Plant Origin Regulates the Response of Solidago canadensis Reproductive Traits to Long-Term Warming and Nitrogen Addition
Abstract
1. Introduction
2. Results
2.1. Reproductive Traits Under Warming, N Addition, and Plant Origin
2.2. Relative Change in Reproductive Traits Under Different Environments
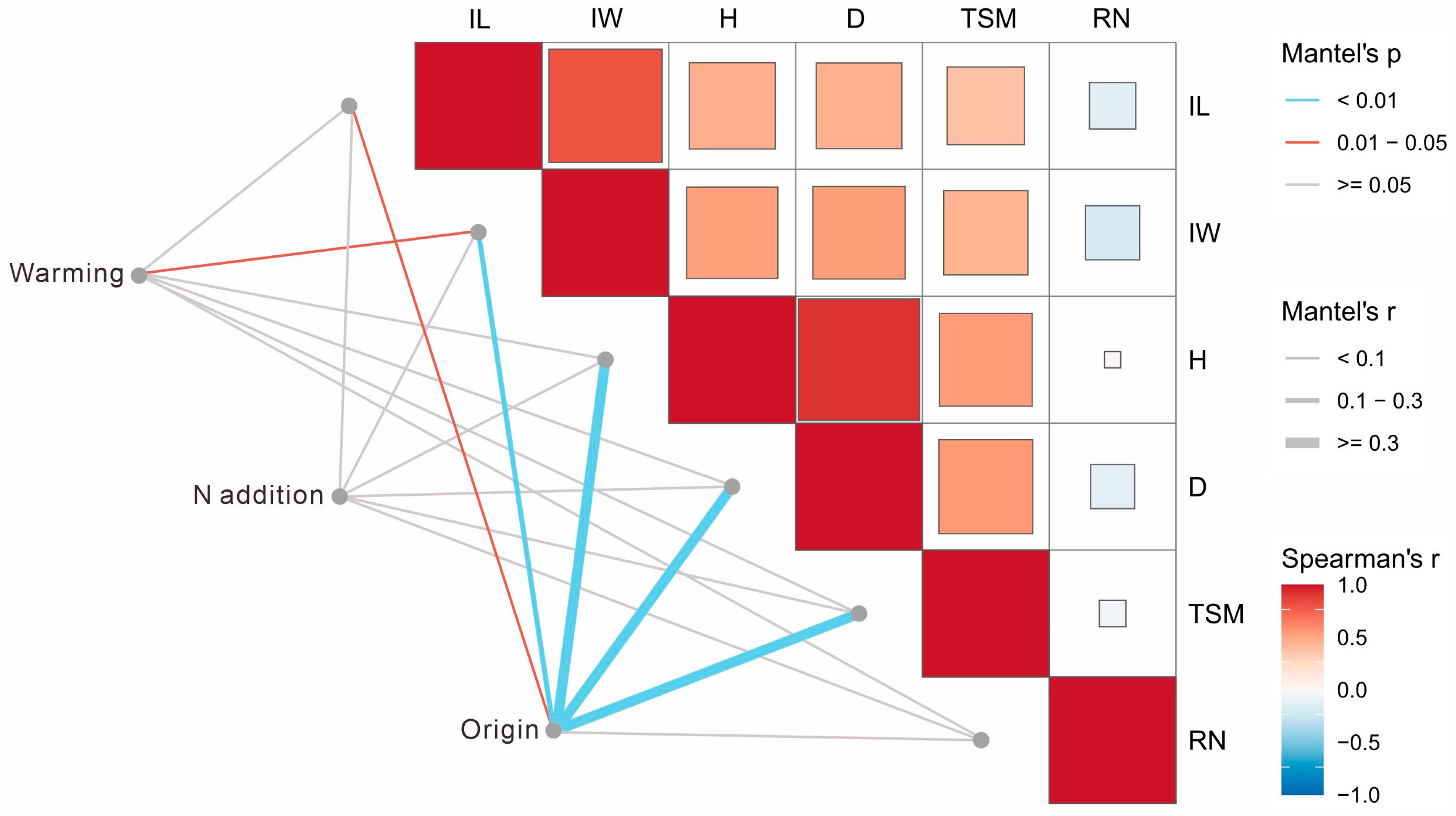
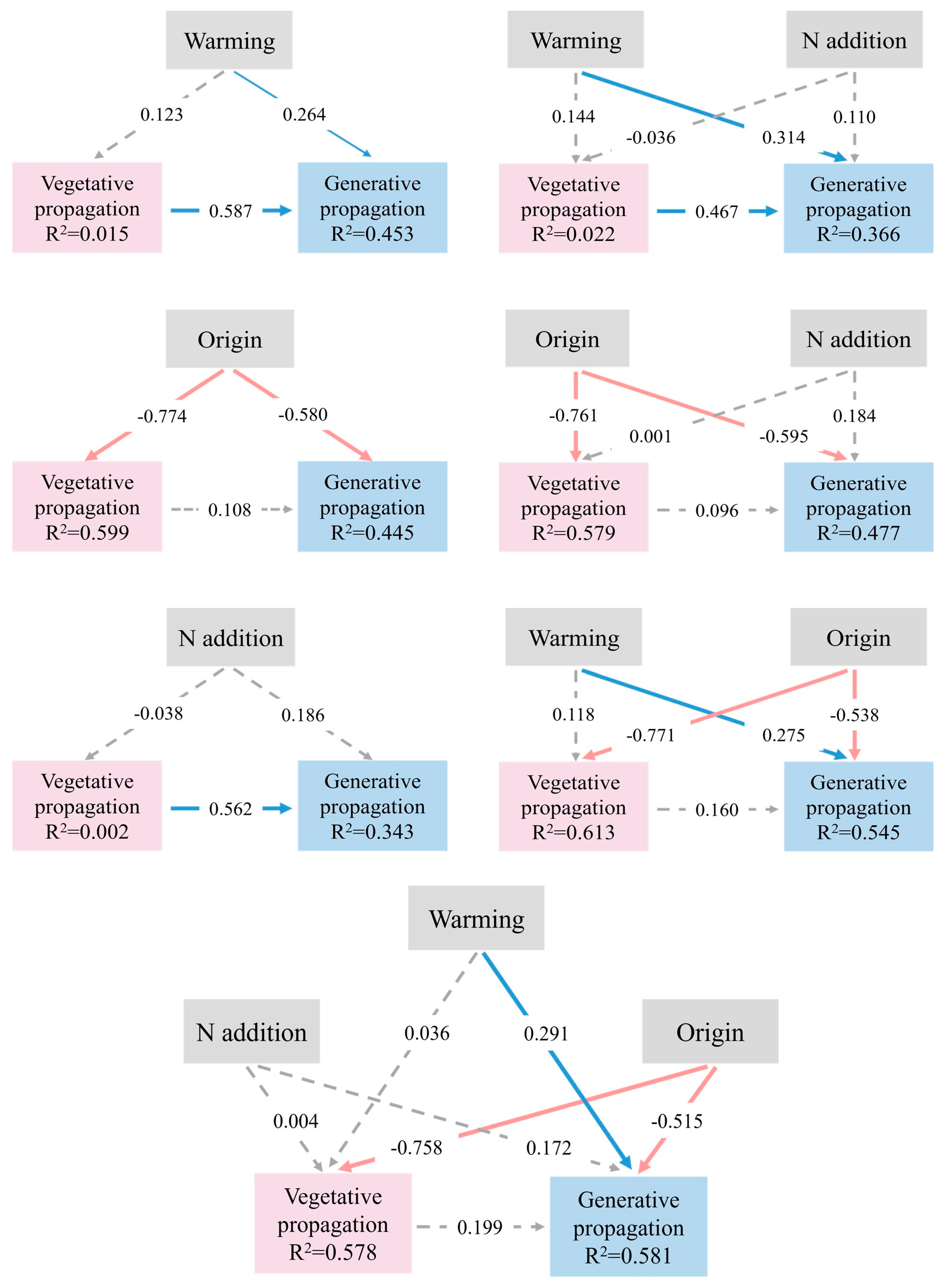
2.3. Potential Associations Among Reproductive Traits
3. Discussion
4. Materials and Methods
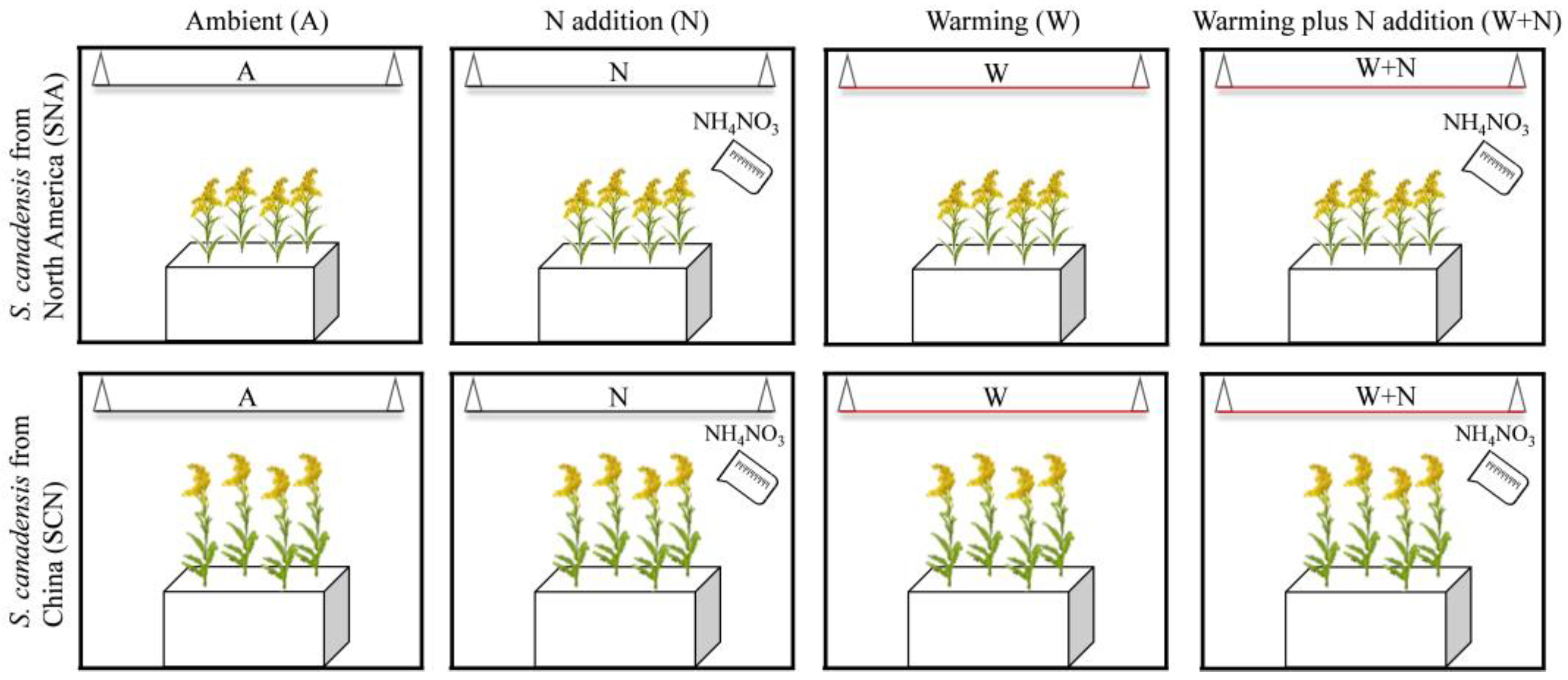
4.1. Experimental Location and Materials
4.2. Long-Term Warming and N Addition Experiment
4.3. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SNA | Solidago canadensis from North America |
| SCN | Solidago canadensis from China |
| A | Ambient |
| W | Warming |
| N | Nitrogen |
| W + N | Warming plus N addition |
| O | Origin |
References
- Diagne, C.; Leroy, B.; Vaissiere, A.C.; Gozlan, R.E.; Roiz, D.; Jaric, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biologi-cal invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.C.; Feng, Y.L.; Zhou, Y.J.; Ao, Y.M.; Chen, C.X.; Xing, Y.J.; Wang, Q.G.; Yan, G.Y. Main hypotheses on mechanisms underlying plant invasion: A review. Chin. J. Appl. Ecol. 2022, 33, 3105–3115. [Google Scholar]
- Ivison, K.; Vange, V.; Speed, J.M.; Dawson, W. Invasive plants grow taller under experimental warming, but mediated effects of biotic interactions are species-specific. Oikos 2025, 2025, e10932. [Google Scholar] [CrossRef]
- Liu, Y.J.; Oduor, A.M.O.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.L.; van Kleunen, M. Do invasive alien plants benefit more from global environmental change than native plants? Glob. Change Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef]
- Deng, B.L.; Liu, Q.; Liu, X.S.; Zheng, L.Y.; Jiang, L.B.; Guo, X.M.; Liu, Y.Q.; Zhang, L. Effects of enhanced UV-B radiation and nitrogen deposition on the growth of invasive plant Triadica sebifera. Chin. J. Plant Ecol. 2017, 41, 471–479. [Google Scholar]
- Zhou, X.H.; Li, J.J.; Gao, Y.Y.; Peng, P.H.; He, W.M. Maternal effects of climate warming and nitrogen deposition vary with home and introduced ranges. Funct. Ecol. 2022, 36, 751–762. [Google Scholar] [CrossRef]
- Ren, G.Q.; Zou, C.B.; Wan, L.Y.; Johnson, J.H.; Li, J.; Zhu, L.; Qi, S.S.; Dai, Z.C.; Zhang, H.Y.; Du, D.L. Interactive effect of climate warming and nitrogen deposi-tion may shift the dynamics of native and invasive species. J. Plant Ecol. 2021, 14, 84–95. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef]
- Patel, P.; Prasad, A.; Srivastava, D.; Niranjian, A.; Saxena, G.; Singh, S.S.; Misra, P.; Chakrabarty, D. Genotype-dependent and temperature-induced modulation of secondary metabolites, antioxidative defense and gene expression profile in Solanum viarum Dunal. Environ. Exp. Bot. 2022, 194, 104686. [Google Scholar] [CrossRef]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.; Frieler, K.; Knutti, K.; Frame, D.J.; Allen, M.R. Green-house-gas emission targets for limiting global warming to 2 °C. Nature 2009, 458, 1158–1162. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; WMO and UNEP: Geneva, Switzerland, 2021. [Google Scholar]
- Sun, Y.; Züst, T.; Silvestro, D.; Erb, M.; Bossdorf, O.; Mateo, P.; Robert, C.; Müllerscharer, H. Climate warming can reduce biocontrol efficacy and promote plant invasion due to both genetic and transient metabolomic changes. Ecol. Lett. 2022, 25, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Laghari, A.H.; Lashiari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Zhang, Y.L.; Leng, Z.R.; Wu, Y.M.; Jia, H.; Yan, C.L.; Wang, X.H.; Ren, G.Q.; Wu, G.R.; Li, J. Interaction between nitrogen, phosphorus, and invasive alien plants. Sustainability 2022, 14, 746. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Borer, E.T.; Stevens, C.J. Nitrogen deposition and climate: An integrated synthesis. Trends Ecol. Evol. 2022, 37, 541–552. [Google Scholar] [CrossRef]
- Correa, P.L.C.; De-la-Cruz-Chacon, I.; Sousa, M.C.; Viera, M.A.R.; Campos, F.G.; Marques, M.O.M.; Boaro, C.S.F.; Ferreira, G. Effect of nitrogen sources on photosynthesis and biosynthesis of alkaloids and leaf volatile compounds in Annona sylvatica A St.-Hil. J. Soil Sci. Plant Nut. 2022, 94, s52729. [Google Scholar] [CrossRef]
- Throop, H.L.; Lerdau, M.T. Effects of nitrogen deposition on insect herbivory: Implications for community and ecosystem processes. Ecosystems 2004, 7, 109–133. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.X.; Zhou, X.H.; Peng, P.H.; Li, J.J.; He, W.M. Warming delays the phenological sequences of an autumn-flowering invader. Ecol. Evol. 2018, 8, 6299–6307. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; McCauley, D.E.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Zenni, R.D.; Bailey, J.K.; Simberloff, D. Rapid evolution and range expansion of an invasive plant are driven by provenance–environment interactions. Ecol. Lett. 2014, 17, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, V.P.; Lislie, S.M.; Mario, V.M.; Leonardo, D.A.M.; Jesús, F.G.C. Reproductive strategy of an invasive buzz-pollinated plant (Solanum rostratum). S. Afr. J. Bot. 2023, 162, 342–352. [Google Scholar]
- Gibson, A.C. Structure-Function Relations of Warm Desert Plants; Springer Science and Business Media: Berlin, Germany, 2012. [Google Scholar]
- Dong, M.; Lu, J.Z.; Zhang, W.J.; Chen, J.K.; Li, B. Canada goldenrod (Solidago canadensis): An invasive alien weed rapidly spreading in China. J. Syst. Evol. 2006, 44, 72–85. [Google Scholar] [CrossRef]
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral, and negative impacts of Solidago canadensis invasion on native plant species richness. Sci. Rep. 2015, 5, 16804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Y.; Sun, S.G.; Dai, C.; Gituru, R.W.; Chen, J.M.; Wang, Q.F. Genetic variation and structure in native and invasive Solidago canadensis populations. Weed Res. 2015, 55, 163–172. [Google Scholar] [CrossRef]
- Wang, Z.X.; He, Z.S.; He, W.M. Nighttime climate warming enhances inhibitory effects of atmospheric nitrogen deposition on the success of invasive Solidago canadensis. Clim. Change 2021, 167, 20. [Google Scholar] [CrossRef]
- Zhou, X.H.; Peng, P.H.; Li, J.J. Simulated climate warming and nitrogen deposition influence leaf traits and leaf trait spectrum in Solidago canadensis from China and North America. Acta Ecol. Sin. 2019, 39, 1605–1615. [Google Scholar]
- Ren, G.Q.; Yang, B.; Cui, M.M.; Dai, Z.C.; Xiang, Y.; Zhang, H.Y.; Li, J.; Javed, Q.; Du, D.L. Warming and elevated nitrogen deposition accelerate the invasion process of Solidago canadensis L. Ecol. Process. 2022, 11, 62. [Google Scholar]
- Schmidtling, R.C. Use of provenance tests to predict response to climate change: Loblolly pine and Norway spruce. Tree Physiol. 1994, 14, 805–817. [Google Scholar] [CrossRef]
- Thebaud, C.; Simberloff, D. Are plants really larger in their introduced ranges? Am. Nat. 2001, 157, 231–236. [Google Scholar] [CrossRef]
- Drake, J.E.; Aspinwall, M.J.; Pfautsch, S.; Rymer, P.D.; Reich, P.B.; Smith, R.A.; Crous, K.Y.; Tissue, D.T.; Ghannoum, O.; Tjoelker, M.G. The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Glob. Change Biol. 2015, 21, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.X.; Zhou, X.H.; Peng, P.H.; Li, J.J.; Zhang, S.M.; He, W.M. An invasive population of Solidago canadensis is less sensitive to warming and nitrogen-addition than its native population in an invaded range. Biol. Invasions 2019, 21, 151–162. [Google Scholar] [CrossRef]
- Zhou, X.H.; He, W.M. Climate warming facilitates seed germination in native but not invasive Solidago canadensis populations. Front. Ecol. Evol. 2020, 8, 595214. [Google Scholar] [CrossRef]
- Luo, L.C.; Lai, X.Q.; Bai, J.; Li, A.X.; Fang, H.F.; Tang, M.; Hu, D.N.; Zhang, L. Effects of soil bacteria and fungi on growth of invasive plant Triadica sebifera with different provenances under nitrogen addition. Chin. J. Plant Ecol. 2023, 47, 206–215. [Google Scholar] [CrossRef]
- Chai, W.L.; Lei, Y.B.; Li, Y.P.; Xiao, H.F.; Feng, Y.L. Responses of invasive Chromolaena odorata and native Eupatorium heterophyllum to atmospheric CO2 enrichment. J. Ecol. 2014, 34, 3744–3751. [Google Scholar]
- Hautier, Y.; Randin, C.F.; Stöcklin, J.; Guisan, A. Changes in reproductive investment with altitude in an alpine plant. J. Plant Ecol. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Wilczek, A.M.; Cooper, M.D.; Korves, T.M.; Schmitt, J. Lagging adaptation to warming climate in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 7906–7913. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhu, M.; Li, G.J. Impact of reproductive traits on the invasive ability of Solidago canadensis L. Coll. Sci. 2015, 26, 324–330. [Google Scholar]
- Yang, J. Warming and Nitrogen Deposition Affect the Invasiveness of Solidago canadensis; Chengdu University of Technology: Chengdu, China, 2014. [Google Scholar]
- Heckman, R.W.; Halliday, F.W.; Mitchell, C.E. A growth-defense trade-off is general across native and exotic grasses. Oecologia 2019, 191, 609–620. [Google Scholar] [CrossRef]
- Ridenour, W.M.; Vivanco, J.M.; Feng, Y.; Horiuchi, J.; Callaway, R.M. No evidence for tradeoffs: Centaurea plants from America are better competitors and defenders. Ecol. Monogr. 2008, 78, 369–386. [Google Scholar] [CrossRef]
- Yu, H.W.; Shen, N.; Yu, S.Q.; Yn, D.; Liu, C.H. Responses of the native species Sparganium angustifolium and the invasive species Egeria densa to warming and interspecific competition. PLoS ONE 2018, 13, e0199478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.; Wang, R.; Liu, J. Roles of clonal integration in both heterogeneous and homogeneous habitats. Front. Plant Sci. 2016, 7, 551. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.T.; Hollister, R.D. Arctic plants are capable of sustained responses to long-term warming. Polar Res. 2016, 35, 25405. [Google Scholar] [CrossRef]
- Kremers, K.; Hollister, R.; Oberbauer, S. Diminished response of Arctic plants to warming over time. PLoS ONE 2015, 10, e0116586. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Dalvand, F.; Fayyaz, P.; Solla, A. Maternal drought stress on Persian oak (Quercus brantii Lindl.) affects susceptibility to single and combined drought and biotic stress in offspring. Environ. Exp. Bot. 2022, 194, 104716. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, J.; Niklas, K.J.; Li, G.; Sun, S. Global warming reduces plant reproductive output for temperate multi-inflorescence species on the Tibetan plateau. New Phytol. 2012, 195, 427–436. [Google Scholar] [CrossRef]
- Saixiyala; Chen, L.L.; Yi, F.Y.; Qiu, X.; Sun, H.L.; Cao, H.X.; Baoyin, T.; Ye, X.H.; Huang, Z.Y. Warming in combination with increased precipitation mediate the sexual and clonal reproduction in the desert steppe dominant species Stipa breviflora. BMC Plant Biol. 2023, 23, 474. [Google Scholar] [CrossRef]
- Guo, J.; Li, H.; Zhou, C.; Yang, Y. Effects of flag leaf and number of vegetative ramets on sexual reproductive performance in the clonal grass Leymus chinensis. Front. Plant Sci. 2020, 11, 534278. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Q.; Zhao, C.Y. Phenotypic plasticity and the successful invasion of alien plants. Chin. J. Ecol. 2020, 39, 3853–3864. [Google Scholar]
- Chun, Y.J.; Collyer, M.L.; Moloney, K.A.; Nason, J.D. Phenotypic plasticity of native vs. invasive Purple loosestrife: A two-state multivariate approach. Ecology 2007, 88, 1499–1512. [Google Scholar] [CrossRef]
- Maron, J.L.; Vila, M.; Bommarco, R.; Elmendorf, S.; Beardsley, P. Rapid evolution of an invasive plant. Ecol. Monogr. 2004, 74, 261–280. [Google Scholar] [CrossRef]
- Mealor, B.A.; Hild, A.L. Post-invasion evolution of native plant populations: A test of biological resilience. Oikos 2007, 116, 1493–1500. [Google Scholar] [CrossRef]
- Cipollini, K.A.; Hurley, S.L. Variation in resistance of experienced and native seedlings of Jewelweed (Impatiens capensis) to invasive garlic mustard (Alliaria petiolata). Ohio J. Sci. 2008, 108, 47–49. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hayes, A.F. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun. Monogr. 2009, 76, 408–420. [Google Scholar] [CrossRef]
- Wetzels, M.; Odekerken-Schroder, G. Oppen CV Using PLS path modeling for assessing hierarchical construct models: Guidelines and empirical illustration. Mis. Quart. 2009, 33, 177–195. [Google Scholar] [CrossRef]
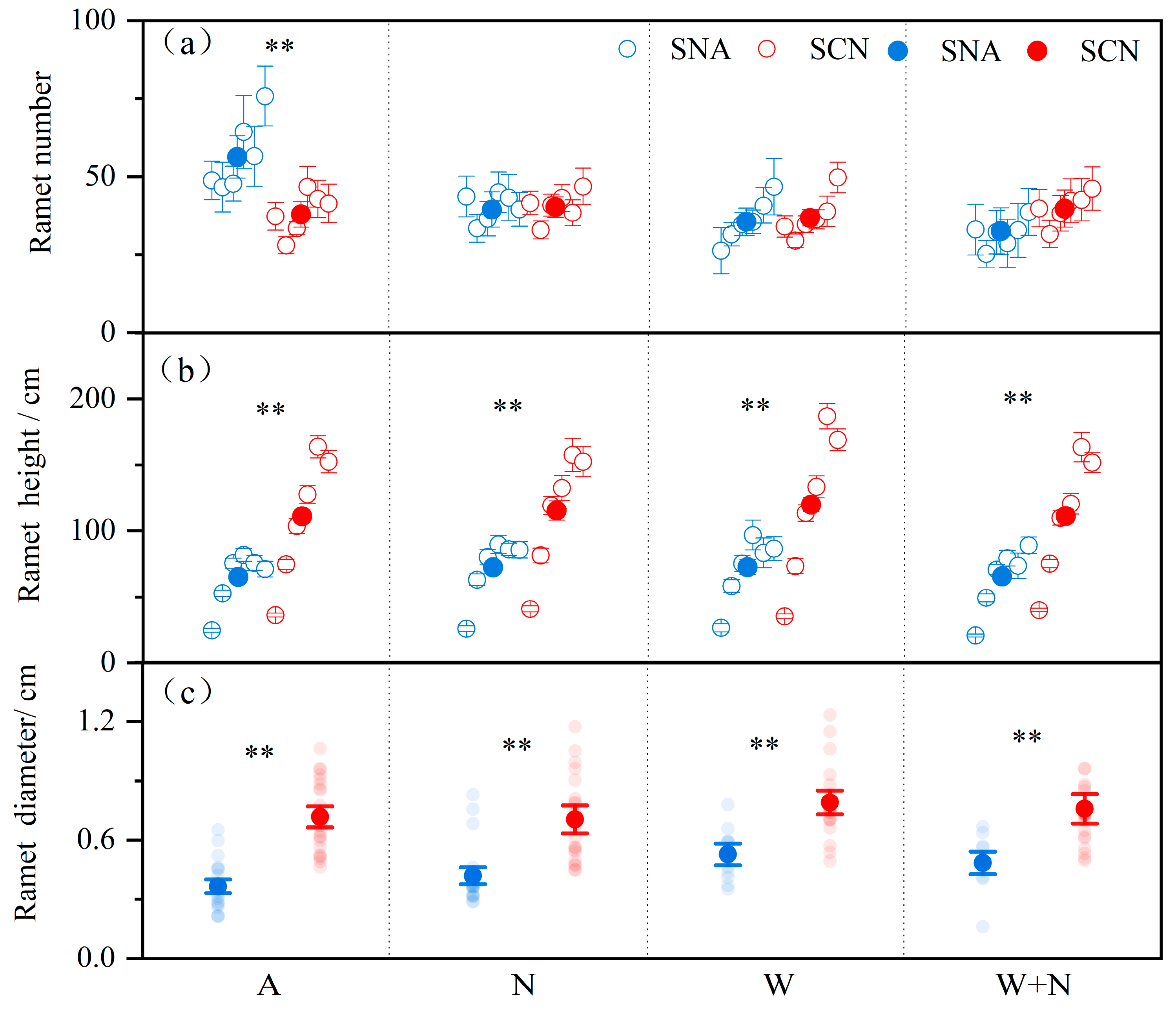
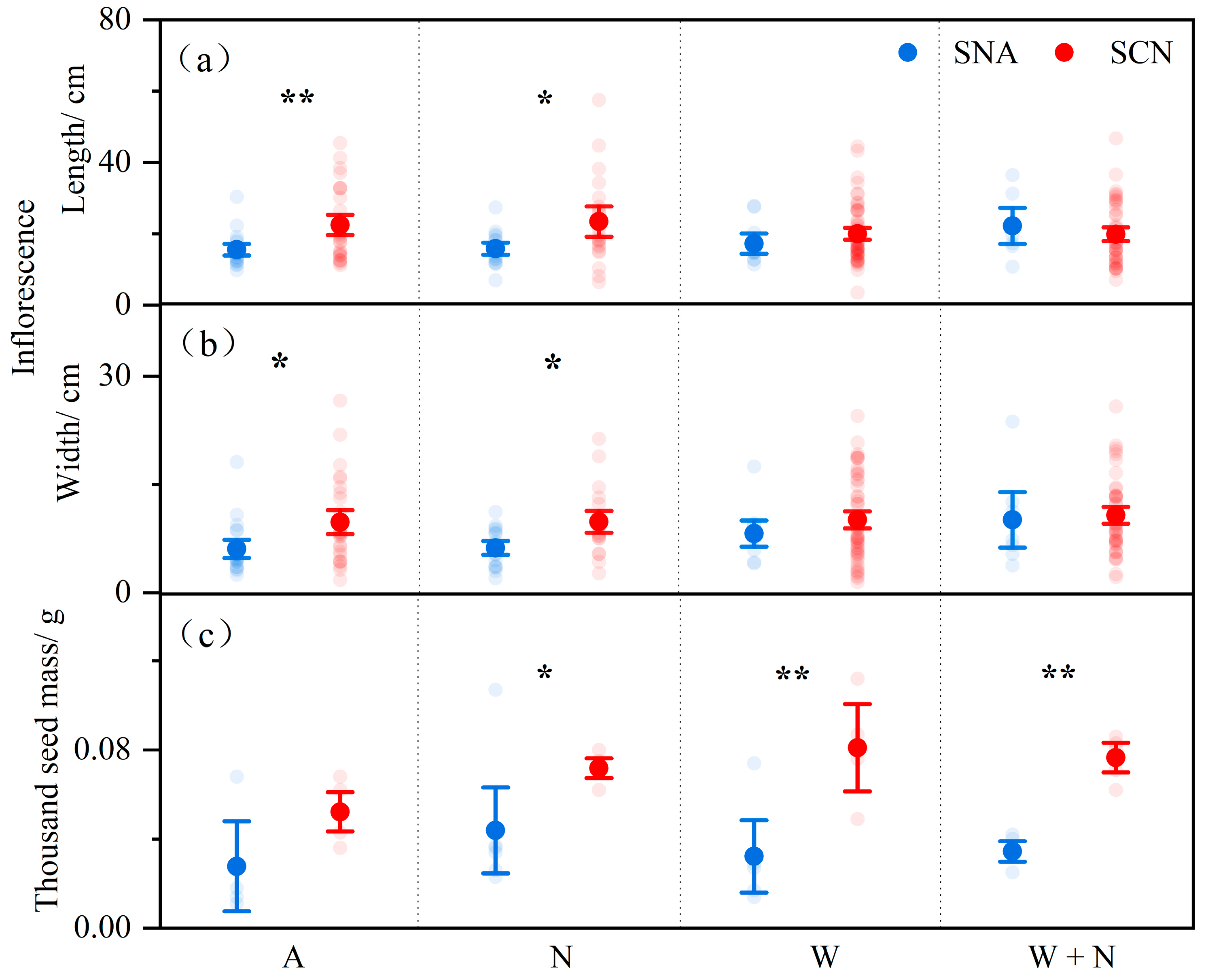
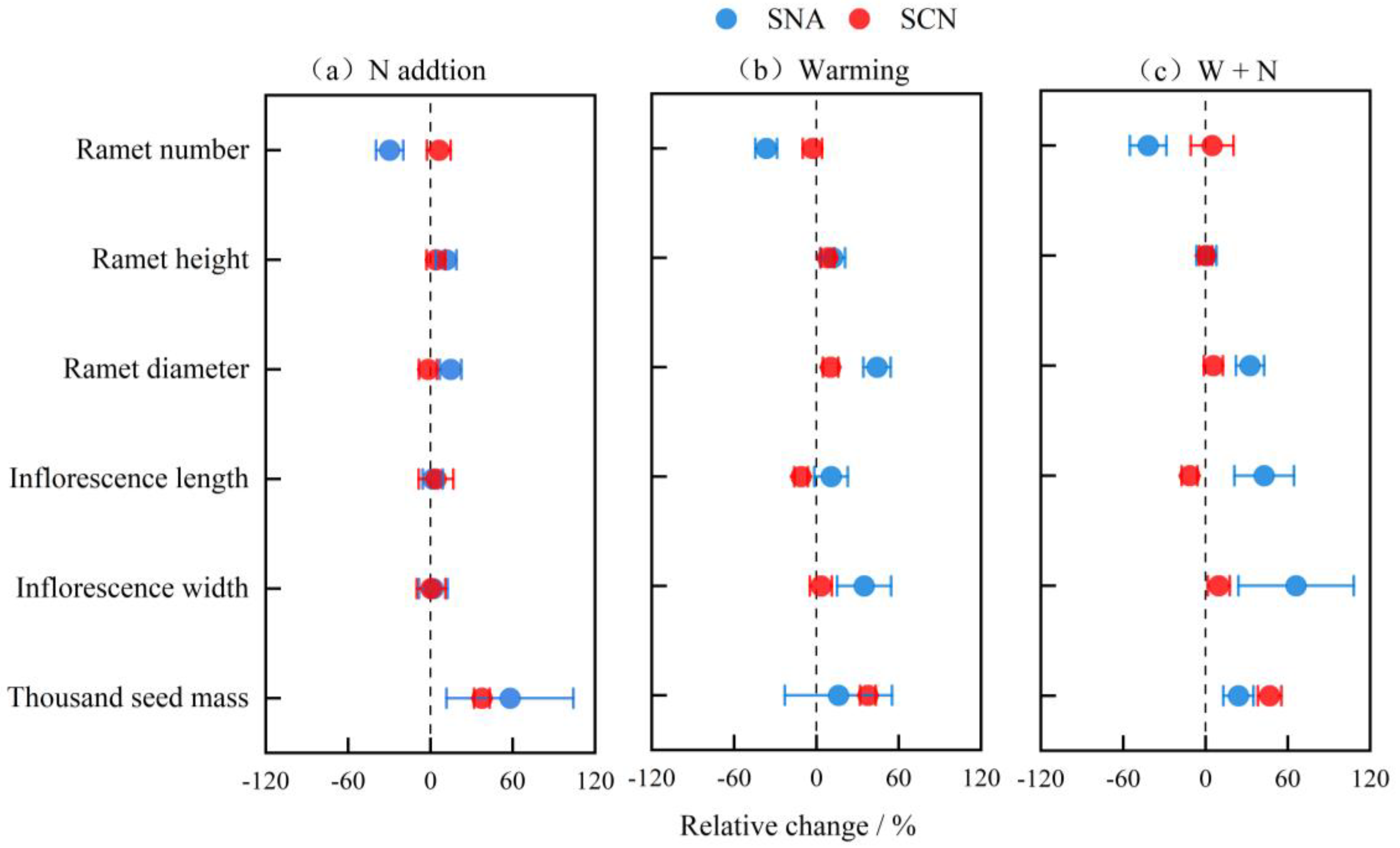
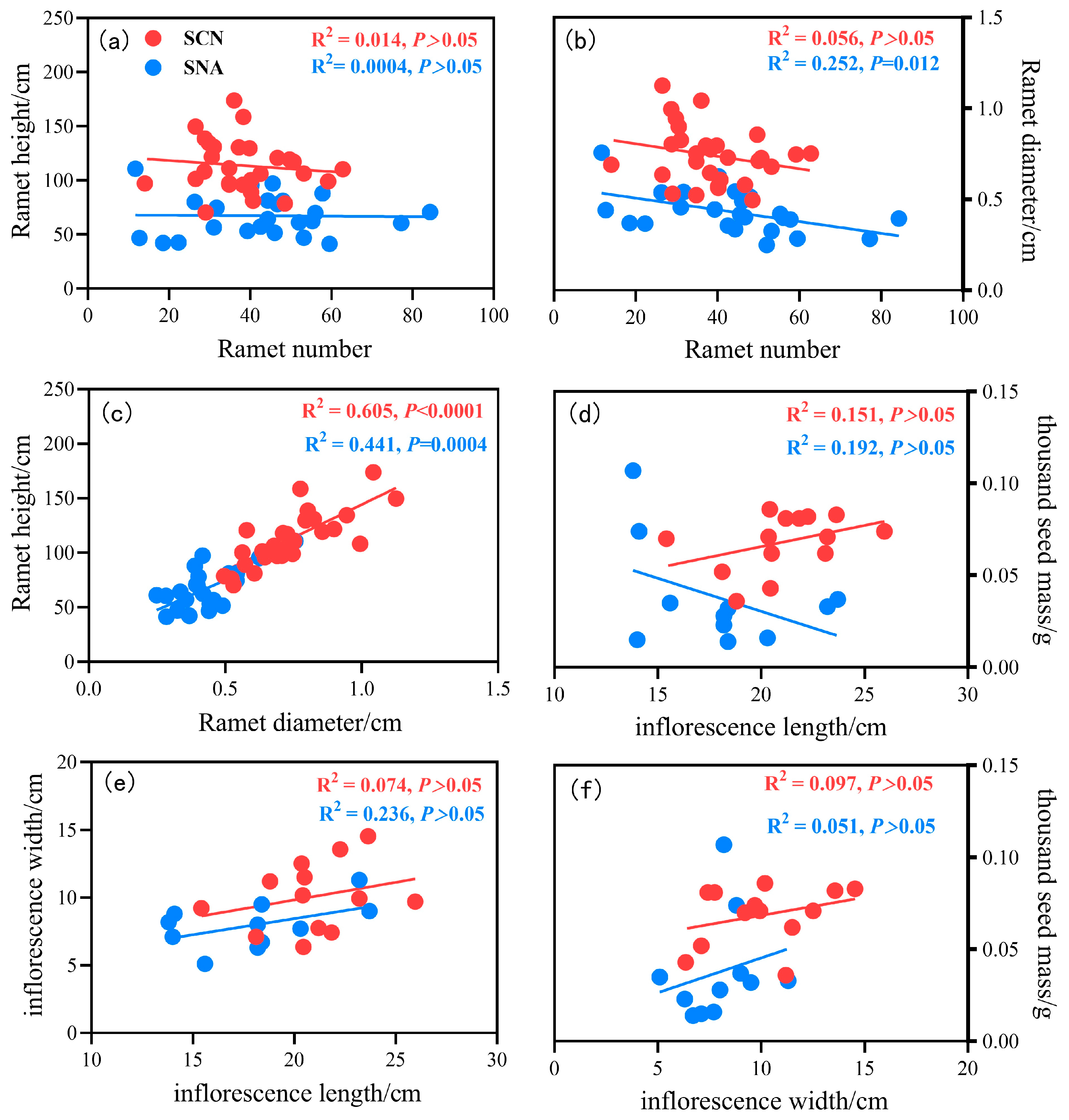
| Ramet Number | Ramet Height | Ramet Diameter | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Warming (W) | 4.461 | 0.040 * | 1.084 | 0.299 | 9.747 | 0.002 ** |
| N addition (N) | 0.883 | 0.352 | 0.000 | 0.996 | 0.092 | 0.762 |
| Origin (O) | 1.585 | 0.214 | 144.941 | <0.001 ** | 106.523 | <0.001 ** |
| W × N | 0.527 | 0.471 | 3.266 | 0.073 | 1.063 | 0.304 |
| W × O | 4.454 | 0.040 * | 0.384 | 0.537 | 0.759 | 0.385 |
| N × O | 3.382 | 0.072 | 0.597 | 0.441 | 0.243 | 0.623 |
| W × N × O | 0.359 | 0.552 | 0.169 | 0.681 | 0.462 | 0.498 |
| Inflorescence Length | Inflorescence Width | 1000 Seed Weight | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Warming (W) | 0.378 | 0.540 | 4.696 | 0.031 * | 1.174 | 0.287 |
| N addition (N) | 0.620 | 0.432 | 0.897 | 0.345 | 1.607 | 0.214 |
| Origin (O) | 4.516 | 0.035 * | 7.759 | 0.006 ** | 29.491 | <0.001 ** |
| W × N | 0.577 | 0.449 | 0.096 | 0.757 | 2.058 | 0.161 |
| W × O | 4.807 | 0.030 * | 2.920 | 0.089 | 2.134 | 0.154 |
| N × O | 0.831 | 0.363 | 0.002 | 0.963 | 0.014 | 0.908 |
| W × N × O | 0.621 | 0.432 | 0.004 | 0.952 | 0.147 | 0.704 |
| N Addition (N) | Warming (W) | W + N | ||||
|---|---|---|---|---|---|---|
| SNA | SCN | SNA | SCN | SNA | SCN | |
| Ramet number | 0.021 * | 0.501 | 0.019 * | 0.695 | 0.051 | 0.767 |
| Ramet height | 0.140 | 0.569 | 0.214 | 0.120 | 0.917 | 0.992 |
| Ramet diameter | 0.072 | 0.792 | 0.001 ** | 0.081 | 0.009 ** | 0.420 |
| Inflorescence length | 0.818 | 0.748 | 0.406 | 0.030 * | 0.097 | 0.048 * |
| Inflorescence width | 0.844 | 0.964 | 0.111 | 0.684 | 0.167 | 0.234 |
| Thousand seed mass | 0.266 | 0.003 ** | 0.702 | 0.115 | 0.094 | 0.005 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Chen, X.; Luo, X.; Wu, Y.; Li, J.; Ren, J.; Li, J. Plant Origin Regulates the Response of Solidago canadensis Reproductive Traits to Long-Term Warming and Nitrogen Addition. Plants 2025, 14, 1711. https://doi.org/10.3390/plants14111711
Zhou X, Chen X, Luo X, Wu Y, Li J, Ren J, Li J. Plant Origin Regulates the Response of Solidago canadensis Reproductive Traits to Long-Term Warming and Nitrogen Addition. Plants. 2025; 14(11):1711. https://doi.org/10.3390/plants14111711
Chicago/Turabian StyleZhou, Xiaohui, Xin Chen, Xin Luo, Yanling Wu, Juanjuan Li, Jianxin Ren, and Jingji Li. 2025. "Plant Origin Regulates the Response of Solidago canadensis Reproductive Traits to Long-Term Warming and Nitrogen Addition" Plants 14, no. 11: 1711. https://doi.org/10.3390/plants14111711
APA StyleZhou, X., Chen, X., Luo, X., Wu, Y., Li, J., Ren, J., & Li, J. (2025). Plant Origin Regulates the Response of Solidago canadensis Reproductive Traits to Long-Term Warming and Nitrogen Addition. Plants, 14(11), 1711. https://doi.org/10.3390/plants14111711






