Integrated Cytological, Physiological, and Comparative Transcriptome Profiling Analysis of the Male Sterility Mechanism of ‘Xinli No.7’ Pear (Pyrus sp.)
Abstract
1. Introduction
2. Results
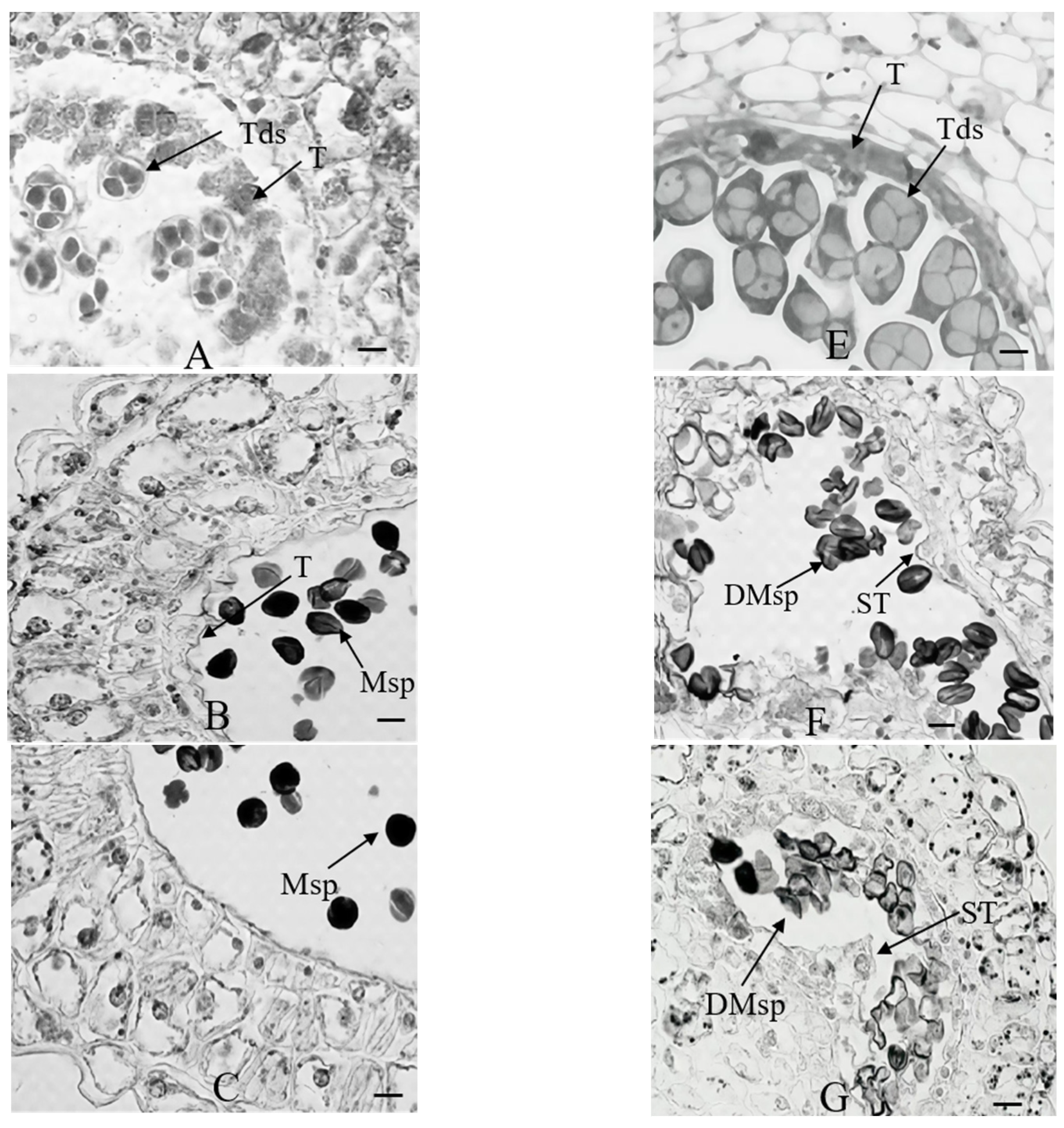
2.1. Morphological and Cytological Comparison
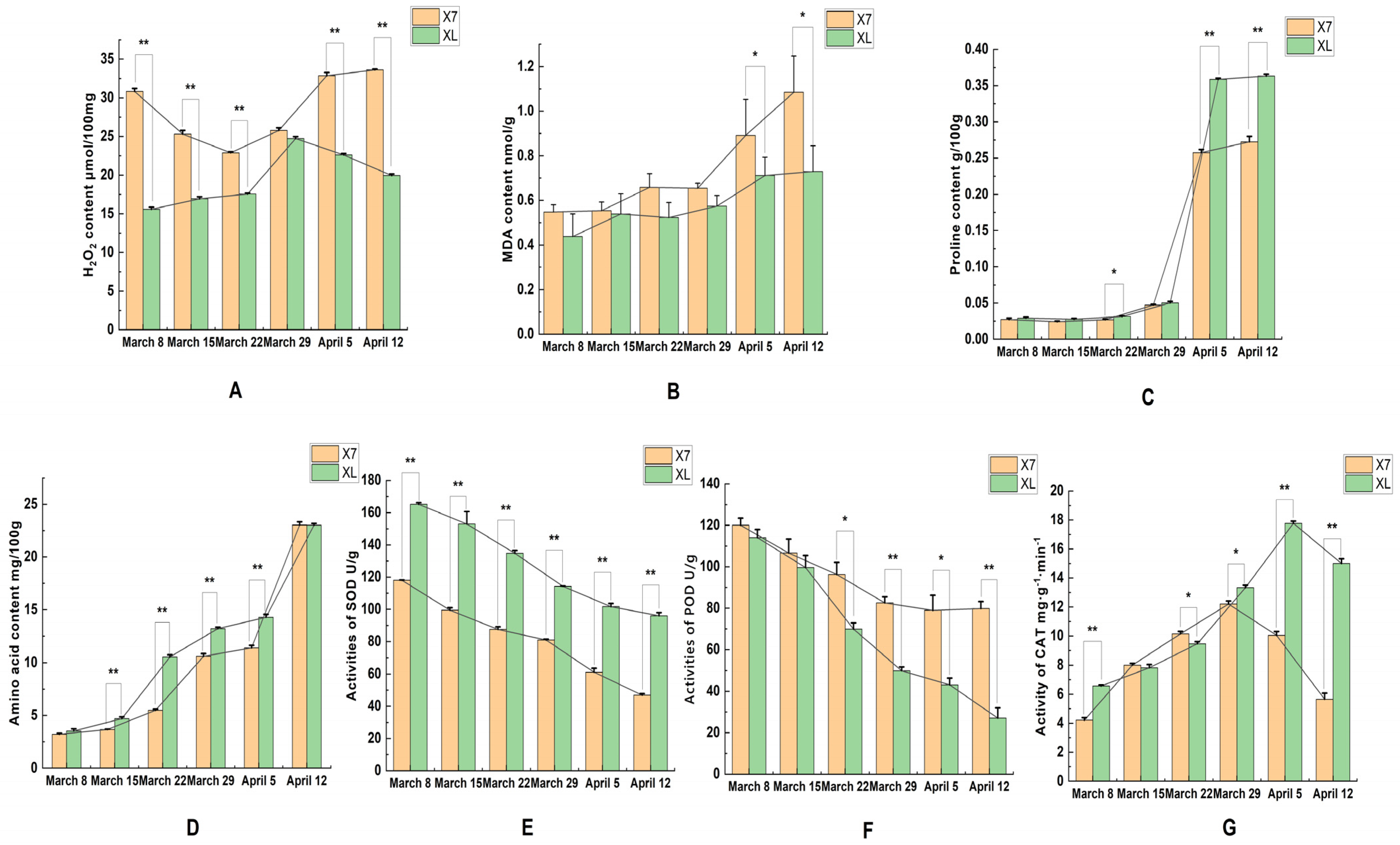
2.2. SOD, POD, CAT, and MDA Activities and Hydrogen Peroxide Content Proline Content and Amino Acid Content
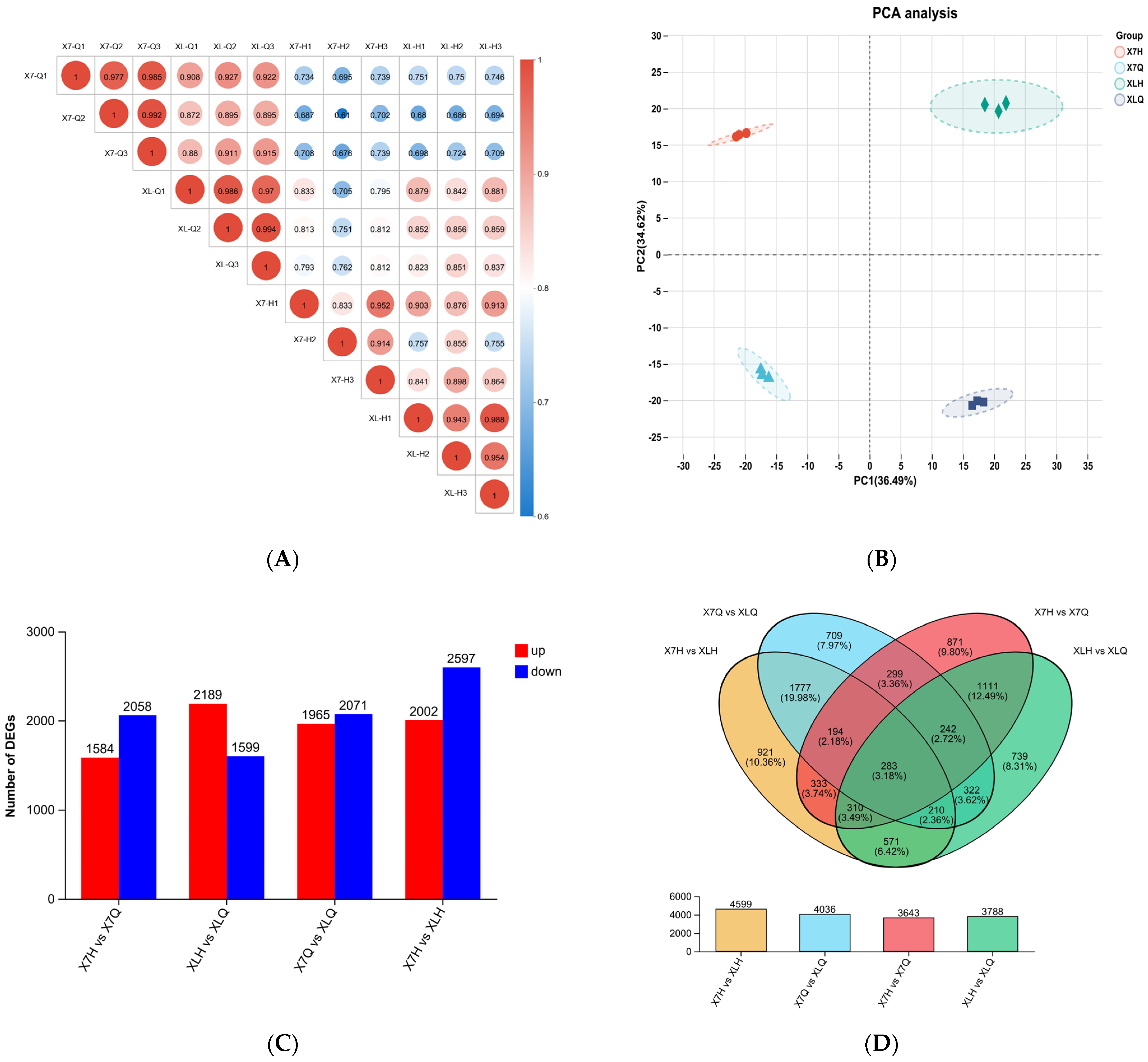
2.3. RNA-Seq Analysis and DEG Comparisons at Different Stages of Anther Development
2.3.1. Evaluation of Sequencing Data of Samples at Different Developmental Stages
2.3.2. Correlation Test Between Samples in Different Periods
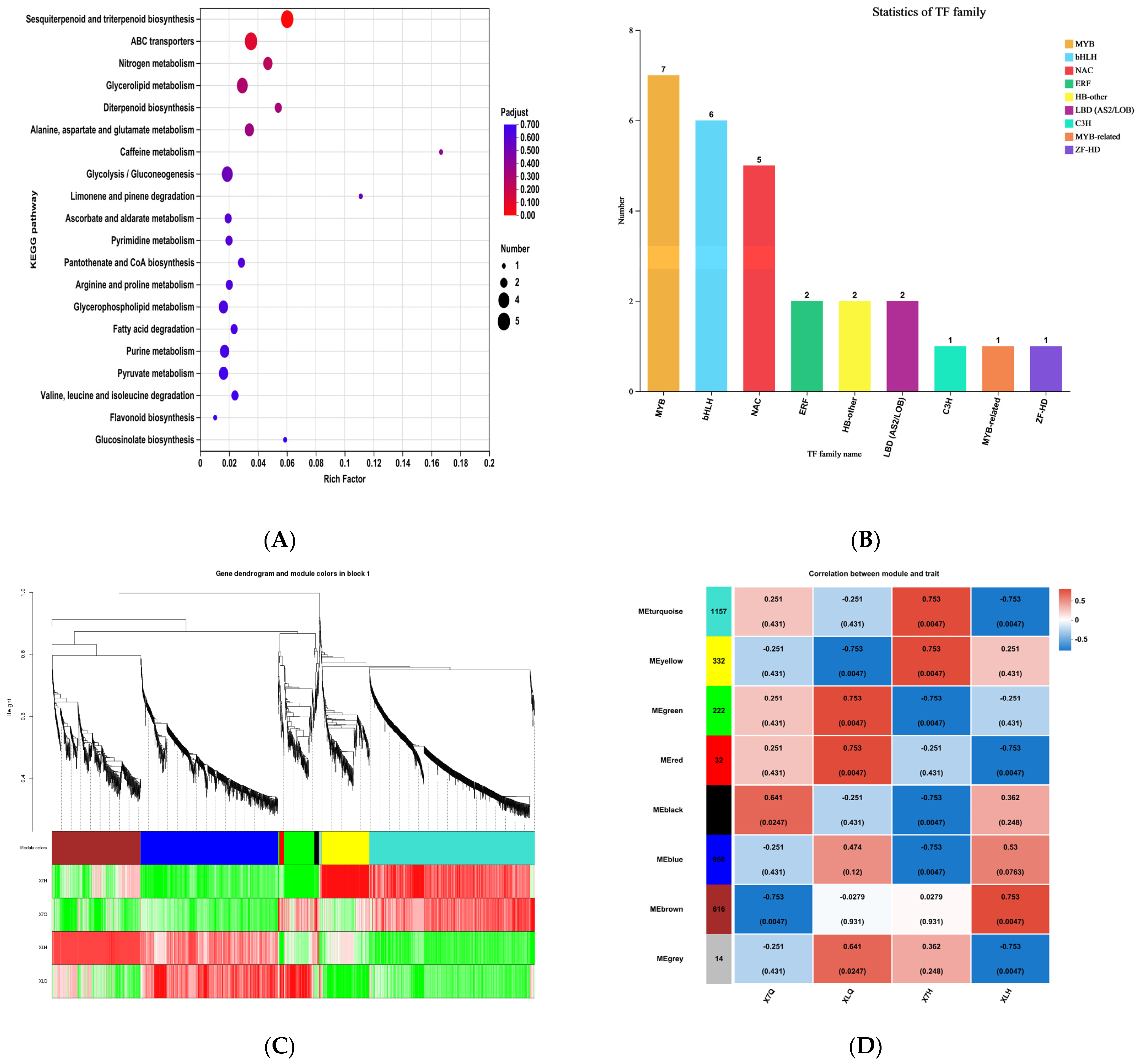
2.3.3. KEGG Enrichment Analysis
2.3.4. Transcription Factor Analysis
2.3.5. WGCNA
2.3.6. ‘Xinli No.7’ Male Sterile Gene Mining
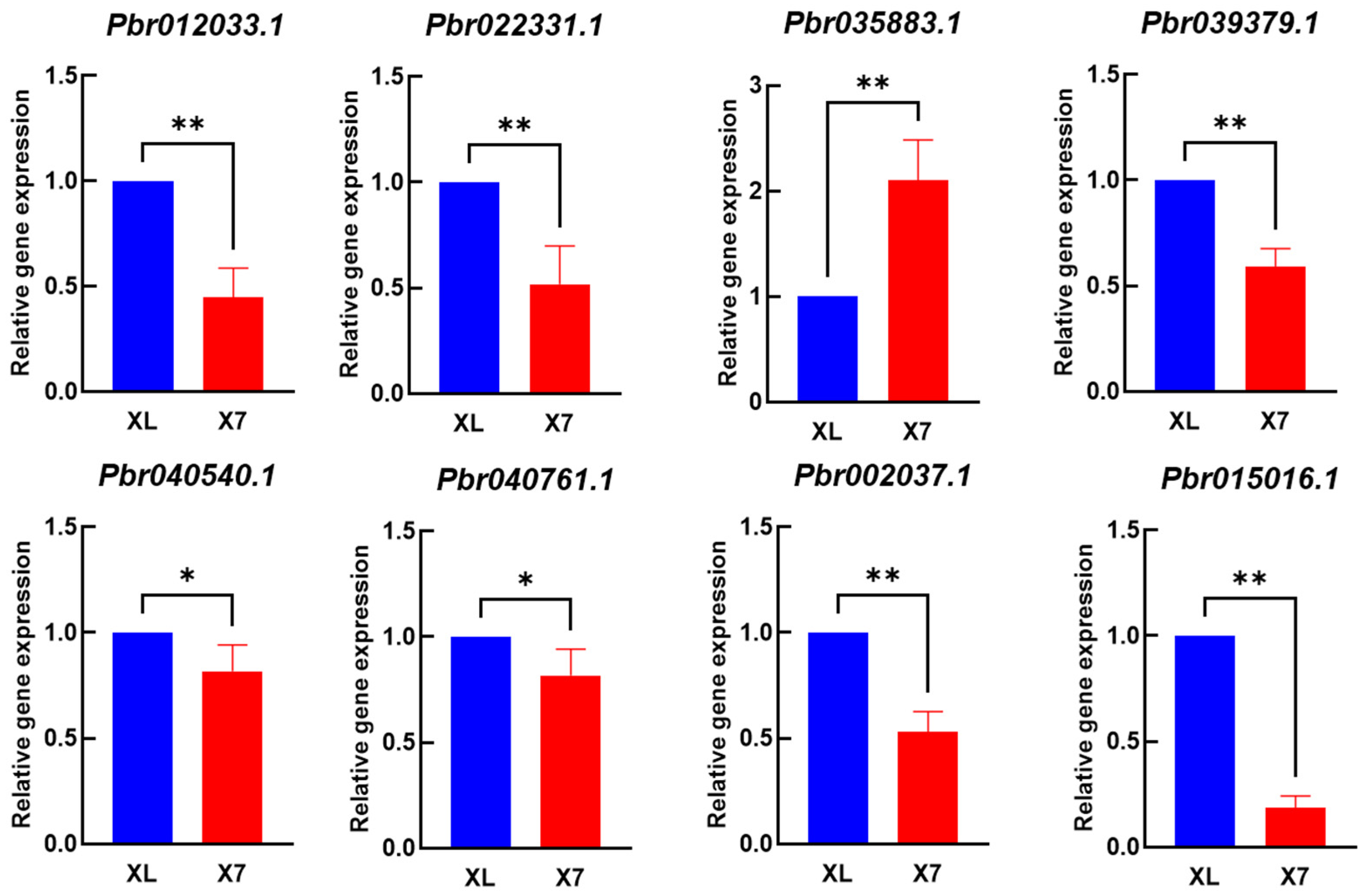
2.3.7. qRT-PCR Validation
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Observation of Cell Development
4.3. Determination of Physiological Indicators
4.4. Transcriptome Sequencing
4.5. Gene Differential Expression Analysis
4.6. Validation of Candidate Genes Using Quantitative Real-Time PCR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.P.; Yan, C.Y.; Cheng, Q.; Wang, X.J.; Wu, C.Y. Breeding of New Pear Variety Xinli No.7 with Characters of Early Maturity, Best Quality and Long Storage Life. J. Fruit Sci. 2002, 19, 36–38. [Google Scholar]
- Zhang, S.L.; Xie, Z.H. Current status, trends, main problems and the suggestions on development of pear industry in China. J. Fruit Sci. 2019, 36, 1067–1072. [Google Scholar]
- Mayr, E. Joseph Gottlieb Kolreuter’s contributions to biology. Osiris 1986, 2, 135–176. [Google Scholar] [CrossRef]
- Kaul, M.L.H. Male Sterility in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1988; pp. 887–892. [Google Scholar]
- Tan, Z.W.; JIN, B.K.; CAO, H.N. Cytological study on male sterility in Pyrus ussuriensis ‘xiaoxiangshui’. Agric. Sci. J. Yanbian Univ. 2013, 35, 28–31. [Google Scholar]
- Wang, B.P.; Wang, K.F.; Zhang, J.W.; Qiao, Y.; Shi, W.H.; Cao, Z. Physiological and biochemical characteristics studies on flower buds of recessive genetic male sterile line in Brassica napus. J. Hebei Agric. Univ. 2017, 40, 6–11. [Google Scholar]
- Zhou, G.C.; Shi, H.C.; Yü, X.J.; Liu, T.X.; Cai, L.; Ke, Y.P. Comparison of Physiological-biochemical Characteristic Between Sterile and Fertile Plants of Male Sterile K932MS in Maize. J. Maize Sci. 2018, 26, 6–11. [Google Scholar]
- Ding, X.; Li, J.; Zhang, H.; He, T.; Han, S.; Li, Y.; Yang, S.; Gai, J. Identification of miRNAs and their targets by high-throughput sequencing and degradome analysis in cytoplasmic male-sterile line NJCMS1A and its maintainer NJCMS1B of soybean. BMC Genom. 2016, 17, 24. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Li, S.; Zhang, L.; Song, X. Oxidative stress and aberrant programmed cell death are associated with pollen abortion in isonuclear alloplasmic male-sterile wheat. Front. Plant Sci. 2018, 9, 595. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, Z.; Ogutu, C.O.; Zhao, L.; Liao, L.; Zheng, B.; Zhang, R.; Wang, L.; Han, Y. Potential association of reactive oxygen species with male sterility in peach. Front. Plant Sci. 2021, 12, 653256. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Zhang, J.; Dong, S.; Liu, Q.; Wang, R. Transcriptomic analysis reveals candidate genes for male sterility in Prunus sibirica. Peer J. 2021, 9, e12349. [Google Scholar] [CrossRef]
- Dai, D.Y.; Yuan, L.W.; Sheng, Y.Y.; Zheng, Q. Transcriptome Sequencing of Stamen in Muskmelon Male Sterile Lines at Different Developmental Stages. Biotechnol. Bull. 2018, 34, 93–100. [Google Scholar]
- Nadeem, M.; Chen, A.; Hong, H.; Li, D.; Li, J.; Zhao, D.; Wang, W.; Wang, X.; Qiu, L. GmMs1 encodes a kinesin-like protein essential for male fertility in soybean (Glycine max L.). J. Integr. Plant Biol. 2021, 63, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, X.; Li, L.; Jiao, Z.; Huang, Q. Metabolism of reactive oxygen species in cytoplasmic male sterility of rice by marking upmost pulvinus interval. Appl. Biochem. Biotechnol. 2014, 175, 1263–1269. [Google Scholar] [CrossRef]
- Chen, L.; Ren, W.; Zhang, B.; Guo, H.; Fang, Z.; Yang, L.; Zhuang, M.; Lü, H.; Wang, Y.; Ji, J.; et al. Comparative transcriptome analysis reveals a potential regulatory network for ogura cytoplasmic male sterility in cabbage (Brassica oleracea L.). Int. J. Mol. Sci. 2023, 24, 6703. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.; Jo, M.; Jeon, J.S. Exploration of sugar and starch metabolic pathway crucial for pollen fertility in rice. Int. J. Mol. Sci. 2022, 23, 14091. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Liu, Y.; Yu, J.; Yao, G.; Yang, H.; Yang, D.; Wu, Y. An integrated transcriptome and metabolome analysis reveals the gene network regulating flower development in Pogostemon cablin. Front. Plant Sci. 2023, 14, 1201486. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.N. Profiling miRNAs Involved in Male Sterility of ‘Qianyang’ Seedless Ponkan and Cybrid HB Pummelo. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Zhang, Z.M. Priliminary Functional Dissection of Male-Sterility Gene MSLP in Citrus. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Wu, Y.Y.; Zhang, C.; Chen, G.F.; Sun, J.L.; Zhu, M.M.; Zhang, M. Cloning and Expression of Cs RAD51 Gene of Citrus suavissima ‘Wuzi Ougan’. J. Zhejiang For. Sci. Technol. 2017, 37, 1–9. [Google Scholar]
- Wang, W.R.; Fan, X.C.; Wang, C.; Liu, C.H.; Fang, J.G. Advances Research on Male Sterility in Fruit Crops. Mol. Plant Breed. 2019, 17, 245–254. [Google Scholar]
- Han, A.H.; Yi, K.L.; Song, L.Q.; Xin, H.; Liu, J.P. Cytological study of male sterility and pollen abortion in pear (Pyrus spp.) variety “Xinli 7”. J. Southwest Univ. (Nat. Sci. Ed.) 2004, 26, 64–67. [Google Scholar]
- Song, X.L.; Sun, X.Z.; Liu, Y.X. Carbohydrate and Free Amino Acids in Anthers of ms5ms6 Genetic Male Sterile Cotton. Cotton Sci. 2001, 13, 334–336. [Google Scholar]
- Liu, H.Y. Study on the Genetic Traits, Biochemical Basis and Molecular Marker of Dominant Genic Male Sterility in Sesame (Sesamum indicum L.); Chinese Academy of Agricultural Sciences: Beijing, China, 2013.
- Akihiro, U.; Shi, W.M.; Kazutsuka, S.; Mariko, S.; Tetruko, T. Functional analysis of salt-inducible proline transporter of barley roots. Plant Cell Physiol 2001, 42, 1282–1289. [Google Scholar]
- Wang, Y.Q. Studies on the Physiological, Biochemical and Molecular Mechanisms of the Sterility Characteristics of Watermelon Genic Male Sterile Line Se18. Ph.D. Dissertation, Northwest A&F University, Xianyang, China, 2020. [Google Scholar]
- Liu, Q.Y.; Zhu, X.W.; Liu, F.H.; Shu, W.Y.; Cui, A.H.; Zhao, T. Relationship Between Free Proline Content and Male Sterility of Tobacco. Tob. Sci. Technol. 2007, 5, 58–61. [Google Scholar]
- Palif, G.; Printer, L.; Palif, Z. The proline content and fertility of the pollen inbreed maize lines. Acta Bet Acad. Sci. Hung. 1981, 27, 179–187. [Google Scholar]
- Feng, X.L.; Fan, G.Y.; Su, X.; Song, G.L.; Shi, G.L.; Wang, F.; Qiu, F.C.; Wang, X.M.; Zhao, Z.H. Advances in Physiological and Biochemical Study on Plant Male Sterility. Crops 2012, 3, 6–11. [Google Scholar]
- Huang, L.; Xiang, J.; Liu, J.Z.; Rong, T.Z.; Wang, J.; Lu, Y.L.; Tang, Q.L.; Wen, W.; Cao, M.J. Expression characterization of genes for CMS-C in maize. Protoplasma 2012, 249, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lopez-Giraldez, F.; Townsend, J.P.; Irish, V.F. RBE controls microRNA164 expression to effect floral organogenesis. Development 2012, 139, 2161–2169. [Google Scholar] [CrossRef]
- Jiang, P.D.; Zhang, X.Q.; Zhu, Y.G.; Zhu, W.; Xie, H.Y.; Wang, X.D. Metabolism of reactive oxygen species in cotton cytoplasmic male sterility and its restoration. Plant Cell Rep. 2007, 26, 1627–1634. [Google Scholar] [CrossRef]
- Wang, K.; Gao, F.; Ji, Y.X.; Liu, Y.; Dan, Z.W.; Yang, P.F.; Zhu, Y.G.; Li, S.Q. ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. New Phytol. 2013, 198, 408–418. [Google Scholar] [CrossRef]
- Yan, J.J.; Tian, H.; Wang, S.Z.; Shao, J.Z.; Zheng, Y.Z.; Zhang, H.Y.; Guo, L.; Ding, Y. Pollen developmental defects in ZD-CMS rice line explored by cytological, molecular and proteomic approaches. J. Proteom. 2014, 108, 110–123. [Google Scholar] [CrossRef]
- Luo, D.P.; Xu, H.; Liu, Z.L.; Guo, J.X.; Li, H.Y.; Chen, L.T.; Fang, C.; Zhang, Q.Y.; Bai, M.; Yao, N.; et al. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573–577. [Google Scholar] [CrossRef]
- Yan, M.Y.; Xie, D.L.; Cao, J.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Zhou, J.; Yu, J.Q. Brassinosteroid-mediated reactive oxygen species are essential for tapetum degradation and pollen fertility in tomato. Plant J. 2020, 102, 931–947. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Li, J.; Ma, L.; Wang, H.L.; Zhou, H.; Jiang, D.G.; Liu, Z.L.; Zhuang, C.X. OsAGO2 controls ROS production and the initiation of tapetal PCD by epigenetically regulating OsHXK1 expression in rice anthers. Proc. Natl. Acad. Sci. USA 2019, 116, 7549–7558. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.A.; Patil, S.B.; Uzair, M.; Fang, J.J.; Zhao, J.F.; Guo, T.T.; Yuan, S.J.; Uzair, M.; Luo, Q.; Shi, J.X.; et al. DEGENERATED PANICLE AND PARTIAL STERILITY 1 (DPS1) encodes a cystathionine β-synthase domain containing protein required for anther cuticle and panicle development in rice. New Phytol. 2020, 225, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Wu, Q.; Xie, Z.Z.; Yu, B.; Zeng, R.F.; Min, Q.; Huang, J.L. OsFPFL4 is Involved in the Root and Flower Development by Affecting Auxin Levels and ROS Accumulation in Rice (Oryza sativa). Rice 2020, 13, 2. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shi, L.; Li, S.H.; Han, H.Z.; Wang, F.; Zhang, N. Changes of Physiological Indexes and Adaptability Evaluation of Changes of Physiological Indexes and Adaptability Evaluation of. Anhui Agric. Sci. Bull. 2021, 27, 10–12+158. [Google Scholar]
- Chen, X.Y.; Shi, J.M.; Peng, C.Q.; Yang, P.; Guan, P. Studies on Physiological and Biochemical Characters of Male Sterile Rosa sterilis during Anther Development Stage. J. Trop. Subtrop. Bot. 2018, 26, 604. [Google Scholar]
- Chen, W.; Fu, w.; Wu, J.M.; Zhong, L.; Wang, X.L.; Han, Y.F.; Shu, J.H. Cytology, physiological and biochemical characterization in microspore abortion of male sterile plant in Festuca arundinacea. Plant Physiol. J. 2017, 53, 531761–531771. [Google Scholar]
- Yuan, J.Y. Study on Cytological and Characteristics of Physiological and Biochemical in the New CMS Line of Non-Heading Chinese Cabbage. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2005. [Google Scholar]
- Nie, Z.X.; Chen, J.; Song, Y.; Fu, H.; Wang, H.; Niu, Q.; Zhu, W. Comparative Transcriptome Analysis of the Anthers from the Cytoplasmic Male-Sterile Pepper Line HZ1A and Its Maintainer Line HZ1B. Horticulturae 2021, 7, 1560–1573. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Z.; Hu, L.; Li, C.; Wang, X.; Yue, Z.; Yin, G. Comparative Transcriptome Analysis of Male Sterile Anthers Induced by High Temperature in Wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 1450–1459. [Google Scholar] [CrossRef]
- Xie, H.; Peng, X.; Qian, M.; Cai, Y.; Ding, X.; Chen, Q.; Cai, Y. The chimeric mitochondrial gene orf182 causes non-pollen-type abortion in Dongxiang cytoplasmic male-sterile rice. Plant J. 2018, 95, 715–726. [Google Scholar] [CrossRef]
- Jacobowitz, J.R.; Doyle, W.C.; Weng, J.K. PRX9 and PRX40 are extensin peroxidases essential for maintaining tapetum and microspore cell wall integrity during Arabidopsis anther development. Plant Cell 2019, 31, 848–861. [Google Scholar] [CrossRef]
- Choi, H.; Jin, J.Y.; Choi, S.; Hwang, J.U.; Kim, Y.Y.; Suh, M.C.; Lee, Y. An ABCG/WBC-type ABC transporter is essential for transport of sporopollenin precursors for exine formation in develo ping pollen. Plant J. 2011, 65, 181–193. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, J.X.; Zhao, G.C.; Zhang, D.B.; Liang, W.Q. Post-meiotic deficient anther1 (PDA1) encodes an ABC transporter required for the development of anther cuticle and pollen exine in rice. J. Plant Biol. 2013, 56, 59–68. [Google Scholar] [CrossRef]
- Qin, P.; Tu, B.; Wang, Y.; Deng, L.; Quilichini, T.D.; Li, T.; Li, S. ABCG15 encodes an ABC transporter protein, and is essential for post-meiotic anther and pollen exine develop ment in rice. Plant Cell Physiol. 2013, 54, 138–154. [Google Scholar] [CrossRef]
- Yang, H.X.; Lu, P.L.; Wang, Y.X.; Ma, H. The transcriptome landscape of Arabidopsis male meiocytes from high-throughput sequencing: The complexity and evolution of the meiotic process. Plant J. 2011, 65, 503–516. [Google Scholar] [CrossRef]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.K.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A b HLH-type tran scription factor, ABA-INDUCIBLE BHLH-TYPE TRAN SCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmon-ate signaling in Arabidopsis. Plant Cell 2013, 25, 1641–1656. [Google Scholar] [CrossRef]
- Sun, X.; Chaomuliga; Sun, Q.; Zhou, L.; Sun, Y.Y.; Zhang, Y.H. Effects of NaCl Stress on Osmoregulation Substances and Hormones in Bromus inermis. J. Xinjiang Agric. Univ. 2023, 46, 180–186. [Google Scholar]
- Kim, H.M.; Jeon, S.; Chung, O.; Jun, J.H.; Kim, H.S.; Blazyte, A.; Lee, H.Y.; Yu, Y.; Cho, Y.S.; Bolser, D.M.; et al. Comparative analysis of 7 short read sequencing platforms using the Korean Reference Genome: MGI and Illumina sequencing benchmark for whole-genome sequencing. GigaScience 2021, 10, giab014. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference ge nome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empiri cal Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef]
- Shi, J.; Chen, L.; Zheng, R.; Guan, C.P.; Wang, Y.J.; Liang, W.Y.; Yang, S.J.; Wang, L.J.; Gong, L.; Zheng, G.B.; et al. Comparative phenotype and microRNAome in developing anthers of wild-type and male-sterile Lycium barbarum L. Plant Sci. 2018, 274, 349–359. [Google Scholar] [CrossRef]

| Sample | Raw Reads/Mb | Clean Reads/Mb | Q20 (%) | Q30 (%) | GC Content (%) | Total Mapped (%) |
|---|---|---|---|---|---|---|
| X7_Q1 | 55.73 | 55.44 | 98.99 | 96.75 | 48.29 | 75.65 |
| X7_Q2 | 50.61 | 50.33 | 98.94 | 96.58 | 47.91 | 75.58 |
| X7_Q3 | 53.07 | 52.75 | 98.96 | 96.67 | 47.75 | 75.25 |
| XL_Q1 | 48.77 | 48.52 | 98.97 | 96.65 | 48.03 | 75.25 |
| XL_Q2 | 51.20 | 50.92 | 98.96 | 96.65 | 48.14 | 76.26 |
| XL_Q3 | 57.08 | 56.76 | 98.98 | 96.74 | 48.37 | 75.60 |
| X7_H1 | 56.37 | 56.04 | 98.96 | 96.66 | 47.82 | 75.24 |
| X7_H2 | 56.04 | 55.71 | 98.96 | 96.66 | 47.76 | 76.96 |
| X7_H3 | 49.42 | 49.13 | 98.9 | 96.44 | 47.34 | 74.13 |
| XL_H1 | 50.57 | 50.36 | 98.99 | 96.75 | 47.49 | 75.63 |
| XL_H2 | 40.69 | 40.46 | 98.92 | 96.53 | 47.60 | 77.01 |
| XL_H3 | 45.00 | 44.76 | 98.96 | 96.67 | 47.98 | 75.23 |
| Gene_id | Module | Regulate | X7H | XLH | Rate (XLH/X7H) | NR_Hit-Name |
|---|---|---|---|---|---|---|
| Pbr012033.1 | green | down | 1.096667 | 11.34667 | 10.3465 | XP_009353802.1 |
| Pbr022331.1 | blue | down | 0.86 | 88.17 | 102.5233 | XP_009378509.2 |
| Pbr035883.1 | green | up | 415.6033 | 184.61 | 0.4442 | XP_009358773.2 |
| Pbr039379.1 | blue | down | 7.143333 | 27.24 | 3.8133 | XP_009360723.2 |
| Pbr040540.1 | yellow | down | 2.25 | 10.65333 | 4.7348 | XP_009346294.1 |
| Pbr040761.1 | brown | down | 1.473333 | 20.89 | 14.1787 | XP_009361467.2 |
| Pbr002037.1 | blue | down | 0.326667 | 2.213333 | 6.7755 | XP_009363200.2 |
| Pbr015016.1 | green | down | 1.263333 | 12.81333 | 10.1425 | XP_009372105.2 |
| Gene_id | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Pbr012033.1 | TGGCCTCATGATTTGCCTGT | CCGTAATGCCCACCAAGTCT |
| Pbr022331.1 | GCTAGACGTCAACCCGCTTTTC | GGAGGAATGATGATTTTGGTGACGG |
| Pbr035883.1 | TTCCTTTCTTGCCAACACCATCCC | TACGCCCGACCTGCTGACTTC |
| Pbr039379.1 | GGAATGGTTTTGGTTTCTCCCG | ACCGGCAGTATCACACATTCTT |
| Pbr040540.1 | CGTCACCGCCGCCATTAAG | CCGCTTCACTACACCATCATCG |
| Pbr040761.1 | CCGCTTCACTACACCATCATCG | CCTCCGAGTGCGGTTAGGAG |
| Pbr002037.1 | CTCCTTCAACAACACCACCACCTC | CCTCTGAGCCTTGCGAACTTCC |
| Pbr015016.1 | CACTATTTCTGGGCGTTGCGA | AGGCTGCCATTCTTGGGTCT |
| Pbr030912.1 | AGCCTTCCTGCCAACGAGT | TTGCTTCTCACCCTTGATGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, X.; Luo, Y.; Ma, Q.; Luo, Z.; Xuan, J.; Wu, C.; Yan, F. Integrated Cytological, Physiological, and Comparative Transcriptome Profiling Analysis of the Male Sterility Mechanism of ‘Xinli No.7’ Pear (Pyrus sp.). Plants 2025, 14, 1783. https://doi.org/10.3390/plants14121783
Li H, Li X, Luo Y, Ma Q, Luo Z, Xuan J, Wu C, Yan F. Integrated Cytological, Physiological, and Comparative Transcriptome Profiling Analysis of the Male Sterility Mechanism of ‘Xinli No.7’ Pear (Pyrus sp.). Plants. 2025; 14(12):1783. https://doi.org/10.3390/plants14121783
Chicago/Turabian StyleLi, Hao, Xiangyü Li, Yüjia Luo, Quanhui Ma, Zhi Luo, Jiayuan Xuan, Cuiyun Wu, and Fenfen Yan. 2025. "Integrated Cytological, Physiological, and Comparative Transcriptome Profiling Analysis of the Male Sterility Mechanism of ‘Xinli No.7’ Pear (Pyrus sp.)" Plants 14, no. 12: 1783. https://doi.org/10.3390/plants14121783
APA StyleLi, H., Li, X., Luo, Y., Ma, Q., Luo, Z., Xuan, J., Wu, C., & Yan, F. (2025). Integrated Cytological, Physiological, and Comparative Transcriptome Profiling Analysis of the Male Sterility Mechanism of ‘Xinli No.7’ Pear (Pyrus sp.). Plants, 14(12), 1783. https://doi.org/10.3390/plants14121783






