Transcriptome Analysis of Apple Leaves with Apple Necrotic Mosaic Virus-Associated Mosaic Symptoms
Abstract
1. Introduction
2. Results
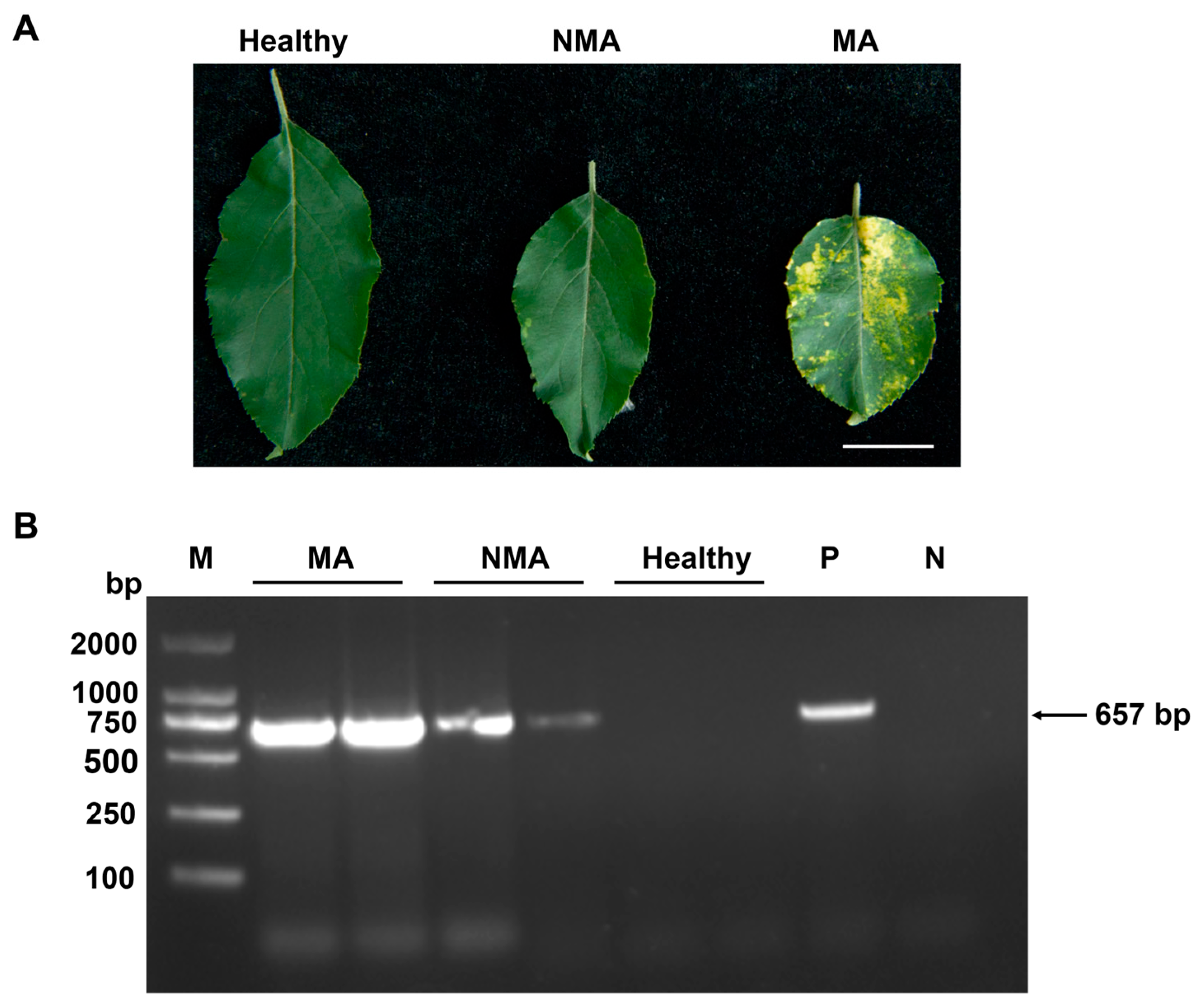
2.1. Confirmation of the Presence of ApNMV in Mosaic and Asymptomatic Apple Leaves
2.2. Overview of RNA-Seq Data
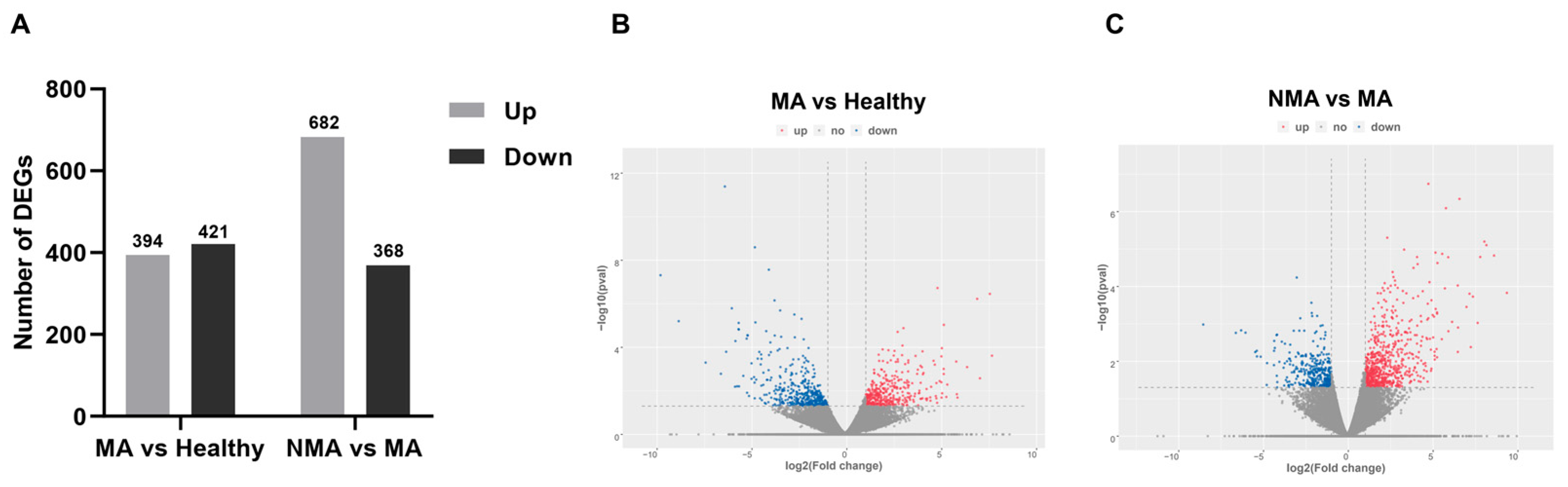
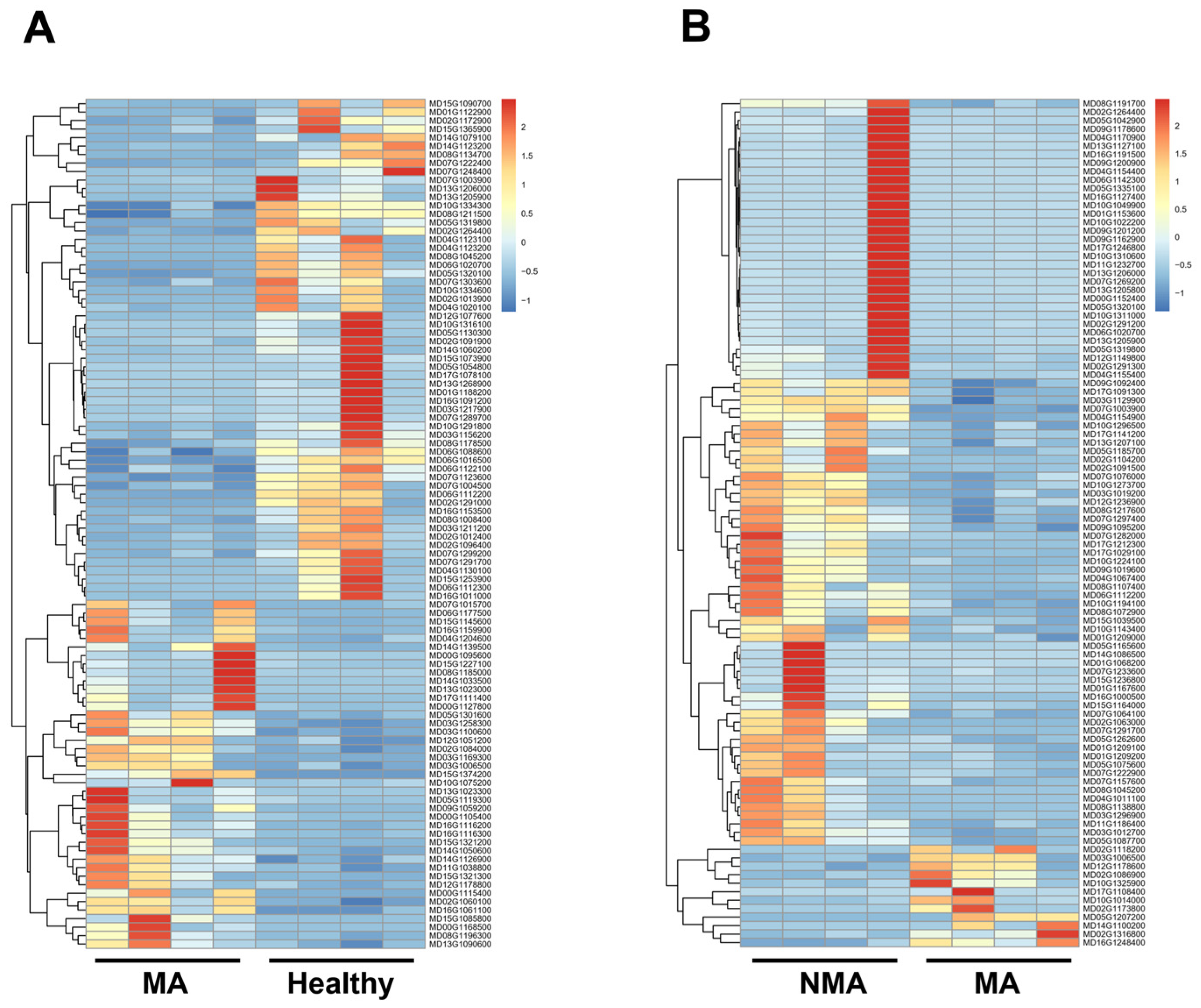
2.3. Analysis of DEGs in Apple Leaves with Healthy, NMA, and MA
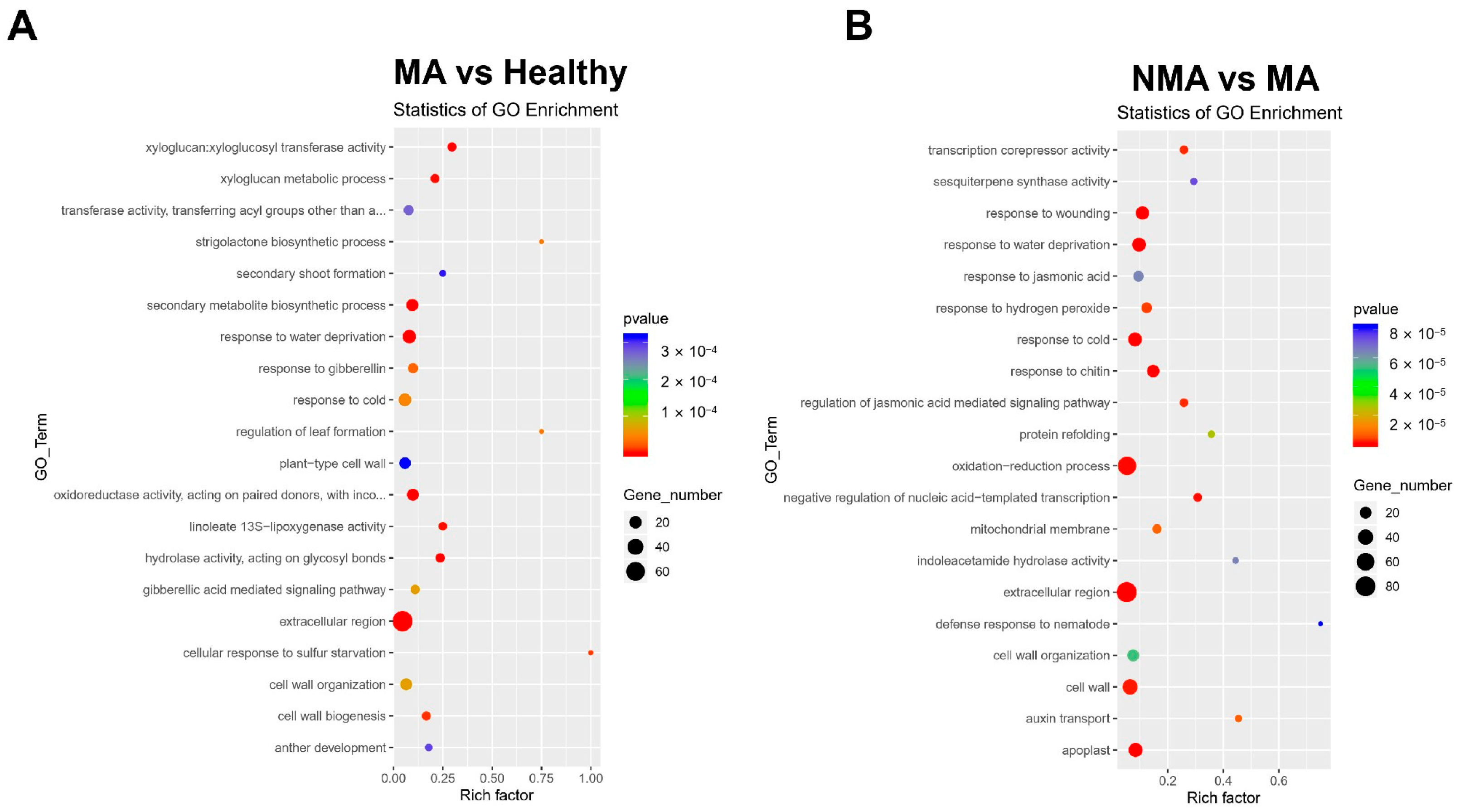
2.4. Functional Enrichment Analysis of DEGs
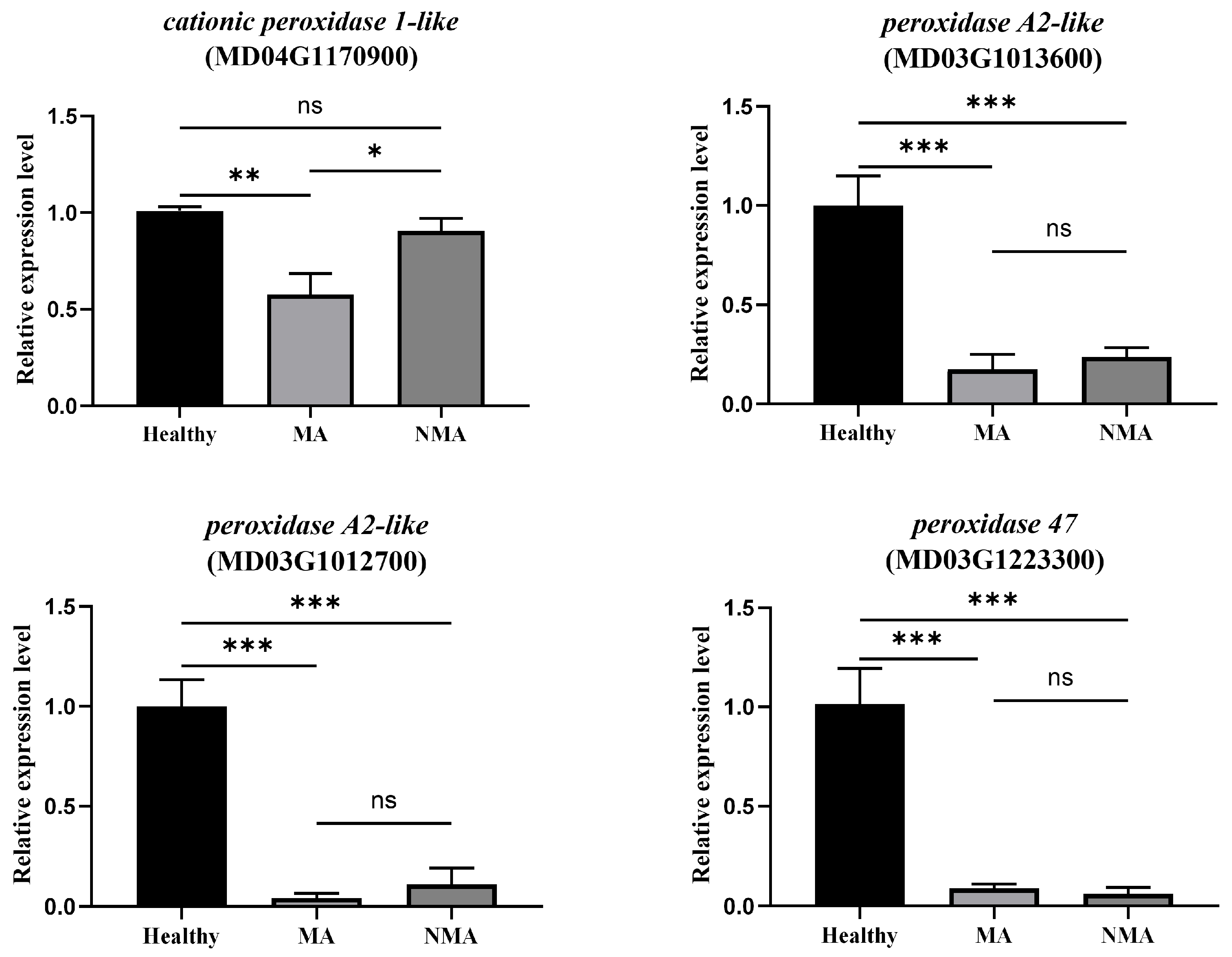
2.5. Validation of the Transcriptome Data by RT-qPCR
2.6. Mosaic Symptoms Development Is Correlated with Down-Regulation of Peroxidases and ROS Production in Apple Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Materials for RNA-Seq
4.2. RNA Extraction, Library Construction and Sequencing
4.3. Quality Control
4.4. RNA-Seq Reads Mapping to Reference Genome
4.5. Analysis of Differential Gene Expression, GO Term and KEGG Pathway Enrichment
4.6. RT-qPCR Analysis of DEGs
4.7. DAB Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzoor, S.; Nabi, S.U.; Baranwal, V.K.; Verma, M.K.; Parveen, S.; Rather, T.R.; Raja, W.H.; Shafi, M. Overview on century progress in research on mosaic disease of apple (Malus domestica Borkh) incited by apple mosaic virus/apple necrotic mosaic virus. Virology 2023, 587, 109846. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.D. Apple stem grooving virus-a review article. Int. J. Mod. Plant Anim. Sci. 2013, 1, 28–42. [Google Scholar]
- Mochizuki, T.; Ogata, Y.; Hirata, Y.; Ohki, S.T. Quantitative transcriptional changes associated with chlorosis severity in mosaic leaves of tobacco plants infected with Cucumber mosaic virus. Mol. Plant Pathol. 2014, 15, 242–254. [Google Scholar] [CrossRef]
- Bradford, F.C.; Joley, L. Infectious variegation in the apple. Agric. Res. 1933, 46, 901–908. [Google Scholar]
- Fukushi, T.; Tahama, Y. On apple mosaic. Mem. Fac. Agric. Hokkaido Univ. 1960, 3, 116–123. [Google Scholar]
- Fulton, R.W. Apple mosaic virus. In CMI/AAB Description Plant Viruses, No. 83; Association of Applied Biologists: Wellesbourne, UK, 1972. [Google Scholar]
- Petrzik, K.; Lenz, O. Apple mosaic virus in pome fruits. In Virus and Viruslike Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: St. Paul, MN, USA, 2011; pp. 25–28. [Google Scholar]
- Grimová, L.; Winkowska, L.; Konrady, M.; Ryšánek, P. Apple mosaic virus. Phytopathol. Mediterr. 2016, 55, 1–19. [Google Scholar]
- Noda, H.; Yamagishi, N.; Yaegashi, H.; Xing, F.; Xie, J.; Li, S.; Zhou, T.; Ito, T.; Yoshikawa, N. Apple necrotic mosaic virus, a novel ilarvirus from mosaic-diseased apple trees in Japan and China. J. Gen Plant Pathol. 2017, 83, 83–90. [Google Scholar] [CrossRef]
- Xing, F.; Robe, B.L.; Zhang, Z.; Wang, H.; Li, S. Genomic Analysis, Sequence Diversity, and Occurrence of Apple necrotic mosaic virus, a Novel Ilarvirus Associated with Mosaic Disease of Apple Trees in China. Plant Dis. 2018, 102, 1841–1847. [Google Scholar] [CrossRef]
- Shi, W.; Yao, R.; Sunwu, R.; Huang, K.; Liu, Z.; Li, X.; Yang, Y.; Wang, J. Incidence and Molecular Identification of Apple Necrotic Mosaic Virus (ApNMV) in Southwest China. Plants 2020, 9, 415. [Google Scholar] [CrossRef]
- Nabi, S.U.; Baranwal, V.K.; Yadav, M.K.; Rao, G.P. Association of Apple necrotic mosaic virus (ApNMV) with mosaic disease in commercially grown cultivars of apple (Malus domestica Borkh) in India. 3 Biotech 2020, 10, 122. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, F.J.; Zheng, P.F.; Xie, Y.H.; You, C.X.; Hao, Y.J. Determination of Protein Interactions among Replication Components of Apple Necrotic Mosaic Virus. Viruses 2020, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, Y.H.; Sun, P.; Zhang, F.J.; Zheng, P.F.; Wang, X.F.; You, C.X.; Hao, Y.J. Nitrate-inducible MdBT2 acts as a restriction factor to limit apple necrotic mosaic virus genome replication in Malus domestica. Mol. Plant Pathol. 2022, 23, 383–399. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xing, F.; Liang, J.; Zhang, Z.; Zhan, B.; Habili, N.; Wang, H.; Li, S. The ABI5-dependent down-regulation of mitochondrial ATP synthase OSCP subunit facilitates apple necrotic mosaic virus infection. J. Exp. Bot. 2023, 74, 4189–4207. [Google Scholar] [CrossRef]
- Roossinck, M.J. Lifestyles of plant viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Hakmaoui, A.; Pérez-Bueno, M.L.; García-Fontana, B.; Camejo, D.; Jiménez, A.; Sevilla, F.; Barón, M. Analysis of the antioxidant response of Nicotiana benthamiana to infection with two strains of Pepper mild mottle virus. J. Exp. Bot. 2012, 63, 5487–5496. [Google Scholar] [CrossRef]
- Lin, K.Y.; Wu, S.Y.; Hsu, Y.H.; Lin, N.S. MiR398-regulated antioxidants contribute to Bamboo mosaic virus accumulation and symptom manifestation. Plant Physiol. 2022, 188, 593–607. [Google Scholar] [CrossRef]
- Jiang, T.; Du, K.; Xie, J.; Sun, G.; Wang, P.; Chen, X.; Cao, Z.; Wang, B.; Chao, Q.; Li, X.; et al. Activated malate circulation contributes to the manifestation of light-dependent mosaic symptoms. Cell Rep. 2023, 42, 112333. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Zhang, M.; Jiao, S.; Guo, Y.; Jiang, T. The role of reactive oxygen species in plant-virus interactions. Plant Cell Rep. 2024, 43, 197. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]
- Clarke, S.F.; Guy, P.L.; Burritt, D.J.; Jameson, P.E. Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment. Physiol. Plant 2002, 114, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, Z.; Zhang, H.; Yang, Y.; Yang, X.; Zhao, X.; Guo, H.; Nagalakshmi, U.; Li, D.; Dinesh-Kumar, S.P.; et al. The MAPK-Alfin-like 7 module negatively regulates ROS scavenging genes to promote NLR-mediated immunity. Proc. Natl. Acad. Sci. USA 2023, 120, e2214750120. [Google Scholar] [CrossRef] [PubMed]
- Riedle-Bauer, M. Role of reactive oxygen species and antioxidant enzymes in systemic virus infections of plants. J. Phytopathol. 2000, 148, 297–302. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef]
- Islam, W.; Naveed, H.; Zaynab, M.; Huang, Z.; Chen, H.Y.H. Plant defense against virus diseases; growth hormones in highlights. Plant Signal. Behav. 2019, 14, 1596719. [Google Scholar] [CrossRef]
- Pacheco, R.; García-Marcos, A.; Manzano, A.; de Lacoba, M.G.; Camañes, G.; García-Agustín, P.; Díaz-Ruíz, J.R.; Tenllado, F. Comparative analysis of transcriptomic and hormonal responses to compatible and incompatible plant-virus interactions that lead to cell death. Mol. Plant-Microbe Interact. 2012, 25, 709–723. [Google Scholar] [CrossRef] [PubMed]
- García-Marcos, A.; Pacheco, R.; Manzano, A.; Aguilar, E.; Tenllado, F. Oxylipin biosynthesis genes positively regulate programmed cell death during compatible infections with the synergistic pair potato virus X-potato virus Y and Tomato spotted wilt virus. J. Virol. 2013, 87, 5769–5783. [Google Scholar] [CrossRef]
- Geri, C.; Love, A.J.; Cecchini, E.; Barrett, S.J.; Laird, J.; Covey, S.N.; Milner, J.J. Arabidopsis mutants that suppress the phenotype induced by transgene-mediated expression of cauliflower mosaic virus (CaMV) gene VI are less susceptible to CaMV-infection and show reduced ethylene sensitivity. Plant Mol. Biol. 2004, 56, 111–124. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. Linking development to defense: Auxin in plant-pathogen interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef]
- Padmanabhan, M.S.; Goregaoker, S.P.; Golem, S.; Shiferaw, H.; Culver, J.N. Interaction of the tobacco mosaic virus replicase protein with the Aux/IAA protein PAP1/IAA26 is associated with disease development. J. Virol. 2005, 79, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, M.S.; Kramer, S.R.; Wang, X.; Culver, J.N. Tobacco mosaic virus replicase-auxin/indole acetic acid protein interactions: Reprogramming the auxin response pathway to enhance virus infection. J. Virol. 2008, 82, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.K.; Kuo, S.Y.; Tu, C.W.; Hsu, Y.H.; Huang, Y.W.; Hu, C.C. The Role of Plant Transcription Factors in the Fight against Plant Viruses. Int. J. Mol. Sci. 2023, 24, 8433. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, W.; Li, J.; Liu, P.; Zhong, K.; Jin, P.; Xu, M.; Yang, J.; Chen, J. NbWRKY40 Positively Regulates the Response of Nicotiana benthamiana to Tomato Mosaic Virus via Salicylic Acid Signaling. Front. Plant Sci. 2021, 11, 603518. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ren, Y.; Wang, D.; Farooq, T.; He, Z.; Zhang, C.; Li, S.; Yang, X.; Zhou, X. A group I WRKY transcription factor regulates mulberry mosaic dwarf-associated virus-triggered cell death in Nicotiana benthamiana. Mol. Plant Pathol. 2022, 23, 237–253. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.Y.; Wu, P.; Xu, Z.S.; Que, F.; Wang, F.; Xiong, A.S. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum). BMC Genom. 2016, 17, 788. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Xi, J.; Wang, Y.; Guo, J.; Liu, Q.; Liu, Y.; Ma, Y.; Zhang, J.; Ma, F.; et al. The MdHSC70-MdWRKY75 module mediates basal apple thermotolerance by regulating the expression of heat shock factor genes. Plant Cell 2024, 36, 3631–3653. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Xing, F.; Yan, Q.; Zhang, Z.; Zhan, B.; Lu, M.; Ma, Y.; Wang, H.; Li, S.; Xie, J. Transcriptome Analysis of Apple Leaves with Apple Necrotic Mosaic Virus-Associated Mosaic Symptoms. Plants 2025, 14, 1787. https://doi.org/10.3390/plants14121787
Gao D, Xing F, Yan Q, Zhang Z, Zhan B, Lu M, Ma Y, Wang H, Li S, Xie J. Transcriptome Analysis of Apple Leaves with Apple Necrotic Mosaic Virus-Associated Mosaic Symptoms. Plants. 2025; 14(12):1787. https://doi.org/10.3390/plants14121787
Chicago/Turabian StyleGao, Dehang, Fei Xing, Qin Yan, Zhixiang Zhang, Binhui Zhan, Meiguang Lu, Yunlong Ma, Hongqing Wang, Shifang Li, and Jipeng Xie. 2025. "Transcriptome Analysis of Apple Leaves with Apple Necrotic Mosaic Virus-Associated Mosaic Symptoms" Plants 14, no. 12: 1787. https://doi.org/10.3390/plants14121787
APA StyleGao, D., Xing, F., Yan, Q., Zhang, Z., Zhan, B., Lu, M., Ma, Y., Wang, H., Li, S., & Xie, J. (2025). Transcriptome Analysis of Apple Leaves with Apple Necrotic Mosaic Virus-Associated Mosaic Symptoms. Plants, 14(12), 1787. https://doi.org/10.3390/plants14121787




