Complexity Meets Risk—The Next Generation of Genome-Edited Plants Challenges Established Concepts for Environmental Risk Assessment in the EU
Abstract
1. Introduction
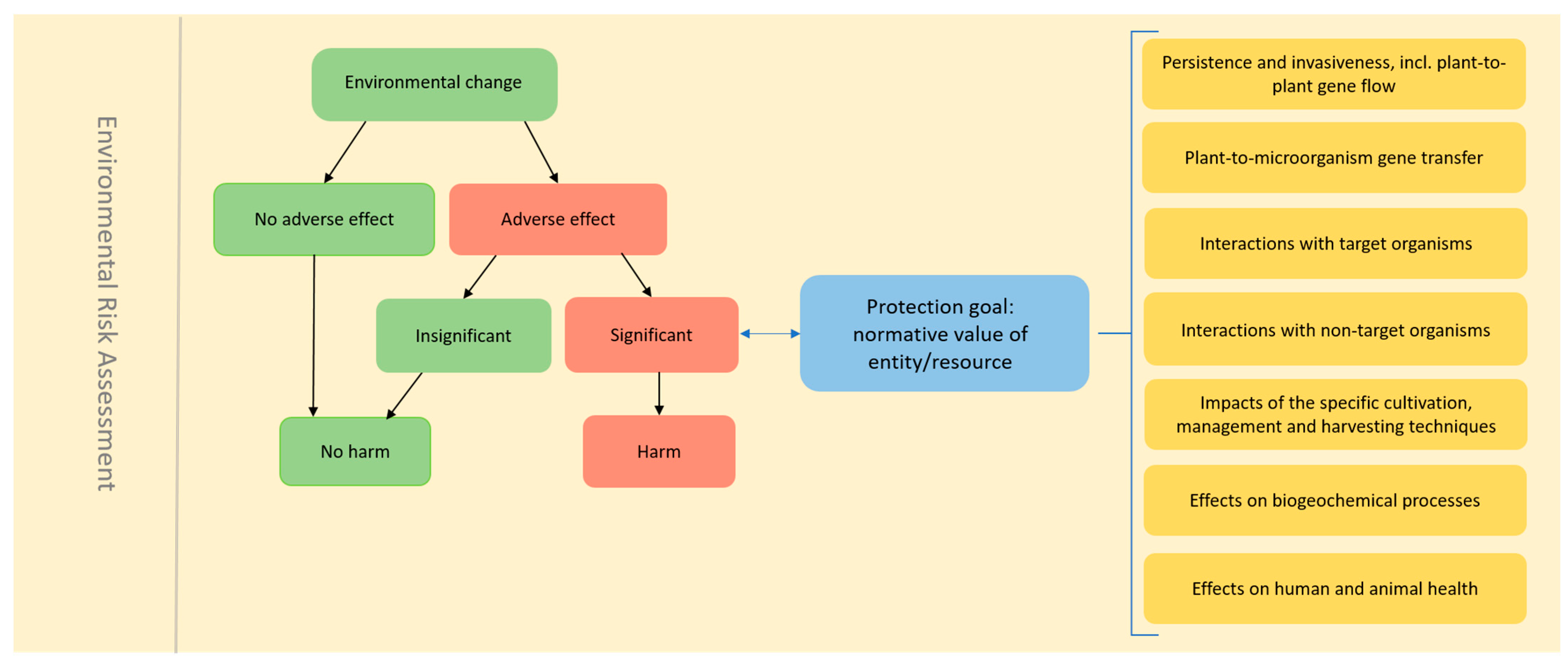
2. The Current ERA Practice in the EU
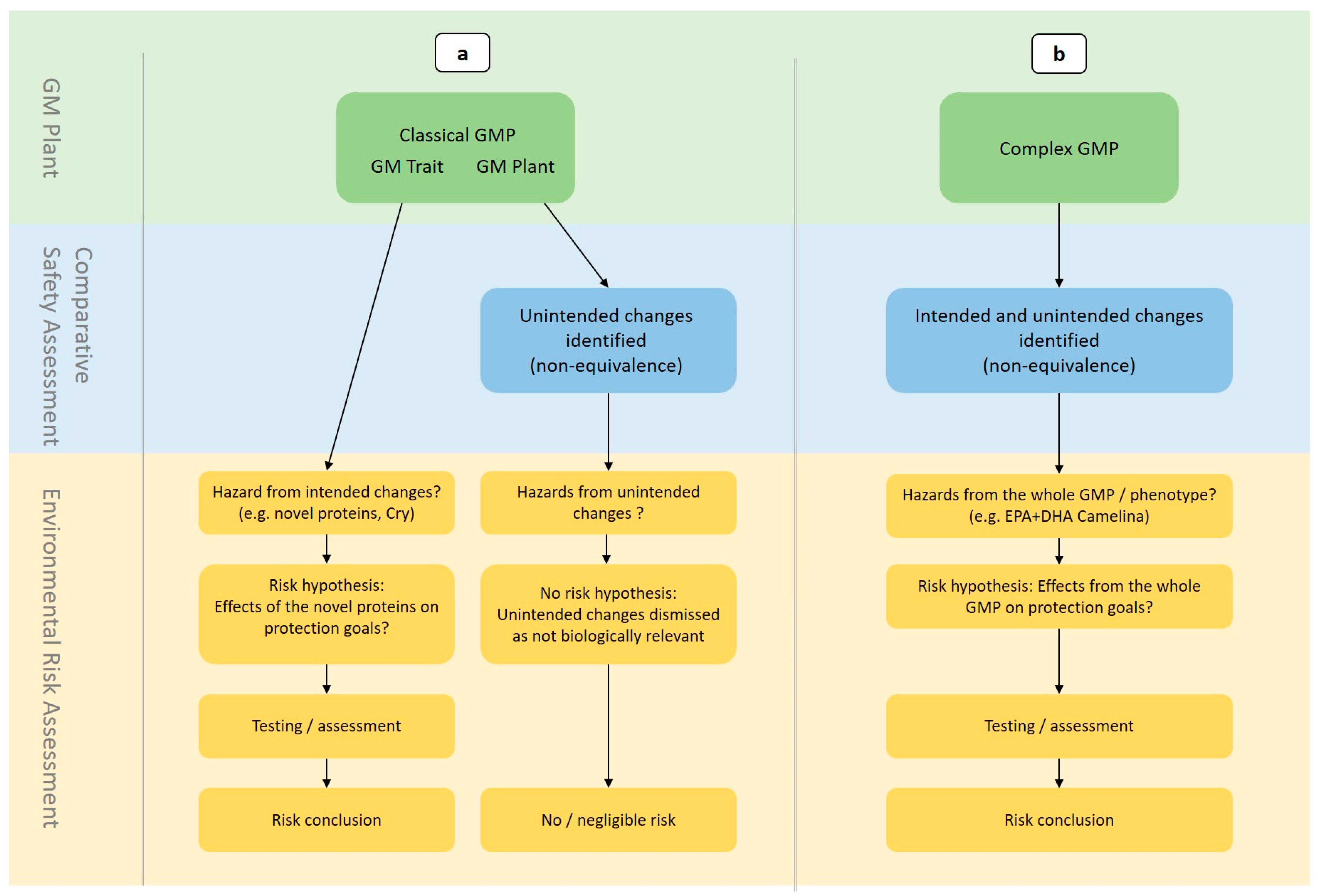
2.1. Consideration of Intended and Unintended Changes in the GMP
2.2. Comparing GMPs with Presumably Safe Plants—History of Safe Use and Familiarity
2.3. Pitfalls of the Comparative Safety Assessment Approach in ERA
Example: GM High Oleic-Acid Soybean
3. Limitations of the Comparative Safety Assessment Approach for Complex GMP Applications
3.1. Complex GMP Applications Lack Equivalence with Non-GM Plants
| No. | Complex GMP Applications | Parental Plants | Intended GM Trait(s) | (Unintended) Changes in the GMP | Potential Environmental Risks | References |
|---|---|---|---|---|---|---|
| 1 | Modification of endogenous seed metabolites | Various oilseed crops, e.g., oilseed rape (Brassica napus), soybean (Glycine max), camelina (Camelina sativa), safflower (Carthamus tinctorius), pennycress (Thlaspi arvense), field cress (Lepidium campestre) | Reduction in unwanted fatty acids (e.g., PUFAs, erucic acid) Enrichment of mono-unsaturated fatty acid (e.g., oleic acid) Increased TAG (triacylglycerol)-oil production Modification of seed coat color | Changes in relative frequency of various (e.g., medium chain) fatty acids Increased PUFA levels Changes in plant growth and development Changes in germination characteristics | Risks for trophic food webs and biodiversity Changes in plant survival and environmental interactions due to changes in biotic or abiotic stress tolerance Risks due to persistence and invasiveness due to changes in seed germination ability | [66,67,68,69,70,71,72,73,74] |
| Cereals (e.g., Triticum sp.) | Modification of seed protein composition, i.e., change in gluten components (e.g., decrease in α, γ gliadins, in-or decrease in ω-gliadins) | Lower gliadin-to-glutenin ratio Production of novel α-gliadins Increased lysine contents | [75] | |||
| 2 | Production of novel fatty acids and oils in seeds | Camelina (Camelina sativa) Crambe (Crambe abyssinica) | Production of long-chain polyunsaturated fatty acids (LCPUFAs, “fish oils”) Production of wax esters (fatty alcohols) | Changes in overall fatty acid composition of seeds Production of intermediate fatty acids | [35,76,77,78,79,80,81,82] | |
| 3 | Production of pharmaceuticals or nutraceuticals | Medicinal herbs, e.g., opium poppy (Papaver somniferum), Madagascar periwinkle (Catharantheus roseus) Food crops (e.g., tomato, rice) | Changed levels of endogenous alkaloids Expression of taste-modifying proteins Production of novel carotenoids (e.g., astaxanthin, canthaxanthin) | Production of various carotenoid intermediates | Risks for trophic food webs and biodiversity Risks for trophic food webs and biodiversity | [83,84,85,86,87,88] |
| 4 | Plants producing new-to-nature substances | Tobacco (Nicotiana benthamiana) | Production of novel biopesticidal molecules (e.g., crucifalexins, chlorobrassinin, bromobrassinin) | Not indicated | Risks to food webs and biodiversity due to novel biotic stress tolerance | [89] |
| 5 | De novo/re-domesticated plants | Wild plants, weeds, e.g., wild rice Oryza alta, barnyard grass Echinocloa crus-galli, E. oryzicola) Crop progenitors, e.g., wild tomato (Solanum pimpinellifolium) Ancient or orphan crops, e.g., ground cherry Physalis pruinosa, wild rice Zizania latifolia) | Modification of key domestication traits (e.g., shoot architecture, fruit characteristics, nutrient content, flower production, day-length sensitivity) | Not indicated | Risks due to persistence and invasiveness of new plants in novel environments or new cultivation techniques | [90,91,92,93,94,95,96,97] |
| 6 | Modification of photosynthetic pathways for more efficient carbon fixation (“green carbon plants”) | Model plants (e.g., Nicotiana tabacum, Arabidopsis sp.) | Increase in carbon assimilation, growth parameters (leaf area, number), biomass yield, light use efficiency | Decreased water use efficiency Changes in plant growth and development | Risks due to persistence and invasiveness due to changes in plant competition | [34,98,99,100] |
3.1.1. Plants with Modifications of Endogenous Seed Metabolites
3.1.2. Plants Producing Novel Oils in Seeds
3.1.3. Plants Producing Pharmaceuticals or Nutraceuticals
3.1.4. Plants Producing New-to-Nature Compounds
3.1.5. De Novo Domestication and Re-Domestication of Plants
3.1.6. Plants with Enhanced Photosynthesis (“Green Carbon Plants”)
3.2. Intended and Unintended Changes Are Blurred
3.3. Lack of Familiarity and Presumed Safety of the Parental Plant
3.4. Unclear Biological Relevance of Observed Differences and Lack of Safety Limits
4. Consequences for the ERA of Complex GMP Applications
4.1. Importance of a Protection-Goal-Focussed ERA
4.2. Potential Environmental Risks of Complex GMP Applications
4.2.1. Risks for Trophic Food Webs and Biodiversity
4.2.2. Risks Due to Changes in Stress Tolerance and Plant Survival
4.2.3. Risks Due to Persistence and Invasiveness
4.3. Development of Test Systems for Assessing the Environmental Performance of New Phenotypes
4.4. Adaptation of the Comparative Assessment of Standard Agronomic and Phenotypic Traits
5. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Miklau, M.; Burn, S.-J.; Eckerstorfer, M.; Dolezel, M.; Greiter, A.; Heissenberger, A.; Hörtenhuber, S.; Zollitsch, W.; Hagen, K. Horizon scanning of potential environmental applications of terrestrial animals, fish, algae and microorganisms produced by genetic modification, including the use of new genomic techniques. Front. Genome Ed. 2024, 6, 1376927. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.F.; Dolezel, M.; Heissenberger, A.; Miklau, M.; Reichenbecher, W.; Steinbrecher, R.A.; Waßmann, F. An EU Perspective on Biosafety Considerations for Plants Developed by Genome Editing and Other New Genetic Modification Techniques (nGMs). Front. Bioeng. Biotechnol. 2019, 7, 31. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Dolezel, M.; Engelhard, M.; Giovannelli, V.; Grabowski, M.; Heissenberger, A.; Lener, M.; Reichenbecher, W.; Simon, S.; Staiano, G.; et al. Recommendations for the Assessment of Potential Environmental Effects of Genome-Editing Applications in Plants in the EU. Plants 2023, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Kawall, K. New Possibilities on the Horizon: Genome Editing Makes the Whole Genome Accessible for Changes. Front. Plant Sci. 2019, 10, 525. [Google Scholar] [CrossRef]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Wei, Z.; Rohila, J.S.; Zhao, K. Multiplex Genome-Editing Technologies for Revolutionizing Plant Biology and Crop Improvement. Front. Plant Sci. 2021, 12, 721203. [Google Scholar] [CrossRef]
- Mushtaq, M.; Ahmad Dar, A.; Skalicky, M.; Tyagi, A.; Bhagat, N.; Basu, U.; Bhat, B.A.; Zaid, A.; Ali, S.; Dar, T.-U.-H.; et al. CRISPR-Based Genome Editing Tools: Insights into Technological Breakthroughs and Future Challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef]
- Armario Najera, V.; Twyman, R.M.; Christou, P.; Zhu, C. Applications of multiplex genome editing in higher plants. Curr. Opin. Biotechnol. 2019, 59, 93–102. [Google Scholar] [CrossRef]
- Mullins, E.; Bresson, J.-L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Firbank, L.G.; Guerche, P.; Hejatko, J.; Moreno, F.J.; Nogue, F.; et al. Evaluation of existing guidelines for their adequacy for the food and feed risk assessment of genetically modified plants obtained through synthetic biology. EFSA J. 2022, 20, e07410. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; Debray, K.; Herwegh, D.; Develtere, W.; Impens, L.; Schaumont, D.; Vandeputte, W.; Aesaert, S.; Coussens, G.; de Boe, Y.; et al. BREEDIT: A multiplex genome editing strategy to improve complex quantitative traits in maize. Plant Cell 2023, 35, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Shelake, R.M.; Kadam, U.S.; Kumar, R.; Pramanik, D.; Singh, A.K.; Kim, J.-Y. Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: Targets, tools, challenges, and perspectives. Plant Commun. 2022, 3, 100417. [Google Scholar] [CrossRef] [PubMed]
- Cardi, T.; Murovec, J.; Bakhsh, A.; Boniecka, J.; Bruegmann, T.; Bull, S.E.; Eeckhaut, T.; Fladung, M.; Galovic, V.; Linkiewicz, A.; et al. CRISPR/Cas-mediated plant genome editing: Outstanding challenges a decade after implementation. Trends Plant Sci. 2023, 28, 1144–1165. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Schindele, P.; Puchta, H. Plant breeding at the speed of light: The power of CRISPR/Cas to generate directed genetic diversity at multiple sites. BMC Plant Biol. 2019, 19, 176. [Google Scholar] [CrossRef]
- Paciorek, T.; Chiapelli, B.J.; Wang, J.Y.; Paciorek, M.; Yang, H.; Sant, A.; Val, D.L.; Boddu, J.; Liu, K.; Gu, C.; et al. Targeted suppression of gibberellin biosynthetic genes ZmGA20ox3 and ZmGA20ox5 produces a short stature maize ideotype. Plant Biotechnol. J. 2022, 20, 1140–1153. [Google Scholar] [CrossRef]
- Kubis, A.; Bar-Even, A. Synthetic biology approaches for improving photosynthesis. J. Exp. Bot. 2019, 70, 1425–1433. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Dai, Q.; Cui, M.; Yang, H.; Cao, P.; Zhao, L. When green carbon plants meet synthetic biology. Mod. Agric. 2023, 1, 98–111. [Google Scholar] [CrossRef]
- Sargent, D.; Conaty, W.C.; Tissue, D.T.; Sharwood, R.E. Synthetic biology and opportunities within agricultural crops. J. Sust. Agric. Environ. 2022, 1, 89–107. [Google Scholar] [CrossRef]
- Scientific Committee on Health and Environmental Risks; Scientific Committee on Emerging and Newly Identified Health Risks; Scientific Committe on Consumer Safety. Opinion on Synthetic Biology I: Definition; European Commission: Luxembourg, 2014; ISBN 978-92-79-30136-0.
- Naegeli, H.; Bresson, J.-L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Firbank, L.G.; Guerche, P.; Hejatko, J.; Moreno, F.J.; Nogue, F.; et al. Evaluation of existing guidelines for their adequacy for the molecular characterisation and environmental risk assessment of genetically modified plants obtained through synthetic biology. EFSA J. 2021, 19, e06301. [Google Scholar] [CrossRef]
- CBD. Guidance on Risk Assessment of Living Modified Organisms and Monitoring in the Context of Risk Assessment UNEP/CBD/BS/COP; CBD: Cancun, Mexico, 2016; Available online: https://www.cbd.int/doc/meetings/bs/mop-08/official/bs-mop-08-08-add1-en.pdf (accessed on 16 April 2025).
- Rozas, P.; Kessi-Pérez, E.I.; Martínez, C. Genetically modified organisms: Adapting regulatory frameworks for evolving genome editing technologies. Biol. Res. 2022, 55, 31. [Google Scholar] [CrossRef]
- EFSA GMO Panel. Guidance on selection of comparators for the risk assessment of genetically modified plants and derived food and feed. EFSA J. 2011, 9, 2149. [Google Scholar] [CrossRef]
- EFSA GMO Panel. Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 2010, 8, 1879. [Google Scholar] [CrossRef]
- EFSA GMO Panel. Scientific Opinion on the assessment of potential impacts of genetically modified plants on non-target organisms. EFSA J. 2010, 8, 1877. [Google Scholar] [CrossRef]
- Halford, N.G.; Hudson, E.; Gimson, A.; Weightman, R.; Shewry, P.R.; Tompkins, S. Safety assessment of genetically modified plants with deliberately altered composition. Plant Biotechnol. J. 2014, 12, 651–654. [Google Scholar] [CrossRef]
- ADAS. Review of the strategies for the comprehensive food and feed safety and nutritional assessment of GM plants per se. EFS3 2013, 10, 480E. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance to develop specific protection goals options for environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services. EFSA J. 2016, 14, 4499. [Google Scholar] [CrossRef]
- Ladyman, J.; Lambert, J.; Wiesner, K. What is a complex system? Eur. J. Philos. Sci. 2013, 3, 33–67. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Grabowski, M.; Lener, M.; Engelhard, M.; Simon, S.; Dolezel, M.; Heissenberger, A.; Lüthi, C. Biosafety of Genome Editing Applications in Plant Breeding: Considerations for a Focused Case-Specific Risk Assessment in the EU. BioTech 2021, 10, 10. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, B.; Tan, J.; Liu, T.; Li, L.; Liu, Y.-G. Plant Synthetic Metabolic Engineering for Enhancing Crop Nutritional Quality. Plant Commun. 2020, 1, 100017. [Google Scholar] [CrossRef]
- EFSA. Guidance for risk assessment of food and feed from genetically modified plants. EFSA J. 2011, 9, 2150. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, eaat9077. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Shrestha, P.; Zhou, X.-R.; Mansour, M.P.; Liu, Q.; Belide, S.; Nichols, P.D.; Singh, S.P. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS ONE 2012, 7, e49165. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; King, G.J.; Borpatragohain, P.; Zou, J. Developing multifunctional crops by engineering Brassicaceae glucosinolate pathways. Plant Commun. 2023, 4, 100565. [Google Scholar] [CrossRef]
- Devos, Y.; Oberkofler, L.; Glandorf, D.C. Genetically modified plants and food/feed: Risk assessment considerations. In Encyclopedia of Toxicology (Fourth Edition); Academic Press: Cambridge, MA, USA, 2023; pp. 951–966. ISBN 9780323854344. [Google Scholar]
- Fang, J.; Nan, P.; Gu, Z.; Ge, X.; Feng, Y.-Q.; Lu, B.-R. Overexpressing Exogenous 5-Enolpyruvylshikimate-3-Phosphate Synthase (EPSPS) Genes Increases Fecundity and Auxin Content of Transgenic Arabidopsis Plants. Front. Plant Sci. 2018, 9, 233. [Google Scholar] [CrossRef]
- Latham, J.R.; Wilson, A.K.; Steinbrecher, R.A. The mutational consequences of plant transformation. J. Biomed. Biotechnol. 2006, 2006, 25376. [Google Scholar] [CrossRef]
- Chu, P.; Agapito-Tenfen, S.Z. Unintended Genomic Outcomes in Current and Next Generation GM Techniques: A Systematic Review. Plants 2022, 11, 2997. [Google Scholar] [CrossRef]
- Koller, F.; Cieslak, M. A perspective from the EU: Unintended genetic changes in plants caused by NGT-their relevance for a comprehensive molecular characterisation and risk assessment. Front. Bioeng. Biotechnol. 2023, 11, 1276226. [Google Scholar] [CrossRef]
- Munger, A.; Coenen, K.; Cantin, L.; Goulet, C.; Vaillancourt, L.-P.; Goulet, M.-C.; Tweddell, R.; Sainsbury, F.; Michaud, D. Beneficial ‘unintended effects’ of a cereal cystatin in transgenic lines of potato, Solanum tuberosum. BMC Plant Biol. 2012, 12, 198. [Google Scholar] [CrossRef]
- EFSA GMO Panel. Guidance on the agronomic and phenotypic characterisation of genetically modified plants. EFSA J. 2015, 13, 4128. [Google Scholar] [CrossRef]
- EFSA GMO Panel. Statistical considerations for the safety evaluation of GMOs. EFSA J. 2010, 8, 1250. [Google Scholar] [CrossRef]
- EFSA. Statistical Significance and Biological Relevance. EFSA J. 2011, 9, 2372. [Google Scholar] [CrossRef]
- Constable, A.; Jonas, D.; Cockburn, A.; Davi, A.; Edwards, G.; Hepburn, P.; Herouet-Guicheney, C.; Knowles, M.; Moseley, B.; Oberdörfer, R.; et al. History of safe use as applied to the safety assessment of novel foods and foods derived from genetically modified organisms. Food Chem. Toxicol. 2007, 45, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- OECD. Consensus Documents: Work on the Safety of Novel Foods and Feeds: Plants: Compositional Considerations (Nutrients, Anti-Nutrients and Toxicants) on Food and Feed Products Using the Following Plants. 2024. Available online: https://www.oecd.org/science/biotrack/consensus-document-for-work-on-safety-novel-and-foods-feeds-plants.htm (accessed on 22 May 2024).
- Ricroch, A.E.; Bergé, J.B.; Kuntz, M. Evaluation of genetically engineered crops using transcriptomic, proteomic, and metabolomic profiling techniques. Plant Physiol. 2011, 155, 1752–1761. [Google Scholar] [CrossRef]
- Capalbo, D.M.F.; Macdonald, P.; Fernandes, P.M.B.; Rubinstein, C.; Vicién, C. Familiarity in the Context of Risk Assessment of Transgenic Crops: Focus on Some Countries in the Americas. Front. Bioeng. Biotechnol. 2019, 7, 463. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques-Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7, 26. [Google Scholar] [CrossRef]
- NAS. Field Testing Genetically Modified Organisms: Framework for Decsions; National Academy Press: Washington, DC, USA, 1989. [Google Scholar]
- Barrett, K.; Abergel, E. Breeding familiarity: Environmental risk assessment for genetically engineered crops in Canada. Sci. Public Policy 2000, 27, 2–12. [Google Scholar] [CrossRef]
- OECD. Safety Considerations for Biotechnology: Scale-Up of Crop Plants; OECD: Paris, France, 1993. [Google Scholar]
- Damgaard, C.; Lokke, H. A critique of the “concept of familiarity” as used in ecological risk assessments of genetically modified plants. BioSafety J. 2001, 6, 1. [Google Scholar]
- Hilbeck, A.; Meyer, H.; Wynne, B.; Millstone, E. GMO regulations and their interpretation: How EFSA’s guidance on risk assessments of GMOs is bound to fail. Environ. Sci. Eur. 2020, 32, 54. [Google Scholar] [CrossRef]
- van der Voet, H.; Perry, J.N.; Amzal, B.; Paoletti, C. A statistical assessment of differences and equivalences between genetically modified and reference plant varieties. BMC Biotechnol. 2011, 11, 15. [Google Scholar] [CrossRef]
- OECD. Revised Consensus Document on Compositional Considerations for New Varieties of Soybean [Glycine max (L.) Merr.]: Key Food and Feed Nutrients, Antinutrients, Toxicants and Allergens ENV/JM/MONO(2012)24. 2012. Available online: https://one.oecd.org/document/env/jm/mono(2012)24/en/pdf (accessed on 14 October 2024).
- OECD. Revised Consensus Document on Compositional Considerations for New Varieties of Low Erucic acid Rapeseed (Canola): Key Food and Feed Nutrients, Anti-Nutrients and Toxicants: Series on the Safety of Novel Foods and Feeds No. 24 ENV/JM/MONO(2011)55. 2011. Available online: https://www.oecd-ilibrary.org/sites/a393a32f-en/index.html?itemId=/content/component/a393a32f-en (accessed on 19 June 2024).
- Engel, J.; van der Voet, H. Equivalence tests for safety assessment of genetically modified crops using plant composition data. Food Chem. Toxicol. 2021, 156, 112517. [Google Scholar] [CrossRef] [PubMed]
- EFSA GMO Panel. Scientific Opinion on application EFSA-GMO-NL-2007-45 for the placing on the market of herbicide-tolerant, high-oleic acid, genetically modified soybean 305423 for food and feed uses, import and processing under Regulation (EC) No 1829/2003 from Pioneer. EFSA J. 2013, 11, 3499. [Google Scholar] [CrossRef][Green Version]
- Perry, J.N.; Braak, C.J.F.t.; Dixon, P.M.; Duan, J.J.; Hails, R.S.; Huesken, A.; Lavielle, M.; Marvier, M.; Scardi, M.; Schmidt, K.; et al. Statistical aspects of environmental risk assessment of GM plants for effects on non-target organisms. Environ. Biosaf. Res. 2009, 8, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, M.; Miklau, M.; Heissenberger, A.; Otto, M. Agronomic and phenotypic plant traits as indicators for environmental risks of genetically modified plants. Environ. Sci. Eur. 2024, 36, 3. [Google Scholar] [CrossRef]
- Andow, D.A.; Lövei, G.L.; Paula, D.P. Equivalence tests to support environmental biosafety decisions: Theory and examples. J. Biosaf. 2016, 25, 77–91. [Google Scholar] [CrossRef]
- Dolezel, M.; Miklau, M.; Heissenberger, A.; Reichenbecher, W. Are Limits of Concern a useful concept to improve the environmental risk assessment of GM plants? Environ. Sci. Eur. 2017, 29, 7. [Google Scholar] [CrossRef]
- Dolezel, M.; Miklau, M.; Heissenberger, A.; Reichenbecher, W. Limits of Concern: Suggestions for the operationalisation of a concept to determine the relevance of adverse effects in the ERA of GMOs. Environ. Sci. Eur. 2018, 30, 39. [Google Scholar] [CrossRef]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogué, F.; Faure, J.-D. Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol. J. 2017, 15, 729–739. [Google Scholar] [CrossRef]
- Jiang, W.Z.; Henry, I.M.; Lynagh, P.G.; Comai, L.; Cahoon, E.B.; Weeks, D.P. Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol. J. 2017, 15, 648–657. [Google Scholar] [CrossRef]
- Ozseyhan, M.E.; Kang, J.; Mu, X.; Lu, C. Mutagenesis of the FAE1 genes significantly changes fatty acid composition in seeds of Camelina sativa. Plant Physiol. Biochem. 2018, 123, 1–7. [Google Scholar] [CrossRef]
- Lee, K.-R.; Jeon, I.; Yu, H.; Kim, S.-G.; Kim, H.-S.; Ahn, S.-J.; Lee, J.; Lee, S.-K.; Kim, H.U. Increasing Monounsaturated Fatty Acid Contents in Hexaploid Camelina sativa Seed Oil by FAD2 Gene Knockout Using CRISPR-Cas9. Front. Plant Sci. 2021, 12, 702930. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.A.; Romsdahl, T.B.; McGinn, M.G.; Nazarenus, T.J.; Cahoon, E.B.; Chapman, K.D.; Sedbrook, J.C. CRISPR/Cas9-Induced fad2 and rod1 Mutations Stacked With fae1 Confer High Oleic Acid Seed Oil in Pennycress (Thlaspi arvense L.). Front. Plant Sci. 2021, 12, 652319. [Google Scholar] [CrossRef]
- Sandgrind, S.; Li, X.; Ivarson, E.; Wang, E.S.; Guan, R.; Kanagarajan, S.; Zhu, L.-H. Improved fatty acid composition of field cress (Lepidium campestre) by CRISPR/Cas9-mediated genome editing. Front. Plant Sci. 2023, 14, 1076704. [Google Scholar] [CrossRef]
- McGinn, M.; Phippen, W.B.; Chopra, R.; Bansal, S.; Jarvis, B.A.; Phippen, M.E.; Dorn, K.M.; Esfahanian, M.; Nazarenus, T.J.; Cahoon, E.B.; et al. Molecular tools enabling pennycress (Thlaspi arvense) as a model plant and oilseed cash cover crop. Plant Biotechnol. J. 2019, 17, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Esfahanian, M.; Nazarenus, T.J.; Freund, M.M.; McIntosh, G.; Phippen, W.B.; Phippen, M.E.; Durrett, T.P.; Cahoon, E.B.; Sedbrook, J.C. Generating Pennycress (Thlaspi arvense) Seed Triacylglycerols and Acetyl-Triacylglycerols Containing Medium-Chain Fatty Acids. Front. Energy Res. 2021, 10, 620118. [Google Scholar] [CrossRef]
- Zhai, Y.; Yu, K.; Cai, S.; Hu, L.; Amoo, O.; Xu, L.; Yang, Y.; Ma, B.; Jiao, Y.; Zhang, C.; et al. Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L. Plant Biotechnol. J. 2020, 18, 1153–1168. [Google Scholar] [CrossRef]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 2014, 77, 198–208. [Google Scholar] [CrossRef]
- Ruiz-López, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef]
- Han, L.; Haslam, R.P.; Silvestre, S.; Lu, C.; Napier, J.A. Enhancing the accumulation of eicosapentaenoic acid and docosahexaenoic acid in transgenic Camelina through the CRISPR-Cas9 inactivation of the competing FAE1 pathway. Plant Biotechnol. J. 2022, 20, 1444–1446. [Google Scholar] [CrossRef]
- Han, L.; Usher, S.; Sandgrind, S.; Hassall, K.; Sayanova, O.; Michaelson, L.V.; Haslam, R.P.; Napier, J.A. High level accumulation of EPA and DHA in field-grown transgenic Camelina—A multi-territory evaluation of TAG accumulation and heterogeneity. Plant Biotechnol. J. 2020, 18, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS ONE 2014, 9, e85061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-H.; Krens, F.; Smith, M.A.; Li, X.; Qi, W.; van Loo, E.N.; Iven, T.; Feussner, I.; Nazarenus, T.J.; Huai, D.; et al. Dedicated Industrial Oilseed Crops as Metabolic Engineering Platforms for Sustainable Industrial Feedstock Production. Sci. Rep. 2016, 6, 22181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, R.; Fan, J.; Zhu, L.-H. Development of Industrial Oil Crop Crambe abyssinica for Wax Ester Production through Metabolic Engineering and Cross Breeding. Plant Cell Physiol. 2019, 60, 1274–1283. [Google Scholar] [CrossRef]
- Alagoz, Y.; Gurkok, T.; Zhang, B.; Unver, T. Manipulating the Biosynthesis of Bioactive Compound Alkaloids for Next-Generation Metabolic Engineering in Opium Poppy Using CRISPR-Cas 9 Genome Editing Technology. Sci. Rep. 2016, 6, 30910. [Google Scholar] [CrossRef]
- Tang, K.; Pan, Q. Strategies for Enhancing Alkaloids Yield in Catharanthus roseus Via Metabolic Engineering Approaches. In Catharanthus roseus; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–16. ISBN 978-3-319-51619-6. [Google Scholar]
- Yano, M.; Hirai, T.; Kato, K.; Hiwasa-Tanase, K.; Fukuda, N.; Ezura, H. Tomato is a suitable material for producing recombinant miraculin protein in genetically stable manner. Plant Sci. 2010, 178, 469–473. [Google Scholar] [CrossRef]
- Hiwasa-Tanase, K.; Hirai, T.; Kato, K.; Duhita, N.; Ezura, H. From miracle fruit to transgenic tomato: Mass production of the taste-modifying protein miraculin in transgenic plants. Plant Cell Rep. 2012, 31, 513–525. [Google Scholar] [CrossRef]
- Zhu, Q.; Zeng, D.; Yu, S.; Cui, C.; Li, J.; Li, H.; Chen, J.; Zhang, R.; Zhao, X.; Chen, L.; et al. From Golden Rice to aSTARice: Bioengineering Astaxanthin Biosynthesis in Rice Endosperm. Mol. Plant 2018, 11, 1440–1448. [Google Scholar] [CrossRef]
- Klocko, A.L. Genetic Containment for Molecular Farming. Plants 2022, 11, 2436. [Google Scholar] [CrossRef]
- Calgaro-Kozina, A.; Vuu, K.M.; Keasling, J.D.; Loqué, D.; Sattely, E.S.; Shih, P.M. Engineering Plant Synthetic Pathways for the Biosynthesis of Novel Antifungals. ACS Cent. Sci. 2020, 6, 1394–1400. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, Z.H.; Reem, N.T.; Dalrymple, J.; Soyk, S.; Swartwood, K.E.; Rodriguez-Leal, D.; van Eck, J.; Lippman, Z.B. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 2018, 4, 766–770. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Zhu, J.-K. Precision genome editing heralds rapid de novo domestication for new crops. Cell 2021, 184, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170.e14. [Google Scholar] [CrossRef]
- Ye, C.-Y.; Fan, L. Orphan Crops and their Wild Relatives in the Genomic Era. Mol. Plant 2021, 14, 27–39. [Google Scholar] [CrossRef]
- Nature Communications. Efficient genetic improvement of orphan crops cannot follow the old path. Nat. Commun. 2024, 15, 321. [Google Scholar] [CrossRef]
- Gutaker, R.M.; Chater, C.C.C.; Brinton, J.; Castillo-Lorenzo, E.; Breman, E.; Pironon, S. Scaling up neodomestication for climate-ready crops. Curr. Opin. Plant Biol. 2022, 66, 102169. [Google Scholar] [CrossRef]
- Lehmeier, C.; Pajor, R.; Lundgren, M.R.; Mathers, A.; Sloan, J.; Bauch, M.; Mitchell, A.; Bellasio, C.; Green, A.; Bouyer, D.; et al. Cell density and airspace patterning in the leaf can be manipulated to increase leaf photosynthetic capacity. Plant J. 2017, 92, 981–994. [Google Scholar] [CrossRef]
- Smith, E.N.; van Aalst, M.; Tosens, T.; Niinemets, Ü.; Stich, B.; Morosinotto, T.; Alboresi, A.; Erb, T.J.; Gómez-Coronado, P.A.; Tolleter, D.; et al. Improving photosynthetic efficiency toward food security: Strategies, advances, and perspectives. Mol. Plant 2023, 16, 1547–1563. [Google Scholar] [CrossRef]
- Simkin, A.J.; McAusland, L.; Headland, L.R.; Lawson, T.; Raines, C.A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. EXBOTJ 2015, 66, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Koller, F.; Cieslak, M.; Bauer-Panskus, A. Environmental risk scenarios of specific NGT applications in Brassicaceae oilseed plants. Environ. Sci. Eur. 2024, 36, 189. [Google Scholar] [CrossRef]
- Fu, M.; Chen, L.; Cai, Y.; Su, Q.; Chen, Y.; Hou, W. CRISPR/Cas9-Mediated Mutagenesis of GmFAD2-1A and/or GmFAD2-1B to Create High-Oleic-Acid Soybean. Agronomy 2022, 12, 3218. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Hernando, A.; Alvarez, J.B.; Shewry, P.R.; Barro, F. Silencing of γ-gliadins by RNA interference (RNAi) in bread wheat. J. Cereal Sci. 2008, 48, 565–568. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Shewry, P.R.; Tosi, P.; Barro, F. Suppression of gliadins results in altered protein body morphology in wheat. J. Exp. Bot. 2011, 62, 4203–4213. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Altamirano-Fortoul, R.; Real, A.; Comino, I.; Sousa, C.; Rosell, C.M.; Barro, F. Reduced-gliadin wheat bread: An alternative to the gluten-free diet for consumers suffering gluten-related pathologies. PLoS ONE 2014, 9, e90898. [Google Scholar] [CrossRef]
- Petrie, J.R.; Zhou, X.-R.; Leonforte, A.; McAllister, J.; Shrestha, P.; Kennedy, Y.; Belide, S.; Buzza, G.; Gororo, N.; Gao, W.; et al. Development of a Brassica napus (Canola) Crop Containing Fish Oil-Like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727. [Google Scholar] [CrossRef]
- Cheng, B.; Wu, G.; Vrinten, P.; Falk, K.; Bauer, J.; Qiu, X. Towards the production of high levels of eicosapentaenoic acid in transgenic plants: The effects of different host species, genes and promoters. Transgenic Res. 2010, 19, 221–229. [Google Scholar] [CrossRef]
- Usher, S.; Haslam, R.P.; Ruiz-Lopez, N.; Sayanova, O.; Napier, J.A. Field trial evaluation of the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: Making fish oil substitutes in plants. Metab. Eng. Commun. 2015, 2, 93–98. [Google Scholar] [CrossRef]
- Colombo, S.M.; Campbell, L.G.; Murphy, E.J.; Martin, S.L.; Arts, M.T. Potential for novel production of omega-3 long-chain fatty acids by genetically engineered oilseed plants to alter terrestrial ecosystem dynamics. Agric. Syst. 2018, 164, 31–37. [Google Scholar] [CrossRef]
- Mipeshwaree Devi, A.; Khedashwori Devi, K.; Premi Devi, P.; Lakshmipriyari Devi, M.; Das, S. Metabolic engineering of plant secondary metabolites: Prospects and its technological challenges. Front. Plant Sci. 2023, 14, 1171154. [Google Scholar] [CrossRef] [PubMed]
- Birchfield, A.S.; McIntosh, C.A. Metabolic engineering and synthetic biology of plant natural products—A minireview. Curr. Plant Biol. 2020, 24, 100163. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Ghorai, M.; Kant, N.; Kumar, M.; Radha; Jha, N.K.; Swamy, M.K.; Proćków, J.; de La Lastra, J.M.P.; et al. Genome editing technologies, mechanisms and improved production of therapeutic phytochemicals: Opportunities and prospects. Biotechnol. Bioeng. 2023, 120, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Eljounaidi, K.; Lichman, B.R. Nature’s Chemists: The Discovery and Engineering of Phytochemical Biosynthesis. Front. Chem. 2020, 8, 596479. [Google Scholar] [CrossRef]
- Courdavault, V.; O’Connor, S.E.; Jensen, M.K.; Papon, N. Metabolic engineering for plant natural products biosynthesis: New procedures, concrete achievements and remaining limits. Nat. Prod. Rep. 2021, 38, 2145–2153. [Google Scholar] [CrossRef]
- Østerberg, J.T.; Xiang, W.; Olsen, L.I.; Edenbrandt, A.K.; Vedel, S.E.; Christiansen, A.; Landes, X.; Andersen, M.M.; Pagh, P.; Sandøe, P.; et al. Accelerating the Domestication of New Crops: Feasibility and Approaches. Trends Plant Sci. 2017, 22, 373–384. [Google Scholar] [CrossRef]
- Rehman, F.; Gong, H.; Bao, Y.; Zeng, S.; Huang, H.; Wang, Y. CRISPR gene editing of major domestication traits accelerating breeding for Solanaceae crops improvement. Plant Mol. Biol. 2022, 108, 157–173. [Google Scholar] [CrossRef]
- Chapman, E.A.; Thomsen, H.C.; Tulloch, S.; Correia, P.M.P.; Luo, G.; Najafi, J.; DeHaan, L.R.; Crews, T.E.; Olsson, L.; Lundquist, P.-O.; et al. Perennials as Future Grain Crops: Opportunities and Challenges. Front. Plant Sci. 2022, 13, 898769. [Google Scholar] [CrossRef]
- Luo, G.; Najafi, J.; Correia, P.M.P.; Trinh, M.D.L.; Chapman, E.A.; Østerberg, J.T.; Thomsen, H.C.; Pedas, P.R.; Larson, S.; Gao, C.; et al. Accelerated Domestication of New Crops: Yield is Key. Plant Cell Physiol. 2022, 63, 1624–1640. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Grandillo, S.; Knaap, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- OECD. Consensus Document on Compositional Considerations for New Varieties of Tomato: Key Food and Feed Nutrients, Toxicants and Allergens: Series on the Safety of Novel Foods and Feeds, No.17. ENV/JM/MONO(2008)26; OECD Environment, Health and Safety Publications: Paris, France, 2008; Available online: https://one.oecd.org/document/env/jm/mono(2008)26/en/pdf (accessed on 2 June 2025).
- Soyk, S.; Lemmon, Z.H.; Oved, M.; Fisher, J.; Liberatore, K.L.; Park, S.J.; Goren, A.; Jiang, K.; Ramos, A.; van der Knaap, E.; et al. Bypassing Negative Epistasis on Yield in Tomato Imposed by a Domestication Gene. Cell 2017, 169, 1142–1155.e12. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.E.; Moyers, B.T.; Man, J.; Subramaniam, B.; Makunga, N.P. The Power and Perils of De Novo Domestication Using Genome Editing. Annu. Rev. Plant Biol. 2023, 74, 727–750. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.M.P.; Najafi, J.; Palmgren, M. De novo domestication: What about the weeds? Trends Plant Sci. 2024, 29, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Muniz, J.; Kretzschmar, A.A.; Rufato, L.; Pelizza, T.R.; Rufato, A.D.R.; de Macedo, T.A. General aspects of physalis cultivation. Cienc. Rural 2014, 44, 964–970. [Google Scholar] [CrossRef]
- Zarzycki, J.; Brecht, V.; Müller, M.; Fuchs, G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc. Natl. Acad. Sci. USA 2009, 106, 21317–21322. [Google Scholar] [CrossRef]
- Theeuwen, T.P.J.M.; Logie, L.L.; Harbinson, J.; Aarts, M.G.M. Genetics as a key to improving crop photosynthesis. EXBOTJ 2022, 73, 3122–3137. [Google Scholar] [CrossRef]
- Walsh, T.A.; Bevan, S.A.; Gachotte, D.J.; Larsen, C.M.; Moskal, W.A.; Merlo, P.A.O.; Sidorenko, L.V.; Hampton, R.E.; Stoltz, V.; Pareddy, D.; et al. Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nat. Biotechnol. 2016, 34, 881–887. [Google Scholar] [CrossRef]
- Menz, J.; Modrzejewski, D.; Hartung, F.; Wilhelm, R.; Sprink, T. Genome Edited Crops Touch the Market: A View on the Global Development and Regulatory Environment. Front. Plant Sci. 2020, 11, 586027. [Google Scholar] [CrossRef]
- Chopra, R.; Johnson, E.B.; Emenecker, R.; Cahoon, E.B.; Lyons, J.; Kliebenstein, D.J.; Daniels, E.; Dorn, K.M.; Esfahanian, M.; Folstad, N.; et al. Identification and stacking of crucial traits required for the domestication of pennycress. Nat. Food 2020, 1, 84–91. [Google Scholar] [CrossRef]
- Gustafsson, C.; Willforss, J.; Lopes-Pinto, F.; Ortiz, R.; Geleta, M. Identification of genes regulating traits targeted for domestication of field cress (Lepidium campestre) as a biennial and perennial oilseed crop. BMC Genet. 2018, 19, 36. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Lu, C.-Y.; Lee, C.-R. The climatic association of population divergence and future extinction risk of Solanum pimpinellifolium. AoB Plants 2020, 12, plaa012. [Google Scholar] [CrossRef] [PubMed]
- Kamenya, S.N.; Mikwa, E.O.; Song, B.; Odeny, D.A. Genetics and breeding for climate change in Orphan crops. Theor. Appl. Genet. 2021, 134, 1787–1815. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, S.; Milla, R. Differential plasticity to water and nutrients between crops and their wild progenitors. Environ. Exp. Bot. 2018, 145, 54–63. [Google Scholar] [CrossRef]
- Tchokponhoué, D.A.; N’Danikou, S.; Houéto, J.S.; Achigan-Dako, E.G. Shade and nutrient-mediated phenotypic plasticity in the miracle plant Synsepalum dulcificum (Schumach. & Thonn.) Daniell. Sci. Rep. 2019, 9, 5135. [Google Scholar] [CrossRef]
- OECD. Consensus Documents: Harmonisation of Regulatory Oversight in Biotechnology 2025. Available online: https://www.oecd.org/env/ehs/biotrack/consensusdocumentsfortheworkonharmonisationofregulatoryoversightinbiotechnologybiologyofcrops.htm (accessed on 2 June 2025).
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA J. 2017, 15, e04970. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar] [CrossRef]
- Carlini, C.R.; Grossi-de-Sá, M.F. Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 2002, 40, 1515–1539. [Google Scholar] [CrossRef]
- OECD. Consensus Document on Compositional Considerations for New Varieties of Bread Wheat (Triticum aestivum): Key Food and Feed Nutrients, Anti-Nutrients and Toxicants ENV/JM/MONO(2003)7. 2003. Available online: https://one.oecd.org/document/ENV/JM/MONO(2003)7/en/pdf (accessed on 2 June 2025).
- Khalid, M.; Rahman, S.U.; Bilal, M.; Huang, D. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Leite Dias, S.; D’Auria, J.C. The Bitter Truth: How Insects Cope with Toxic Plant Alkaloids. EXBOTJ 2024, 76, 5–15. [Google Scholar] [CrossRef]
- EFSA GMO Panel. Scientific Opinion on Current Practice, Challenges, and Future Opportunities in the Safety Assessment of Newly Expressed Proteins in Genetically Modified Plants: Draft Scientific Opinion, EFSA-Q-2023-00664. 2025. Available online: https://connect.efsa.europa.eu/RM/s/consultations/publicconsultation2/a0lTk000003SPsz/pc1278 (accessed on 12 February 2025).
- Commission Decision of 24 July 2002 Establishing Guidance Notes Supplementing AnnexII to Directive 2001/18/EC of the European Parliament and of the Council on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC: C(2002) 2715. 2002. Available online: https://eur-lex.europa.eu/eli/dec/2002/623/oj/eng (accessed on 2 June 2025).
- Naegeli, H.; Bresson, J.L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Firbank, L.G.; Guerche, P.; Hejatko, J.; Moreno, F.J.; Mullins, E.; et al. Assessment of genetically modified soybean GMB151 for food and feed uses, under Regulation (EC) No 1829/2003 (application EFSA-GMO-NL-2018-153). EFSA J. 2021, 19, e06424. [Google Scholar] [CrossRef]
- Devos, Y.; Romeis, J.; Luttik, R.; Maggiore, A.; Perry, J.N.; Schoonjans, R.; Streissl, F.; Tarazona, J.V.; Brock, T.C.M. Optimising environmental risk assessments: Accounting for ecosystem services helps to translate broad policy protection goals into specific operational ones for environmental risk assessments. EMBO Rep. 2015, 16, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, P.; Arce, A.; Focks, A.; Ingels, B.; Jölli, D.; Lambin, S.; Rundlöf, M.; Süßenbach, D.; Del Aguila, M.; Ercolano, V.; et al. Revised guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2023, 21, e07989. [Google Scholar] [CrossRef]
- Kowarik, I.; Heink, U.; Bartz, R. “Ökologische Schäden” in Folge der Ausbringung Gentechnisch Veränderter Organismen im Freiland—Entwicklung einer Begriffsdefinition und eines Konzeptes zur Operationalisierung. BfN Schriften No. 166. 2006. Available online: https://www.bfn.de/publikationen/bfn-schriften/bfn-schriften-166-oekologische-schaeden-folge-der-ausbringung (accessed on 2 September 2024).
- Kowarik, I.; Bartz, R.; Heink, U. Bewertung “ökologischer Schäden” Infolge des Anbaus Gentechnisch Veränderter Organismen (GVO) in der Landwirtschaft; Bundesamt für Naturschutz: Bonn-Bad Godesberg, Germany, 2008; ISBN 978-3-7843-3956-6. [Google Scholar]
- Bartz, R.; Heink, U.; Kowarik, I. Proposed definition of environmental damage illustrated by the cases of genetically modified crops and invasive species. Conserv. Biol. 2010, 24, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.J.; McWilliams, S.R.; Place, A.R.; Huguenin, M.A. Diet preferences for specific fatty acids and their effect on composition of fat reserves in migratory Red-eyed Vireos (Vireo olivaceous). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 503–514. [Google Scholar] [CrossRef]
- Pierce, B.J.; McWilliams, S.R. The fat of the matter: How dietary fatty acids can affect exercise performance. Integr. Comp. Biol. 2014, 54, 903–912. [Google Scholar] [CrossRef]
- Maillet, D.; Weber, J.-M. Relationship between n-3 PUFA content and energy metabolism in the flight muscles of a migrating shorebird: Evidence for natural doping. J. Exp. Biol. 2007, 210, 413–420. [Google Scholar] [CrossRef]
- Kawall, K. Genome-edited Camelina sativa with a unique fatty acid content and its potential impact on ecosystems. Environ. Sci. Eur. 2021, 33, 38. [Google Scholar] [CrossRef]
- Hixson, S.M.; Sharma, B.; Kainz, M.J.; Wacker, A.; Arts, M.T. Production, distribution, and abundance of long-chain omega-3 polyunsaturated fatty acids: A fundamental dichotomy between freshwater and terrestrial ecosystems. Environ. Rev. 2015, 23, 414–424. [Google Scholar] [CrossRef]
- Hixson, S.M.; Shukla, K.; Campbell, L.G.; Hallett, R.H.; Smith, S.M.; Packer, L.; Arts, M.T. Long-Chain Omega-3 Polyunsaturated Fatty Acids Have Developmental Effects on the Crop Pest, the Cabbage White Butterfly Pieris rapae. PLoS ONE 2016, 11, e0152264. [Google Scholar] [CrossRef]
- Patil, A.D.; Kasabe, P.J.; Dandge, P.B. Pharmaceutical and nutraceutical potential of natural bioactive pigment: Astaxanthin. Nat. Prod. Bioprospect. 2022, 12, 25. [Google Scholar] [CrossRef]
- Atarashi, M.; Manabe, Y.; Kishimoto, H.; Sugawara, T.; Osakabe, M. Antioxidant Protection by Astaxanthin in the Citrus Red Mite (Acari: Tetranychidae). Environ. Entomol. 2017, 46, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Evergetis, E.; Papachristos, D.; Vangelatou, O.; Antonatos, S.; Milonas, P.; Haroutounian, S.A.; Machera, K. An essay on ecosystem availability of Nicotiana glauca graham alkaloids: The honeybees case study. BMC Ecol. 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Tiedeken, E.J.; Egan, P.A.; Stevenson, P.C.; Wright, G.A.; Brown, M.J.F.; Power, E.F.; Farrell, I.; Matthews, S.M.; Stout, J.C. Nectar chemistry modulates the impact of an invasive plant on native pollinators. Funct. Ecol. 2016, 30, 885–893. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Abbott, S.K. Nutritional ecology of essential fatty acids: An evolutionary perspective. Aust. J. Zool. 2011, 59, 369. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Shrestha, P.; Callahan, D.L.; Singh, S.P.; Petrie, J.R.; Zhou, X.-R. Reduced Triacylglycerol Mobilization during Seed Germination and Early Seedling Growth in Arabidopsis Containing Nutritionally Important Polyunsaturated Fatty Acids. Front. Plant Sci. 2016, 7, 1402. [Google Scholar] [CrossRef]
- Aznar-Moreno, J.A.; Durrett, T.P. Simultaneous Targeting of Multiple Gene Homeologs to Alter Seed Oil Production in Camelina sativa. Plant Cell Physiol. 2017, 58, 1260–1267. [Google Scholar] [CrossRef]
- Darwin, S.C.; Knapp, S.; Peralta, I.E. Taxonomy of tomatoes in the Galápagos Islands: Native and introduced species of Solanum section Lycopersicon (Solanaceae). Syst. Biodivers. 2003, 1, 29–53. [Google Scholar] [CrossRef]
- Peralta, I.E.; Spooner, D.M.; Knapp, S. Taxonomy of Wild Tomatoes and Their Relatives (Solanum Sect. Lycopersicoides, Sect. Juglandifolia, Sect. Lycopersicon; Solanaceae); American Society of Plant Taxonomists: Ann Arbor, MI, USA, 2008; ISBN 978-0-912861-84-5. [Google Scholar]
- Knapp, S. Solanum Pimpinellifolium—New for the Alien Flora of Austria, with Comments on Austrian Records of S. Triflorum and S. Nitidibaccatum. Neilreichia 2018, 9, 49–53. [Google Scholar] [CrossRef]
- McAlpine, K.G.; Jesson, L.K.; Kubien, D.S. Photosynthesis and water-use efficiency: A comparison between invasive (exotic) and non-invasive (native) species. Austral Ecol. 2008, 33, 10–19. [Google Scholar] [CrossRef]
- McDowell, S.C.L. Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am. J. Bot. 2002, 89, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Song, X.; He, B.; Valverde, B.E.; Qiang, S. Enhanced photosynthesis endows seedling growth vigour contributing to the competitive dominance of weedy rice over cultivated rice. Pest. Manag. Sci. 2017, 73, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, M.; Miklau, M.; Greiter, G.; Heissenberger, A.; Ribarits, A.; Manhalter, S.; Mechtler, K.; Stepanek, W.; van Gehren, P.; Wechselberger, K.; et al. Use of Phenotypic Plant Traits to Support the Environmental Risk Assessment of Genetically Modified Plants. BfN Schriften No. 708, Bonn. 2024. Available online: https://www.bfn.de/en/publications/bfn-schriften/bfn-schriften-708-use-phenotypic-plant-traits-support-environmental-risk (accessed on 16 April 2025).
- Damgaard, C.; Kjær, C. Competitive interactions and the effect of herbivory on Bt-Brassica napus, Brassica rapa and Lolium perenne. J. Appl. Ecol. 2009, 46, 1073–1079. [Google Scholar] [CrossRef]
- Liu, Y.; Stewart, C.N.; Li, J.; Huang, H.; Zhang, X. The presence of Bt-transgenic oilseed rape in wild mustard populations affects plant growth. Transgenic Res. 2015, 24, 1043–1053. [Google Scholar] [CrossRef]
- Mercer, K.L.; Andow, D.A.; Wyse, D.L.; Shaw, R.G. Stress and domestication traits increase the relative fitness of crop-wild hybrids in sunflower. Ecol. Lett. 2007, 10, 383–393. [Google Scholar] [CrossRef]
- Mercer, K.L.; Wyse, D.L.; Shaw, R.G. Effects of competition on the fitness of wild and crop-wild hybrid sunflower from a diversity of wild populations and crop lines. Evolution 2006, 60, 2044. [Google Scholar] [CrossRef]
- Guadagnuolo, R.; Clegg, J.; Ellstrand, N.C. Relative fitness of transgenic vs. non-transgenic maize × teosinte hybrids: A field evaluation. Ecol. Appl. 2006, 16, 1967–1974. [Google Scholar] [CrossRef]
- Yang, X.; Xia, H.; Wang, W.; Wang, F.; Su, J.; Snow, A.A.; Lu, B.-R. Transgenes for insect resistance reduce herbivory and enhance fecundity in advanced generations of crop-weed hybrids of rice. Evol. Appl. 2011, 4, 672–684. [Google Scholar] [CrossRef]
- Guan, Z.-J.; Zhang, P.-F.; Wei, W.; Mi, X.-C.; Kang, D.-M.; Liu, B. Performance of hybrid progeny formed between genetically modified herbicide-tolerant soybean and its wild ancestor. AoB Plants 2015, 7, 1–8. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sheng, Z.W.; Hu, Y.Q.; Liu, Q.; Qiang, S.; Song, X.L.; Liu, B. Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean. Transgenic Res. 2021, 30, 105–119. [Google Scholar] [CrossRef]
- Vacher, C.; Weis, A.E.; Hermann, D.; Kossler, T.; Young, C.; Hochberg, M.E. Impact of ecological factors on the initial invasion of Bt transgenes into wild populations of birdseed rape (Brassica rapa). Theor. Appl. Genet. 2004, 109, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.A.; Andersen, B.; Jørgensen, R.B. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy B. rapa. Mol. Ecol. 1999, 8, 605–615. [Google Scholar] [CrossRef]
- Waschmann, R.S.; Watrud, L.S.; Reece, L.R.; Shiroyama, T. Sunlit mesocosms designed for pollen confinement and risk assessment of transgenic crops. Aerobiologia 2010, 26, 311–325. [Google Scholar] [CrossRef]
- Londo, J.P.; Bollman, M.A.; Sagers, C.L.; Lee, E.H.; Watrud, L.S. Changes in fitness-associated traits due to the stacking of transgenic glyphosate resistance and insect resistance in Brassica napus L. Heredity 2011, 107, 328–337. Heredity 2011, 107, 328–337. [Google Scholar] [CrossRef]
- Moon, H.S.; Halfhill, M.D.; Good, L.L.; Raymer, P.L.; Neal Stewart, C. Characterization of directly transformed weedy Brassica rapa and introgressed B. rapa with Bt cry1Ac and gfp genes. Plant Cell Rep. 2007, 26, 1001–1010. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; Raybould, A. Protection goals in environmental risk assessment: A practical approach. Transgenic Res. 2014, 23, 945–956. [Google Scholar] [CrossRef]
- Raybould, A.; Macdonald, P. Policy-Led Comparative Environmental Risk Assessment of Genetically Modified Crops: Testing for Increased Risk Rather Than Profiling Phenotypes Leads to Predictable and Transparent Decision-Making. Front. Bioeng. Biotechnol. 2018, 6, 43. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Biodiversity for Food and Agriculture. Available online: https://www.fao.org/state-of-biodiversity-for-food-agriculture/en (accessed on 7 March 2025).
- OECD. Safety Assessment of Transgenic Organisms in the Environment: OECD Consensus Documents; OECD Publishing: Paris, France, 2017; ISBN 978-92-64-27972-8. [Google Scholar]
- Bohle, F.; Schneider, R.; Mundorf, J.; Zühl, L.; Simon, S.; Engelhard, M. Where does the EU-path on new genomic techniques lead us? Front. Genome Ed. 2024, 6, 1377117. [Google Scholar] [CrossRef]
- European Commission. Regulation of the European Parliament and of the Council on Plants Obtained by Certain New Genomic Techniques and Their Food and Feed, and Amending Regulation (EU) 2017/625 COM(2023) 411 Final, Brussels. 2023. Available online: https://food.ec.europa.eu/plants/genetically-modified-organisms/new-techniques-biotechnology_en (accessed on 11 September 2024).
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on Methods for Assessing the Health and Environmental Risks and Socio-Economic Issues Associated with Plants Obtained Using Certain New Genomic Techniques (NGTs): ANSES Opinion, Request No 2021-SA-0019, Maisons-Alfort. 2024. Available online: www.anses.fr (accessed on 11 September 2024).
- EFSA. Relevance of new scientific evidence on the occurrence of teosinte in maize fields in Spain and France for previous environmental risk assessment conclusions and risk management recommendations on the cultivation of maize events MON810, Bt11, 1507 and GA21: Technical Report. EFSA Support. Publ. 2016, 13, EN-1094. [Google Scholar] [CrossRef]
- Devos, Y.; Aiassa, E.; Muñoz-Guajardo, I.; Messéan, A.; Mullins, E. Update of environmental risk assessment conclusions and risk management recommendations of EFSA (2016) on EU teosinte. EFSA J. 2022, 20, e07228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolezel, M.; Miklau, M.; Heissenberger, A.; Kroeger, I.; Otto, M. Complexity Meets Risk—The Next Generation of Genome-Edited Plants Challenges Established Concepts for Environmental Risk Assessment in the EU. Plants 2025, 14, 1723. https://doi.org/10.3390/plants14111723
Dolezel M, Miklau M, Heissenberger A, Kroeger I, Otto M. Complexity Meets Risk—The Next Generation of Genome-Edited Plants Challenges Established Concepts for Environmental Risk Assessment in the EU. Plants. 2025; 14(11):1723. https://doi.org/10.3390/plants14111723
Chicago/Turabian StyleDolezel, Marion, Marianne Miklau, Andreas Heissenberger, Iris Kroeger, and Mathias Otto. 2025. "Complexity Meets Risk—The Next Generation of Genome-Edited Plants Challenges Established Concepts for Environmental Risk Assessment in the EU" Plants 14, no. 11: 1723. https://doi.org/10.3390/plants14111723
APA StyleDolezel, M., Miklau, M., Heissenberger, A., Kroeger, I., & Otto, M. (2025). Complexity Meets Risk—The Next Generation of Genome-Edited Plants Challenges Established Concepts for Environmental Risk Assessment in the EU. Plants, 14(11), 1723. https://doi.org/10.3390/plants14111723