Interactions Between the Transcription Factor BOL/DRNL/ESR2 and the Jasmonate Pathway
Abstract
1. Introduction
2. Results
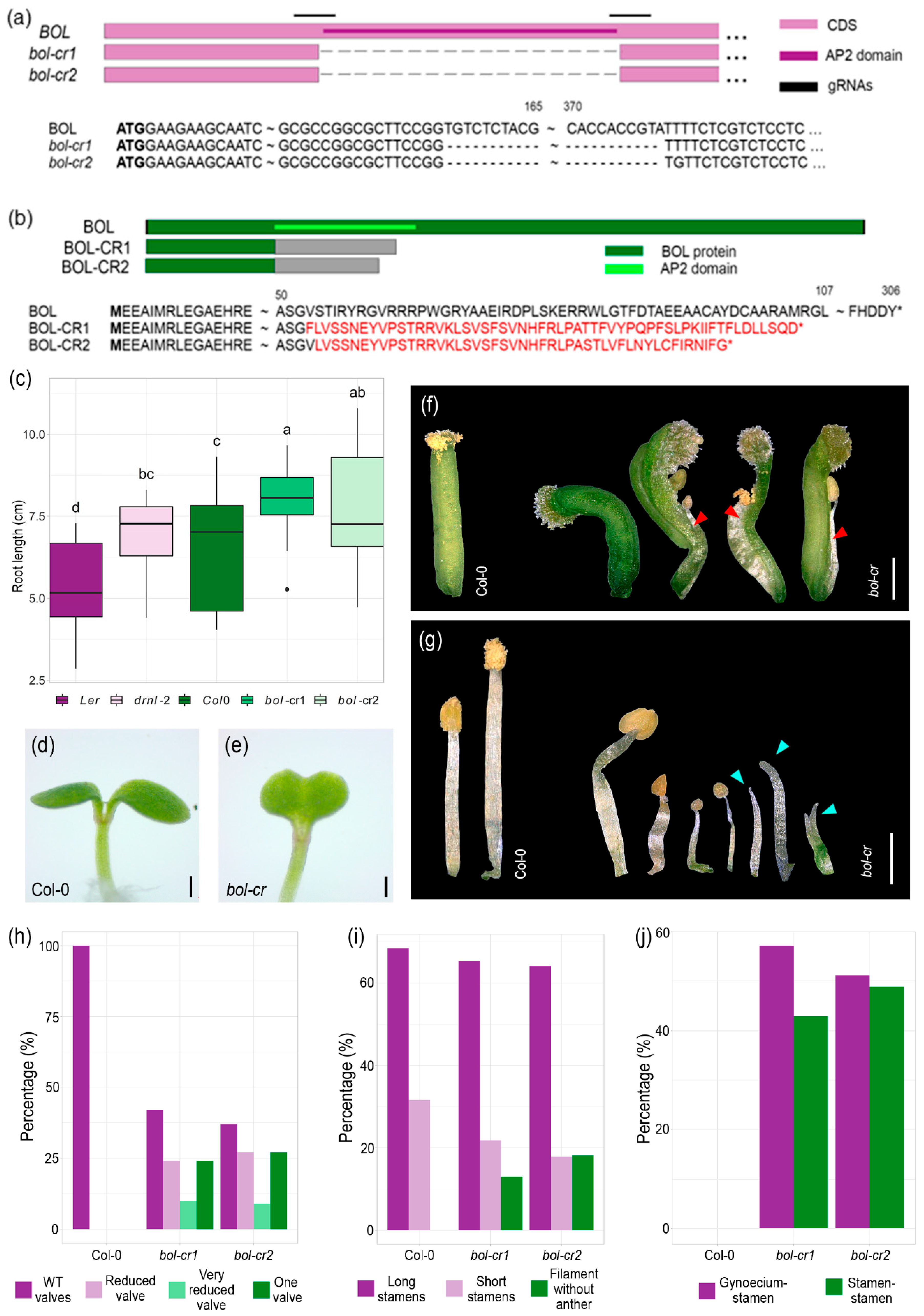
2.1. Gene-Edited bol-cr Mutant Alleles Show Stamen, Gynoecium, and Cotyledon Defects
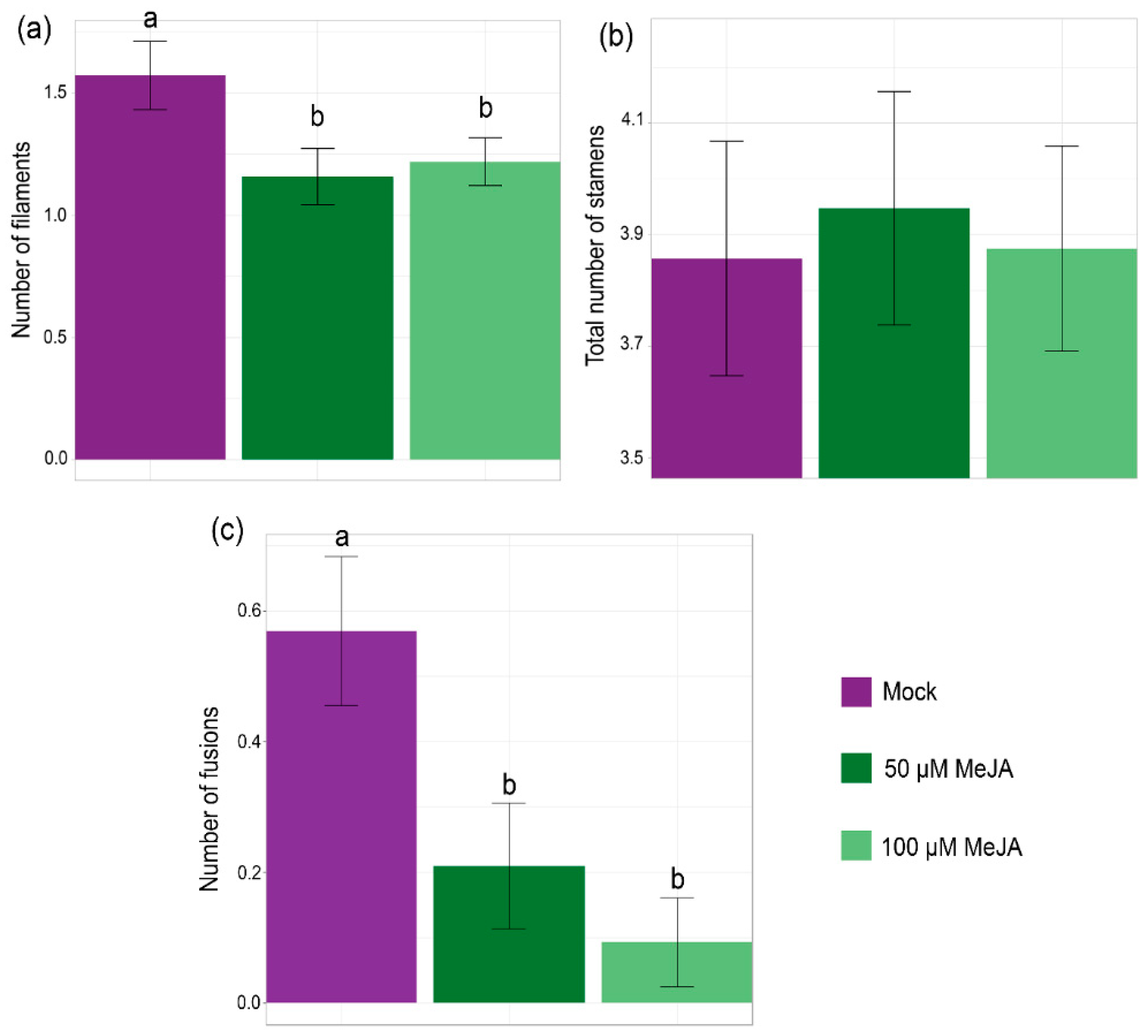
2.2. Exogenous Application of MeJA to Bol Loss-of-Function Mutants Partially Recovers the Wild-Type Stamen Phenotype
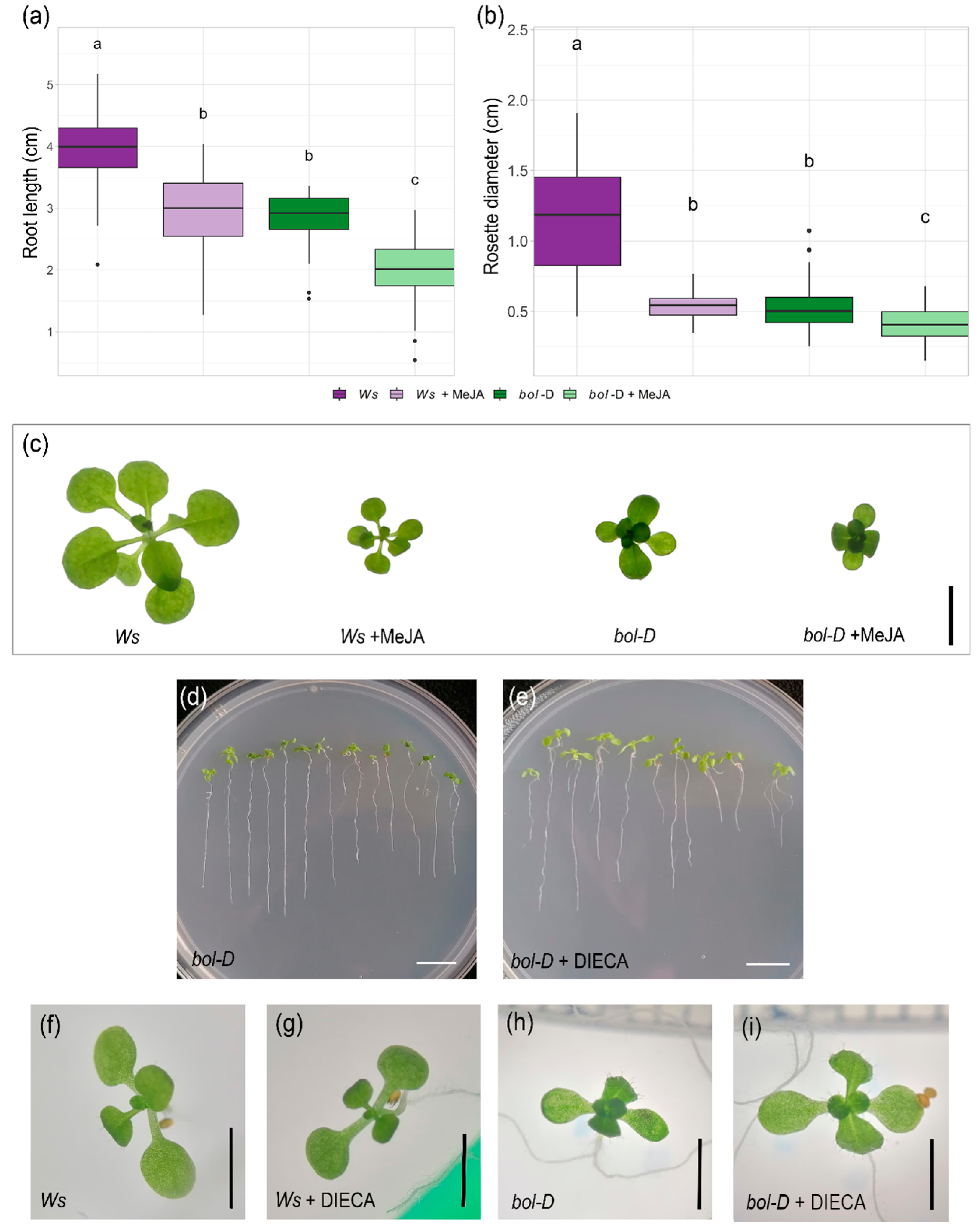
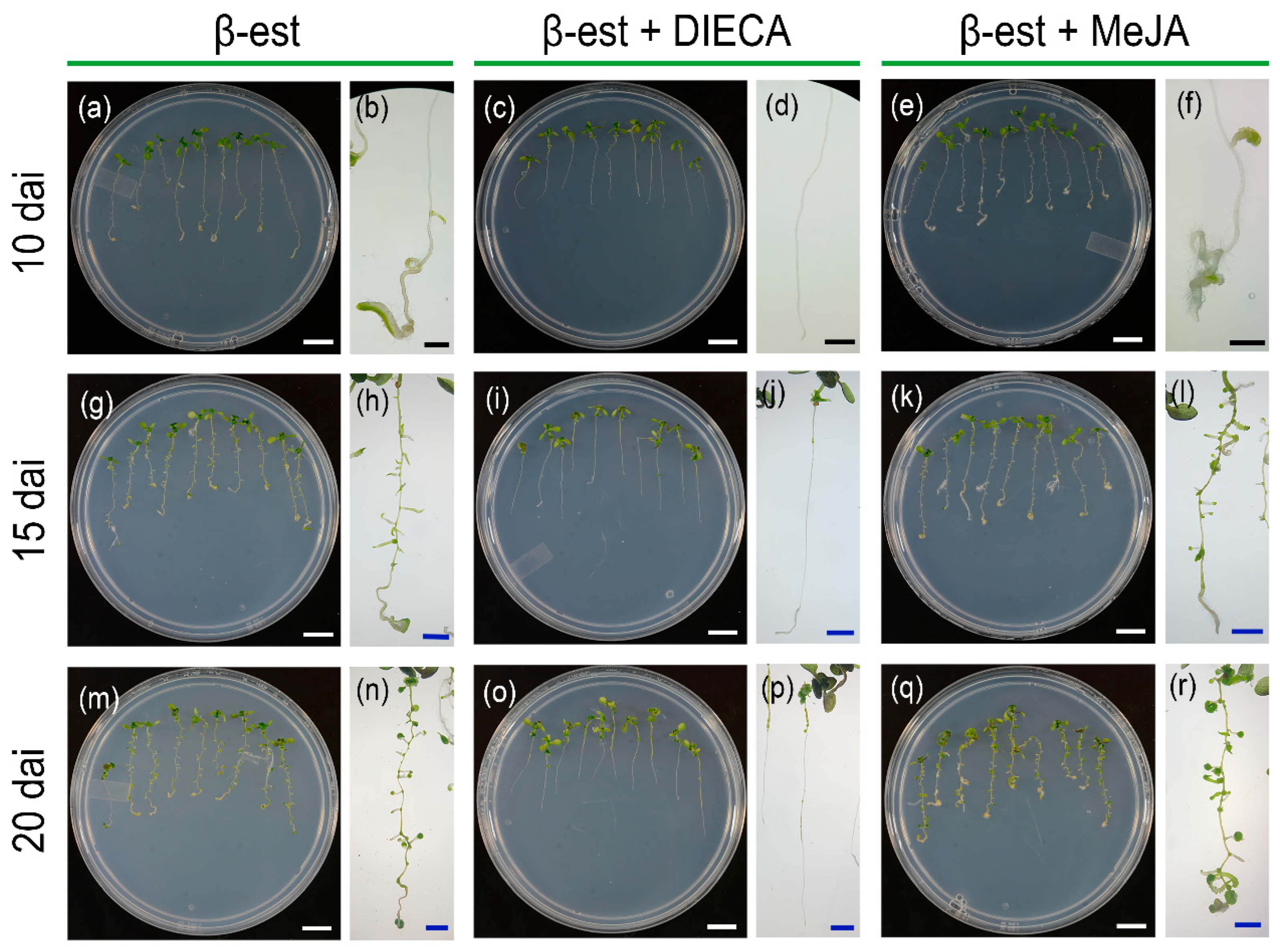
2.3. MeJA Application or Inhibition Mimic or Partially Restore Bol-D Phenotypes
2.4. Changes in BOL Expression Cause Differences in Jasmonic Acid Accumulation
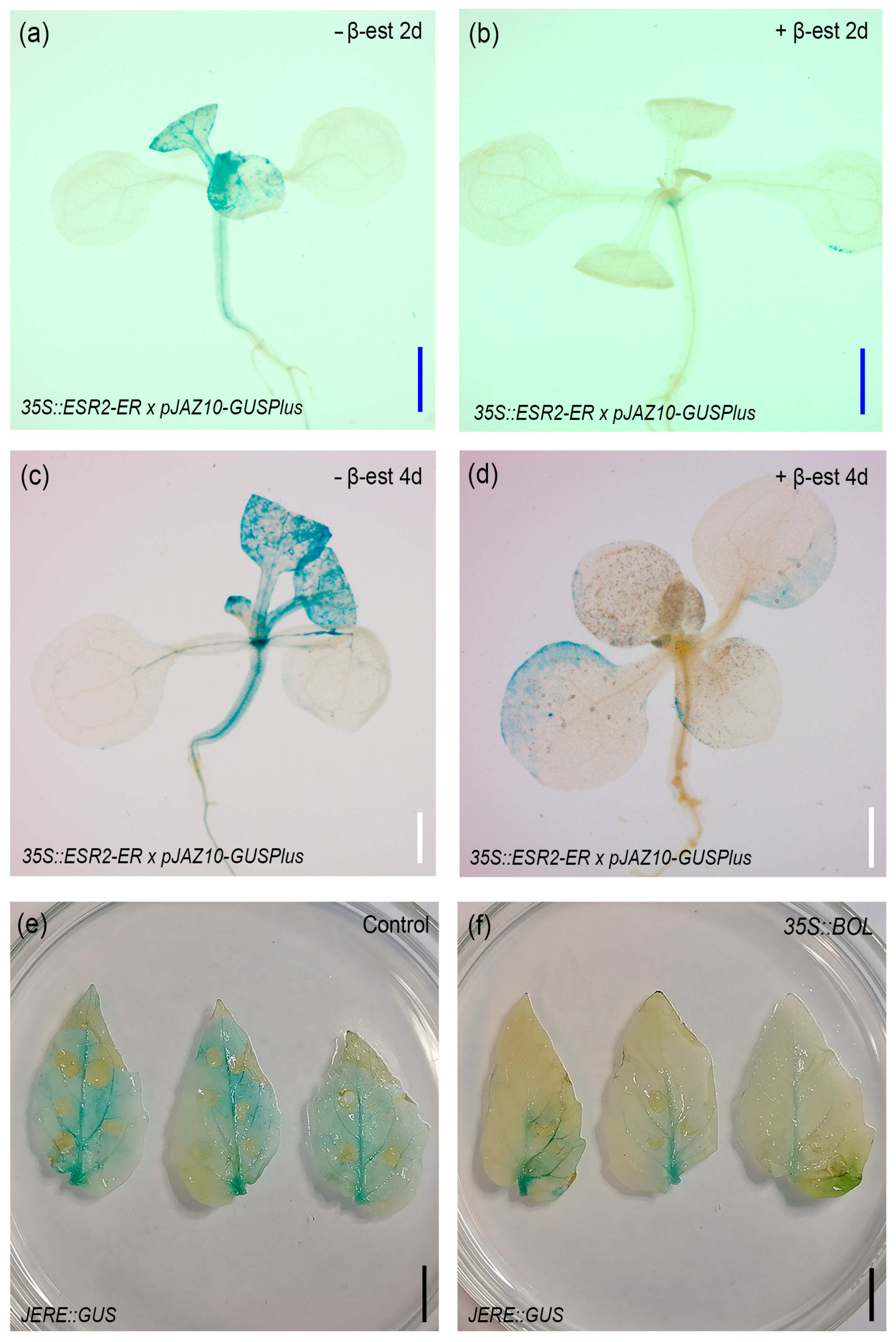
2.5. BOL Affects the Expression of the JA Response Marker pJAZ10::GUSPlus
2.6. Jasmonate Inhibition Impairs the Formation of BOL-Induced Green Calli
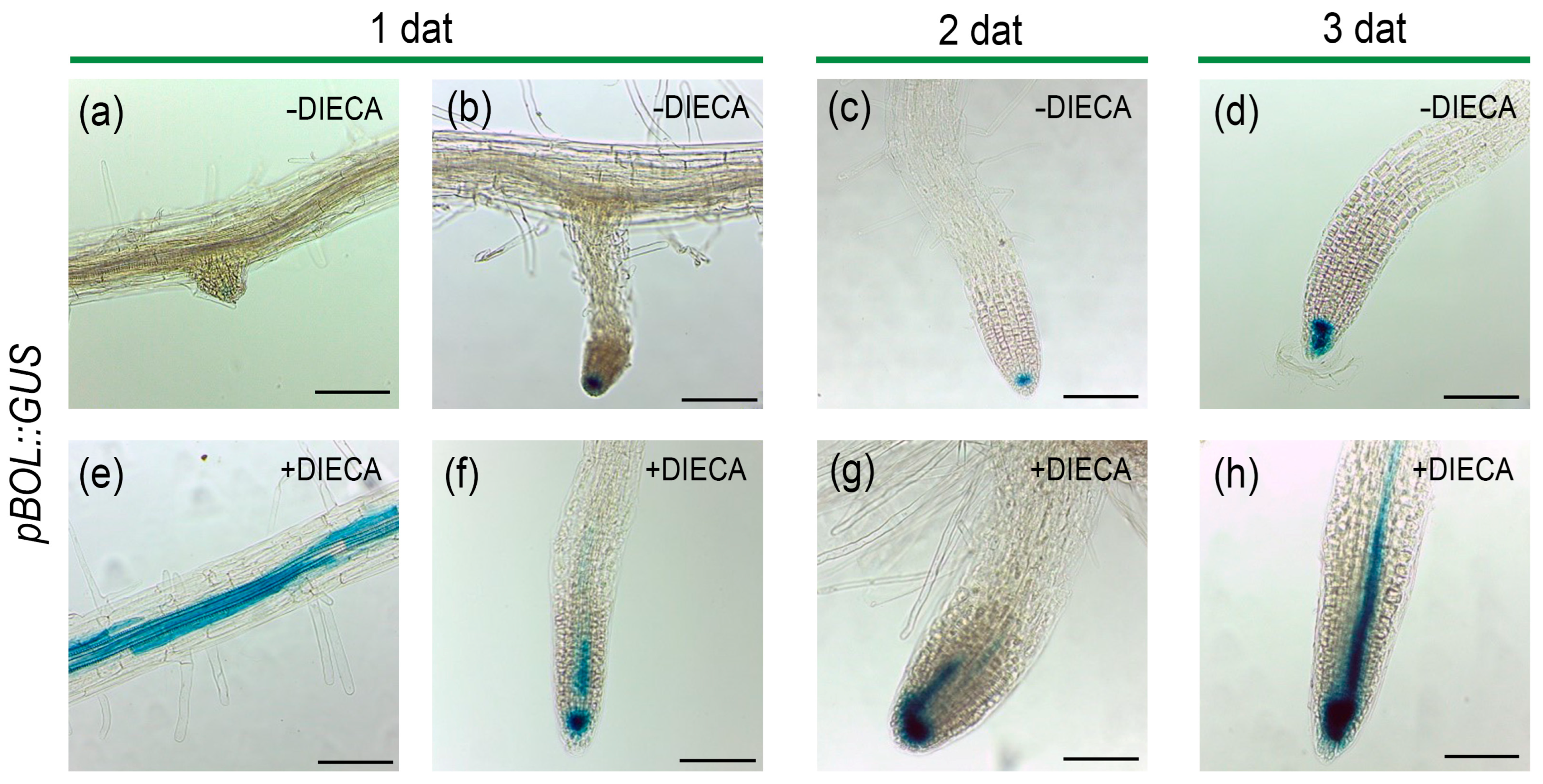
2.7. The Inhibition of JA Biosynthesis Alters the Pattern of BOL Expression
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Plant Growth
4.3. Generation of a CRISPR-Cas9 Induced New Allele of BOL
4.4. GUS Expression Analysis
4.5. Phenotypic Analyses and Documentation
4.6. BOL Transient Expression Assays
4.7. MeJA and DIECA Treatments
4.8. Jasmonic Acid Quantification
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traas, J. Organogenesis at the Shoot Apical Meristem. Plants 2019, 8, 6. [Google Scholar] [CrossRef]
- Marsch-Martinez, N.; Greco, R.; Becker, J.D.; Dixit, S.; Bergervoet, J.H.W.; Karaba, A.; De Folter, S.; Pereira, A. BOLITA, an Arabidopsis AP2/ERF-like Transcription Factor That Affects Cell Expansion and Proliferation/Differentiation Pathways. Plant Mol. Biol. 2006, 62, 825–843. [Google Scholar] [CrossRef]
- Durán-Medina, Y.; Serwatowska, J.; Reyes-Olalde, J.I.; De Folter, S.; Marsch-Martínez, N. The AP2/ERF Transcription Factor DRNL Modulates Gynoecium Development and Affects Its Response to Cytokinin. Front. Plant Sci. 2017, 8, 1841. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Banno, H.; Niu, Q.W.; Howell, S.H.; Chua, N.H. The Enhancer of SHOOT REGENERATION 2 Gene in Arabidopsis Regulates CUP-SHAPED COTYLEDON 1 at the Transcriptional Level and Controls Cotyledon Development. Plant Cell Physiol. 2006, 47, 1443–1456. [Google Scholar] [CrossRef]
- Nag, A.; Yang, Y.; Jack, T. DORNRÖSCHEN-LIKE, an AP2 Gene, Is Necessary for Stamen Emergence in Arabidopsis. Plant Mol. Biol. 2007, 65, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.W.; Cole, M.; Flier, A.; Grewe, B.; Werr, W. The AP2 Transcription Factors DORNRÖSCHEN and DORNRÖSCHEN-LIKE Redundantly Control Arabidopsis Embryo Patterning via Interaction with PHAVOLUTA. Development 2007, 134, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.W.; Jacobs, B.; Cole, M.; Comelli, P.; Werr, W. DORNRÖSCHEN-LIKE Expression Marks Arabidopsis Floral Organ Founder Cells and Precedes Auxin Response Maxima. Plant Mol. Biol. 2011, 76, 171–185. [Google Scholar] [CrossRef]
- Capua, Y.; Eshed, Y. Coordination of Auxin-Triggered Leaf Initiation by Tomato LEAFLESS. Proc. Natl. Acad. Sci. USA 2017, 114, 3246–3251. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Wenkel, S.; Chandler, J.W.; Werr, W.; Jiao, Y. Spatiotemporal Control of Axillary Meristem Formation by Interacting Transcriptional Regulators. Development 2018, 145, dev158352. [Google Scholar] [CrossRef]
- Frerichs, A.; Thoma, R.; Abdallah, A.T.; Frommolt, P.; Werr, W.; Chandler, J.W. The Founder-Cell Transcriptome in the Arabidopsis Apetala1 Cauliflower Inflorescence Meristem. BMC Genom. 2016, 17, 855. [Google Scholar] [CrossRef]
- Dai, Y.; Luo, L.; Zhao, Z. Genetic Robustness Control of Auxin Output in Priming Organ Initiation. Proc. Natl. Acad. Sci. USA 2023, 120, e2221606120. [Google Scholar] [CrossRef] [PubMed]
- Durán-Medina, Y.; Díaz-Ramírez, D.; Herrera-Ubaldo, H.; Di Marzo, M.; Cruz-Valderrama, J.E.; Guerrero-Largo, H.; Ruiz-Cortés, B.E.; Gómez-Felipe, A.; Reyes-Olalde, J.I.; Colombo, L.; et al. The Transcription Factor ENHANCER OF SHOOT REGENERATION 2 Orchestrates Cytokinin Dynamics Leading to Developmental Reprograming and Green Callus Formation. Plant Physiol. 2025, 198, kiaf182. [Google Scholar] [CrossRef]
- Comelli, P.; Glowa, D.; Chandler, J.W.; Werr, W. Founder-Cell-Specific Transcription of the DORNRÖSCHEN-LIKE Promoter and Integration of the Auxin Response. J. Exp. Bot. 2016, 67, 143–155. [Google Scholar] [CrossRef][Green Version]
- Pauwels, L.; Inzé, D.; Goossens, A. Jasmonate-Inducible Gene: What Does It Mean? Trends Plant Sci. 2009, 14, 87–91. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Jasmonic Acid Distribution and Action in Plants: Regulation during Development and Response to Biotic and Abiotic Stress. Proc. Natl. Acad. Sci. USA 1995, 92, 4114–4119. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Creelman, R.A.; Mullet, J.E.; Zenk, M.H. A Chloroplast Lipoxygenase Is Required for Wound-Induced Jasmonic Acid Accumulation InArabidopsis Communicated By. Plant Biol. 1995, 92, 8675–8679. [Google Scholar]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-Iso-Jasmonoyl-L-Isoleucine Is the Endogenous Bioactive Jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef]
- Gfeller, A.; Dubugnon, L.; Liechti, R.; Farmer, E.E. Jasmonate Biochemical Pathway. Sci. Signal 2010, 3, cm3. [Google Scholar] [CrossRef]
- Hamberg’, M.; Fahlstadius, P. Allene Oxide Cyclase: A New Enzyme in Plant Lipid Metabolism. Arch. Biochem. Biophys. 1990, 276, 5–526. [Google Scholar] [CrossRef]
- Kombrink, E. Chemical and Genetic Exploration of Jasmonate Biosynthesis and Signaling Paths. Planta 2012, 236, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Halitschke, R.; Kim, H.B.; Baldwin, I.T.; Feldmann, K.A.; Feyereisen, R. A Knock-out Mutation in Allene Oxide Synthase Results in Male Sterility and Defective Wound Signal Transduction in Arabidopsis Due to a Block in Jasmonic Acid Biosynthesis. Plant J. 2002, 31, 1–12. [Google Scholar] [CrossRef]
- Schaller, A.; Stintzi, A. Enzymes in Jasmonate Biosynthesis—Structure, Function, Regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Yan, Y.; Borrego, E.; Kolomiets, M.V. Jasmonate Biosynthesis, Perception and Function in Plant Development and Stress Responses. In Lipid Metabolism; InTech: London, UK, 2013. [Google Scholar]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ Repressor Proteins Are Targets of the SCFCOI1 Complex during Jasmonate Signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Fonseca, S.; Chico, J.M.; Solano, R. The Jasmonate Pathway: The Ligand, the Receptor and the Core Signalling Module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. An Update to the 2007 Review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of Jasmonate-Mediated Stamen Development and Seed Production by a BHLH-MYB Complex in Arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Turner, J.G. Wound-Induced Endogenous Jasmonates Stunt Plant Growth by Inhibiting Mitosis. PLoS ONE 2008, 3, e3699. [Google Scholar] [CrossRef]
- Staswick, P.E.; Sut, W.; Howell, S.H. Methyl Jasmonate Inhibition of Root Growth and Induction of a Leaf Protein Are Decreased in an Arabidopsis Thaliana Mutant. Proc. Natl. Acad. Sci. USA 1992, 89, 6837–6840. [Google Scholar] [CrossRef]
- Noir, S.; Bömer, M.; Takahashi, N.; Ishida, T.; Tsui, T.L.; Balbi, V.; Shanahan, H.; Sugimoto, K.; Devoto, A. Jasmonate Controls Leaf Growth by Repressing Cell Proliferation and the Onset of Endoreduplication While Maintaining a Potential Stand-by Mode. Plant Physiol. 2013, 161, 1930–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-Mediated Wound Signalling Promotes Plant Regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef]
- Kamińska, M. Role and Activity of Jasmonates in Plants under in Vitro Conditions. Plant Cell Tissue Organ. Cult. 2021, 146, 425–447. [Google Scholar] [CrossRef]
- Yan, Y.; Stolz, S.; Chételat, A.; Reymond, P.; Pagni, M.; Dubugnon, L.; Farmer, E.E. A Downstream Mediator in the Growth Repression Limb of the Jasmonate Pathway. Plant Cell 2007, 19, 2470–2483. [Google Scholar] [CrossRef]
- Farmer, E.E.; Caldelari, D.; Pearce, C.; Walker-Simmons, M.K.; Ryan, C.A. Diethyldithiocarbamic Acid Lnhibits the Octadecanoid Signaling Pathway for the Wound Lnduction of Proteinase Lnhibitors in Tomato Leaves. Plant Physiol. 1994, 106, 337–342. [Google Scholar] [CrossRef]
- Eklund, D.M.; Cierlik, I.; Ståldal, V.; Claes, A.R.; Vestman, D.; Chandler, J.; Sundberg, E. Expression of Arabidopsis SHORT INTERNODES/STYLISH Family Genes in Auxin Biosynthesis Zones of Aerial Organs Is Dependent on a GCC Box-like Regulatory Element. Plant Physiol. 2011, 157, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Gasperini, D.; Chételat, A.; Stolz, S.; Santuari, L.; Farmer, E.E. Role of NINJA in Root Jasmonate Signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 15473–15478. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Reinstädler, A.; Lipka, V.; Lippok, B.; Somssich, I.E. Synthetic Plant Promoters Containing Defined Regulatory Elements Provide Novel Insights into Pathogen- and Wound-Induced Signaling. Plant Cell 2002, 14, 749–762. [Google Scholar] [CrossRef]
- Sanders, P.M.; Lee, P.Y.; Biesgen, C.; Boone, J.D.; Beals, T.P.; Weiler, E.W.; Goldberg, R.B. The Arabidopsis DELAYED DEHISCENCE1 Gene Encodes an Enzyme in the Jasmonic Acid Synthesis Pathway. Plant Cell 2000, 12, 1041–1061. [Google Scholar] [CrossRef]
- Ishiguro, S.; Kawai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The DEFECTIVE IN ANTHER DEHISCENCE1 Gene Encodes a Novel Phospholipase A1 Catalyzing the Initial Step of Jasmonic Acid Biosynthesis, Which Synchronizes Pollen Maturation, Anther Dehiscence, and Flower Opening in Arabidopsis. Plant Cell 2001, 13, 2191–2209. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Przybyl, M. Jasmonate Signaling during Arabidopsis Stamen Maturation. Plant Cell Physiol. 2019, 60, 2648–2659. [Google Scholar] [CrossRef]
- Nguyen, H.T.; To, H.T.M.; Lebrun, M.; Bellafiore, S.; Champion, A. Jasmonates—The Master Regulator of Rice Development, Adaptation and Defense. Plants 2019, 8, 339. [Google Scholar] [CrossRef]
- Reeves, P.H.; Ellis, C.M.; Ploense, S.E.; Wu, M.F.; Yadav, V.; Tholl, D.; Chételat, A.; Haupt, I.; Kennerley, B.J.; Hodgens, C.; et al. A Regulatory Network for Coordinated Flower Maturation. PLoS Genet. 2012, 8, e1002506. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qi, T.; Huang, H.; Xie, D. Regulation of Stamen Development by Coordinated Actions of Jasmonate, Auxin, and Gibberellin in Arabidopsis. Mol. Plant 2013, 6, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, K.; Przedniczek, K. Comprehensive Insight into Gibberellin- and Jasmonate-Mediated Stamen Development. Genes 2019, 10, 811. [Google Scholar] [CrossRef]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis Xylem Pericycle Underlies Shoot Regeneration from Root and Hypocotyl Explants Grown in Vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis Regeneration from Multiple Tissues Occurs via a Root Development Pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Hayashi, K.; Suzuki, T.; Fukaki, H.; Prusinska, J.; Meester, C.; Quareshy, M.; Egoshi, S.; Matsuura, H.; Takahashi, K.; et al. Jasmonic Acid Inhibits Auxin-Induced Lateral Rooting Independently of the CORONATINE INSENSITIVE1 Receptor. Plant Physiol. 2018, 177, 1704–1716. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of Adventitious Root Formation: Insights into Synergistic and Antagonistic Hormonal Interactions. Physiol. Plant 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Uyehara, A.N.; Del Valle-Echevarria, A.R.; Hunter, C.T.; Nelissen, H.; Demuynck, K.; Cahill, J.F.; Gorman, Z.; Jander, G.; Muszynski, M.G. Cytokinin Promotes Jasmonic Acid Accumulation in the Control of Maize Leaf Growth. Plants 2023, 12, 3014. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; Kang, J.H.; Howe, G.A. Jasmonate-Triggered Plant Immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef]
- Kloth, K.J.; Dicke, M. Rapid Systemic Responses to Herbivory. Curr. Opin. Plant Biol. 2022, 68, 102242. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Caldelari, D.; Wolfender, J.L.; Farmer, E.E. Four 13-Lipoxygenases Contribute to Rapid Jasmonate Synthesis in Wounded Arabidopsis Thaliana Leaves: A Role for Lipoxygenase 6 in Responses to Long-Distance Wound Signals. New Phytol. 2013, 197, 566–575. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yu, Y.; Feng, L.; Jia, J.; Liu, B.; Li, B.; Guo, H.; Zhai, J. A Comprehensive Online Database for Exploring ∼20,000 Public Arabidopsis RNA-Seq Libraries. Mol. Plant 2020, 13, 1231–1233. [Google Scholar] [CrossRef]
- Brown, R.L.; Kazan, K.; McGrath, K.C.; Maclean, D.J.; Manners, J.M. A Role for the GCC-Box in Jasmonate-Mediated Activation of the PDF1.2 Gene of Arabidopsis. Plant Physiol. 2003, 132, 1020–1032. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A Web Tool for Synthetic Single-Guide RNA Design of CRISPR-System in Plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Engler, C.; Weber, E.; Gruetzner, R.; Marillonnet, S. Fast Track Assembly of Multigene Constructs Using Golden Gate Cloning and the MoClo System. Bioeng. Bugs 2012, 3, 38–43. [Google Scholar] [CrossRef]
- Brooks, C.; Nekrasov, V.; Lipppman, Z.B.; Van Eck, J. Efficient Gene Editing in Tomato in the First Generation Using the Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated9 System. Plant Physiol. 2014, 166, 1292–1297. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis Thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Hopke, J.; Donath, J.; Blechert, S.; Boland, W. Herbivore-Induced Volatiles: The Emission of Acyclic Homoterpenes from Leaves of Phaseolus Lunatus and Zea Mays Can Be Triggered by a β-Glucosidase and Jasmonic Acid. FEBS Lett. 1994, 352, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Koch, T.; Hilpert, A.; Fiala, B.; Boland, W.; Linsenmair, K.E. Extrafloral Nectar Production of the Ant-Associated Plant, Macaranga Tanarius, Is an Induced, Indirect, Defensive Response Elicited by Jasmonic Acid. Proc. Natl. Acad. Sci. USA 2000, 98, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.; Krumm, T.; Jung, V.; Rgen Engelberth, J.; Boland, W. Differential Induction of Plant Volatile Biosynthesis in the Lima Bean by Early and Late Intermediates of the Octadecanoid-Signaling Pathway. Plant Physiol. 1999, 121, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Ibarra-Laclette, E.; Adame-Álvarez, R.M.; Martínez, O.; Ramirez-Chávez, E.; Molina-Torres, J.; Herrera-Estrella, L. How Plants Sense Wounds: Damaged-Self Recognition Is Based on Plant-Derived Elicitors and Induces Octadecanoid Signaling. PLoS ONE 2012, 7, e30537. [Google Scholar] [CrossRef]
- López-García, C.M.; Ávila-Hernández, C.A.; Quintana-Rodríguez, E.; Aguilar-Hernández, V.; Lozoya-Pérez, N.E.; Rojas-Raya, M.A.; Molina-Torres, J.; Araujo-León, J.A.; Brito-Argáez, L.; González-Sánchez, A.A.; et al. Extracellular Self- and Non-Self DNA Involved in Damage Recognition in the Mistletoe Parasitism of Mesquite Trees. Int. J. Mol. Sci. 2024, 25, 457. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Cortés, B.E.; Durán-Medina, Y.; Ramos-Tamayo, C.C.; Guerrero-Largo, H.; Elizarraraz-Anaya, M.I.C.; Hernández-Zepeda, O.F.; Ramírez-Chávez, E.; Lammers, M.; de Maagd, R.A.; Molina-Torres, J.; et al. Interactions Between the Transcription Factor BOL/DRNL/ESR2 and the Jasmonate Pathway. Plants 2025, 14, 1757. https://doi.org/10.3390/plants14121757
Ruiz-Cortés BE, Durán-Medina Y, Ramos-Tamayo CC, Guerrero-Largo H, Elizarraraz-Anaya MIC, Hernández-Zepeda OF, Ramírez-Chávez E, Lammers M, de Maagd RA, Molina-Torres J, et al. Interactions Between the Transcription Factor BOL/DRNL/ESR2 and the Jasmonate Pathway. Plants. 2025; 14(12):1757. https://doi.org/10.3390/plants14121757
Chicago/Turabian StyleRuiz-Cortés, Beatriz E., Yolanda Durán-Medina, C. Cecilia Ramos-Tamayo, Herenia Guerrero-Largo, Ma. Isabel Cristina Elizarraraz-Anaya, Omar Fabián Hernández-Zepeda, Enrique Ramírez-Chávez, Michiel Lammers, Ruud A. de Maagd, Jorge Molina-Torres, and et al. 2025. "Interactions Between the Transcription Factor BOL/DRNL/ESR2 and the Jasmonate Pathway" Plants 14, no. 12: 1757. https://doi.org/10.3390/plants14121757
APA StyleRuiz-Cortés, B. E., Durán-Medina, Y., Ramos-Tamayo, C. C., Guerrero-Largo, H., Elizarraraz-Anaya, M. I. C., Hernández-Zepeda, O. F., Ramírez-Chávez, E., Lammers, M., de Maagd, R. A., Molina-Torres, J., de Folter, S., & Marsch-Martínez, N. (2025). Interactions Between the Transcription Factor BOL/DRNL/ESR2 and the Jasmonate Pathway. Plants, 14(12), 1757. https://doi.org/10.3390/plants14121757






