.; Song, L.L.; Wu, X.; Pan, A.H.; Xu, Q.Q.; et al. Environmental Behaviors of Bacillus thuringiensis (Bt) Insecticidal Proteins and Their Effects on Microbial Ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Arpaia, S.; Birch, A.N.E.; Kiss, J.; Loon, J.J.A.V.; Messean, A.; Nuti, M.; Perry, J.N.; Sweet, J.B.; Tebbe, C.C. Assessing environmental impacts of genetically modified plants on non-target organisms: The relevance of in planta studies. Sci. Total Environ. 2017, 583, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Mohamed, B.A.; Ran, Y.; Yang, Y.; Pezzuolo, A.; Samer, M.; Ai, P. A comparative environmental life cycle assessment of rice straw-based bioenergy projects in China. Environ. Res. 2022, 212, 113404. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.Q.; Yue, B.W. Research Progress on Direct Straw Returning Technology and Equipment in the Northeast Rice Region. China Rice 2024, 30, 37–42. [Google Scholar]
- Pathak, H.; Singh, R.; Bhatia, A.; Jain, N. Recycling of rice straw to improve wheat yield and soil fertility and reduce atmospheric pollution. Paddy Water Environ. 2006, 4, 111–117. [Google Scholar] [CrossRef]
- Chen, H.G.; Cao, Q.G.; Xiong, G.L.; Li, W.; Zhang, A.X.; Yu, H.S.; Wang, J.S. Composition of Wheat Rhizosphere Antagonistic Bacteria and Wheat Sharp Eyespot as Affected by Rice Straw Mulching. Pedosphere 2010, 20, 505–514. [Google Scholar] [CrossRef]
- Wang, F.R. Effects of Direct Straw Returning and Carbonization Returning on the Stoichiometric Ratios of Carbon, Nitrogen, and Phosphorus in Paddy Soil Aggregates and the Utilization of Nitrogen and Phosphorus Nutrients in Rice. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2023. [Google Scholar]
- Che, Y.X.; Zhang, B.Y.; Liu, B.Y.; Wang, J.C.; Zhang, H.L. Effects of Straw Return Rate on Soil Physicochemical Properties and Yield in Paddy Fields. Agronomy 2024, 14, 1668. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.; Singh, S.P.; Ellur, R.K.; Choudhary, V.; Sarkel, S.; Singh, D.; Krishnan, S.G.; Nagarajan, M.; Vinod, K.K.; et al. Incorporation of blast resistance into “PRR78”, an elite Basmati rice restorer line, through marker assisted backcross breeding. Field Crop. Res. 2012, 128, 8–16. [Google Scholar] [CrossRef]
- Liu, Y.M.; Liu, Q.S.; Wang, Y.A.; Chen, X.P.; Song, X.Y.; Romeis, J.; Li, Y.H.; Peng, Y.F. Ingestion of Bt corn pollen containing Cry1Ab/2Aj or Cry1Ac does not harm Propylea japonica larvae. Sci. Rep. 2016, 6, 23507. [Google Scholar] [CrossRef]
- Wang, B.F.; Wu, F.C.; Yin, J.Q.; Jiang, Z.L.; Song, X.Y.; Reddy, V.P. Use of Taxonomic and Trait-Based Approaches to Evaluate the Effect of Bt maize Expressing Cry1Ie Protein on Non-Target Collembola: A Case Study in Northeast China. Insects 2021, 12, 88. [Google Scholar] [CrossRef]
- Li, P.; Ye, S.F.; Chen, J.; Wang, L.Y.; Li, Y.J.; Wu, G.G.; Song, L.L.; Wang, C.; Sun, Y.; Wang, J.B.; et al. Combined metagenomic and metabolomic analyses reveal that Bt rice planting alters soil C-N metabolism. ISME Commun. 2023, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, G.W.; Chen, X.Y.; Wang, X.F.; Lu, Y.W.; Liang, Z.H.; Xu, J.F.; Peng, C. Analysis of the Unintended Effects of the Bacillus thuringiensis Insecticidal Protein in Genetically Modified Rice Using Untargeted Transcriptomics. Processes 2023, 11, 3202. [Google Scholar] [CrossRef]
- Li, C. Complexity and Density of Rhizosphere Microbiota in Bt Insect-Resistant Rice Remain Unchanged. China Science Daily, 9 August 2021. [Google Scholar]
- Qiu, L.M.; Liu, Q.Q.; Lin, R.K.; Shi, L.Q.; Zhan, Z.X. Research Progress on the Ecological Safety of Bt-Transgenic Rice on Major Non-Target Insects. Fujian J. Agric. Sci. 2018, 33, 326–333. [Google Scholar]
- Wu, Y.; Weng, Z.J.; Yan, H.X.; Yao, Z.T.; Li, Z.Z.; Sun, Y.J.; Ma, K.S.; Huil, J.J.; Zhang, D.L.; Ma, W.H.; et al. The microRNA-7322-5p/p38/Hsp19 axis modulates Chilo suppressalis cell-defences against Cry1Ca: An effective target for a stacked transgenic rice approach. Plant Biotechnol. J. 2023, 21, 1827–1838. [Google Scholar] [CrossRef]
- Sun, C. Determination and Analysis of the Hazard Quotient (HQ) of Three Bt Rice Varieties on Non-Target Organisms. Master’s Thesis, Zhejiang University, Hangzhou, China, 2019. [Google Scholar]
- Lu, H.H.; Wu, W.X.; Chen, Y.X.; Wang, H.L.; Devare, M.; Thies, J.E. Soil microbial community responses to Bt transgenic rice residue decomposition in a paddy field. J. Soils Sediments 2010, 10, 1598–1605. [Google Scholar] [CrossRef]
- Fang, H.; Dong, B.; Yan, H.; Tang, F.F.; Wang, B.C.; Yu, Y.L. Effect of vegetation of transgenic Bt rice lines and their straw amendment on soil enzymes, respiration, functional diversity and community structure of soil microorganisms under field conditions. J. Environ. Sci. 2012, 24, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Raubuch, M.; Behr, K.; Roose, K.; Joergensen, R.G. Specific respiration rates, adenylates, and energy budgets of soil microorganisms after addition of transgenic Bt-maize straw. Pedobiologia 2010, 53, 191–196. [Google Scholar] [CrossRef]
- Čerevková, A.; Miklisová, D.; Szoboszlay, M.; Tebbe, C.C.; Cagan, L. The responses of soil nematode communities to Bt maize cultivation at four field sites across Europe. Soil Biol. Biochem. 2018, 119, 194–202. [Google Scholar] [CrossRef]
- Wang, S. Effects of Straw Returning on Seed Germination and Maize Seedling Growth. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2021. [Google Scholar]
- Nakano, H.; Morita, S.; Shigemori, H.; Hasegawa, K. Plant Growth Inhibitory Compounds from Aqueous Leachate of Wheat Straw. Plant Growth Regul. 2006, 48, 215–219. [Google Scholar]
- Tang, Z.L.; Guo, R.Y.; Yang, X.; Min, J.Y.; He, P.Y.; Huang, X.Y.; Huang, K.F. Appropriate Concentration of Rice Straw Decomposition Liquid Prompted Germination and Seedling Growth of Common Buckwheat. J. Soil Sci. Plant Nutr. 2024, 24, 3861–3872. [Google Scholar] [CrossRef]
- Dong, S.Q.; Yuan, X.Y.; Hu, C.Y.; Wen, Y.Y.; Guo, P.Y.; Nie, L.Y.; Qiao, X.F. Effects of Foxtail Millet Straw Aqueous Extract on Allelopathy in Winter Wheat Seedlings. J. China Agric. Univ. 2015, 20, 31–38. [Google Scholar]
- Crawford, L.E.; Ii, M.M.W.; Wortman, S.E. An Early-Killed Rye (Secale cereale) Cover Crop Has Potential for Weed Management in Edamame (Glycine max). Weed Sci. 2018, 66, 502–507. [Google Scholar] [CrossRef]
- Yang, C.J.; Liu, R.Q.; Gu, Y.Y. Allelopathic Effects of Chokecherry Leaf Aqueous Extract on Four Plant Species. J. High. Norm. Sci. 2024, 44, 87–91. [Google Scholar]
- Tang, M.M.; Dong, N.; Liang, Z.H.; Zhang, T.H.; Li, S.M. Effects of Basil Extract on Wheat Seedling Growth. Anhui Agric. Bull. 2023, 29, 1–4. [Google Scholar]
- Li, B.; Wu, W.W.; Shen, W.Y.; Xiong, F.; Wang, K.H. Allelochemicals Released from Rice Straw Inhibit Wheat Seed Germination and Seedling Growth. Agronomy 2024, 14, 2376. [Google Scholar] [CrossRef]
- Xie, X.L.; Jiang, B.; Zhou, X.R.; Zhao, L.X.; Wang, J.H.; Shang, J. Allelopathic Effects of Aqueous Extract of Torreya fargesii Aril on Seed Germination and Seedling Growth of Radish, Mustard and Cabbage. Agric. Biotechnol. 2016, 5, 11–17. [Google Scholar]
- Qian, P.Z.; Sha, W.; Ma, T.Y.; Dong, J.H.; Wang, T.; Ao, J.; Zhang, J.; Zhang, M.J. Effects of Peony Seed Extract on Seed Germination and Seedling Growth of Two Plant Species. Mol. Plant Breed. 2023, 21, 642–648. [Google Scholar]
- Guo, H.G.; Ai, T.S.; Sun, Q.; Jin, D.; Sun, W.X.; Wu, Y.B. Effects of Different Crop Straw Extracts on Rapeseed Seed Germination and Seedling Growth. Mol. Plant Breed. 2024, 1–13. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20240514.1102.004.html (accessed on 26 February 2025).
- Feng, W.M.; Zhang, J.S.; Jiao, N.; Chen, H.F.; Yang, J.T.; Gao, D.D.; Guo, P.H. Effects of Codonopsis pilosula Straw Water Extract on Seed Germination and Seedling Growth of Medicinal Plants. Henan Agric. Sci. 2024, 53, 57–64. [Google Scholar]
- Liu, Y.P.; Xi, K.P.; Wang, H.J.; Wang, M.; Wu, L.L. Allelopathic Effects of Cotton Straw Water Extract on Lettuce. Shanxi Agric. Sci. 2018, 46, 1643–1645. [Google Scholar]
- Zhao, X.L.; Li, J.; Gu, W.R.; Ai, J.G.; Kong, L.Z.; Qiao, T.C.; Wei, S. Effects of Maize Straw Decomposition Solution on Seed Germination under Continuous Cropping. Crops 2013, 137–141. [Google Scholar] [CrossRef]
- Liu, X.M.; Meng, Q.M.; Wang, X.Q.; Wang, G.Q. Allelopathic Effects of Water Extracts from Different Parts of Maize Straw on Shepherd’s Purse. Hebei Agric. Sci. 2013, 17, 36–39. [Google Scholar]
- Shen, X.F.; Hu, X.Y.; Chen, Y.; Li, Y.B.; Luo, Q.Y. Effects of Rice Straw Extract on Seed Germination and Seedling Growth of Sweet Corn. Crops 2015, 158–160. [Google Scholar] [CrossRef]
- Li, F.Y.; Sun, X.F.; Feng, W.Q.; Qin, Y.S.; Wang, C.Q.; Tu, S.H. Allelopathic Effects of Rice Straw Water Extract on Wheat. Southwest China J. Agric. Sci. 2008, 21, 960–964. [Google Scholar]
- Zhang, B.; Yu, J.H.; Xie, J.M.; Feng, Z.; Zhang, G.B. Effects of Decomposed Maize Straw Water Extract on Germination Characteristics of Cucumber Seeds. J. Gansu Agric. Univ. 2012, 47, 82–87. [Google Scholar]
- Hua, Z.R.; Li, X.L. Allelopathic Effects of Maize Straw Water Extract on Shangmai 5226. J. Jiangxi Agric. 2017, 29, 28–32. [Google Scholar]
- Zhang, W.; Lu, L.Y.; Hu, L.Y.; Cao, W.; Sun, K.; Sun, Q.B.; Siddikee, A.; Shi, R.H.; Dai, C.C. Evidence for the Involvement of Auxin, Ethylene and ROS Signaling During Primary Root Inhibition of Arabidopsis by the Allelochemical Benzoic Acid. Plant Cell Physiol. 2018, 59, 1889–1904. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhang, G.W.; Yang, C.Q.; Zhang, L.; Ni, W.C. Allelopathic Effects of Wheat Straw Extract and Decomposition Solution on Germination and Seedling Growth of Cotton. J. Cotton Sci. 2016, 28, 375–383. [Google Scholar]
- Elisante, F.; Tarimo, M.T.; Ndakidemi, P.A. Allelopathic effect of seed and leaf aqueous extracts of datura stramonium on leaf chlorophyll content, shoot and root elongation of Cenchrus ciliaris and Neonotonia wightii. Am. J. Plant Sci. 2013, 4, 2332. [Google Scholar] [CrossRef]
- Jaballah, S.B.; Zribi, I.; Haouala, R. Physiological and biochemical responses of two lentil varieties to chickpea (Cicer arietinum L.) aqueous extracts. Sci. Hortic. 2017, 225, 74–80. [Google Scholar] [CrossRef]
- Gong, Z.Y. Effects of Straw Residue Return on Cucumber Seedling Growth and Soil Microbial Community. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2016. [Google Scholar]
- Chung, W.H. Unraveling new functions of superoxide dismutase using yeast model system: Beyond its conventional role in superoxide radical scavenging. J. Microbiol. 2017, 55, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, L.H.; Huang, Y.Z.; Geng, C.M.; Yin, B.H. Physiological and visible injury responses in different growth stages of winter wheat to ozone stress and the protection of spermidine. Atmos. Pollut. Res. 2015, 6, 596–604. [Google Scholar] [CrossRef]
- Hu, M.J.; Zhu, Y.Y.; Liu, G.S.; Gao, Z.Y.; Li, M.; Su, Z.H.; Zhang, Z.K. Inhibition on anthracnose and induction of defense response by nitric oxide in pitaya fruit. Sci. Hortic. 2019, 245, 224–230. [Google Scholar] [CrossRef]
- Zhao, Y. Study on the Activity of Endogenous Inhibitory Substances in Paris polyphylla Seeds and Their Identification by GC-MS. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2018. [Google Scholar]
- Chen, Y.; Wang, H.Y.; Lv, X.F.; Ma, H.J.; Li, Q.Z.; Cui, J.H. Effects of Corn Straw Extract on Seed Germination and Seedling Physiological Metabolism of Three Crops. Maize Sci. 2016, 24, 98–104. [Google Scholar]
- Wang, X.K. Principles and Techniques of Plant Physiology and Biochemistry Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Gao, J.F. Guide to Plant Physiology Experiments; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2003. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).