Molecular Insights into Rice Immunity: Unveiling Mechanisms and Innovative Approaches to Combat Major Pathogens
Abstract
1. Introduction
2. Rice–Pathogen Interactions at the Genetic Level
2.1. Qualitative Resistance Mechanisms in Rice
2.2. Quantitative Resistance in Rice: Key Genetic Advances
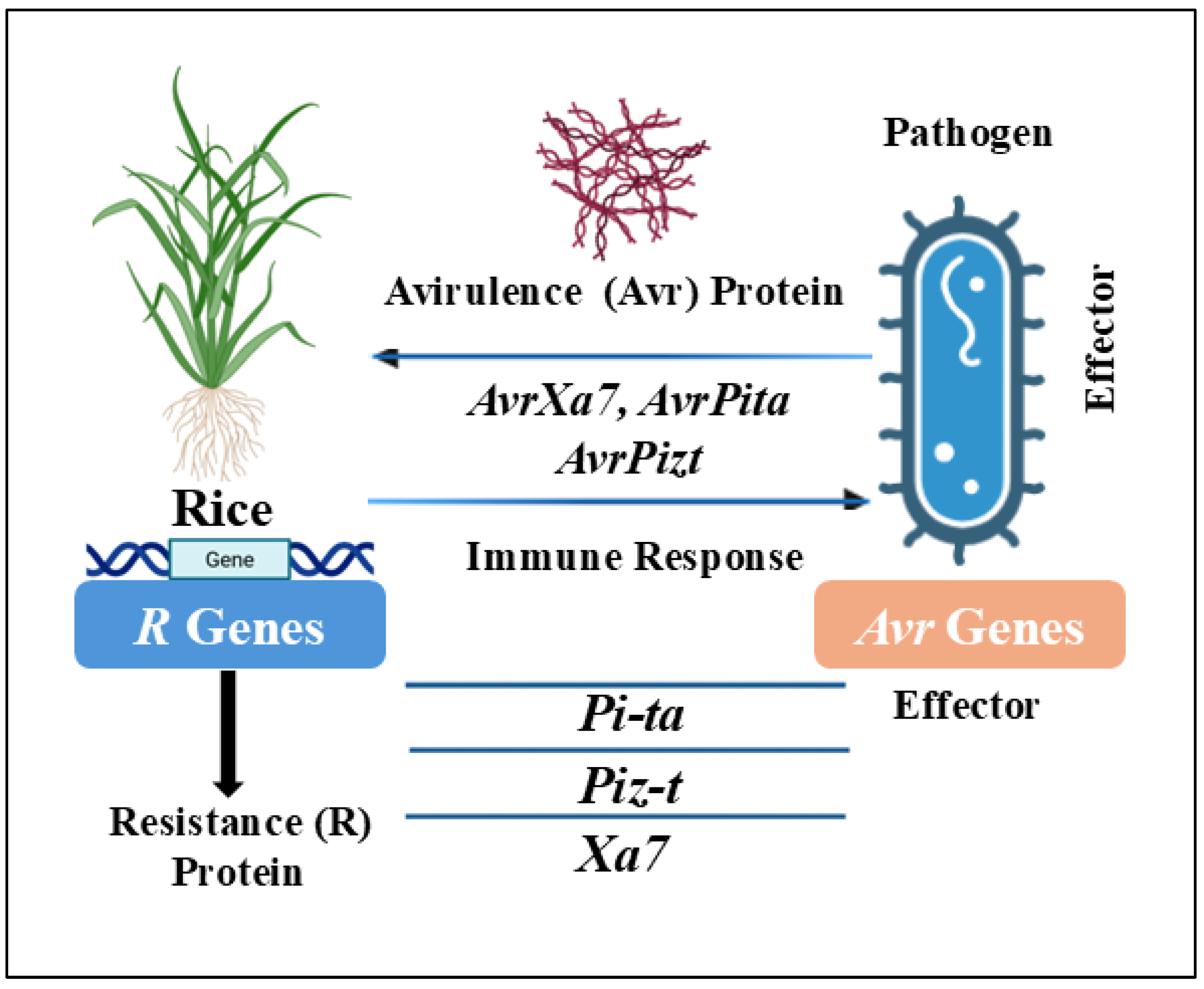
3. Gene-for-Gene Concept in Rice Disease Resistance
3.1. Gene-for-Gene Resistance Mechanisms in Rice Against Xanthomonas oryzae
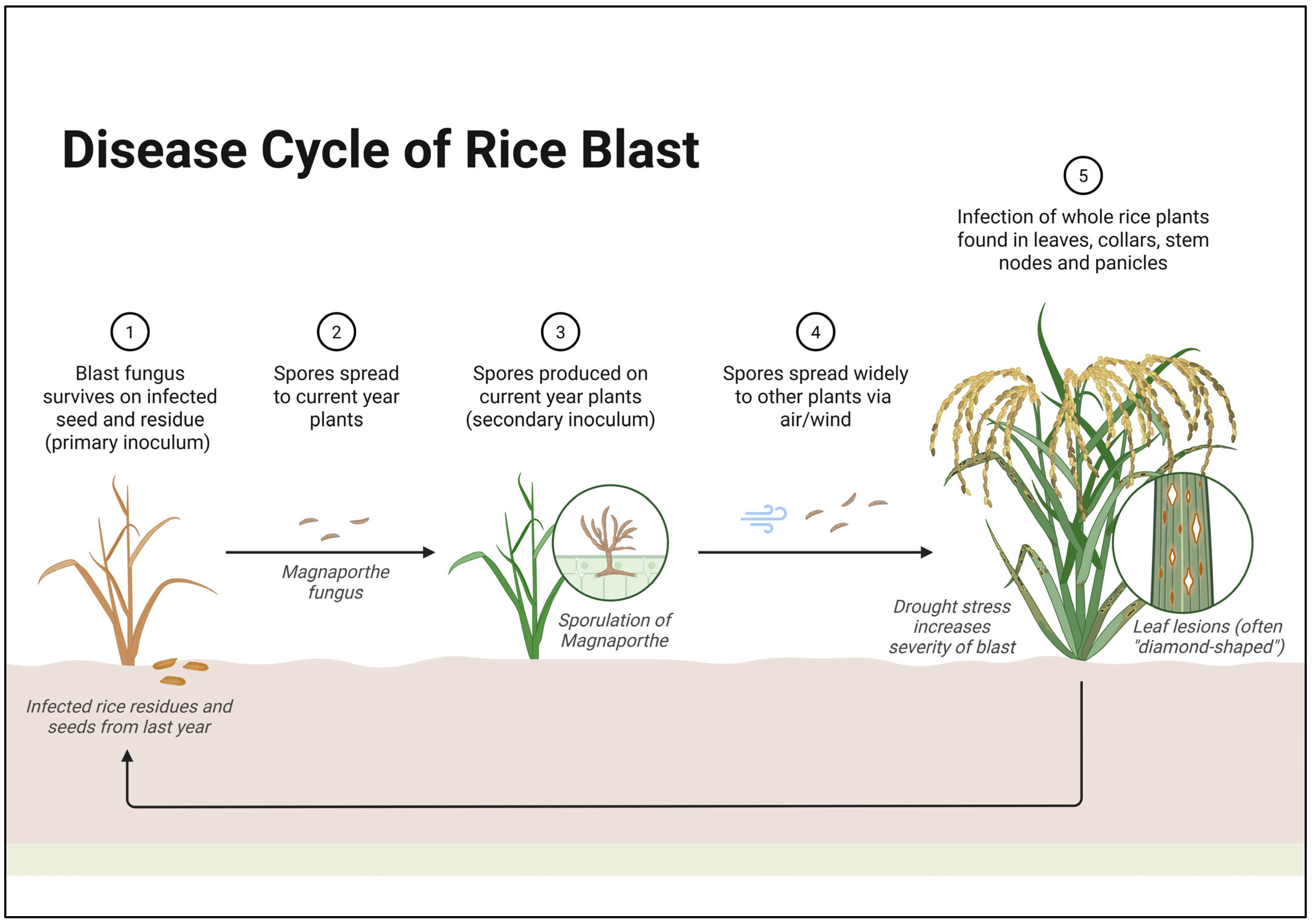
3.2. Gene-for-Gene Resistance Mechanisms in Rice Against Magnaporthe oryzae
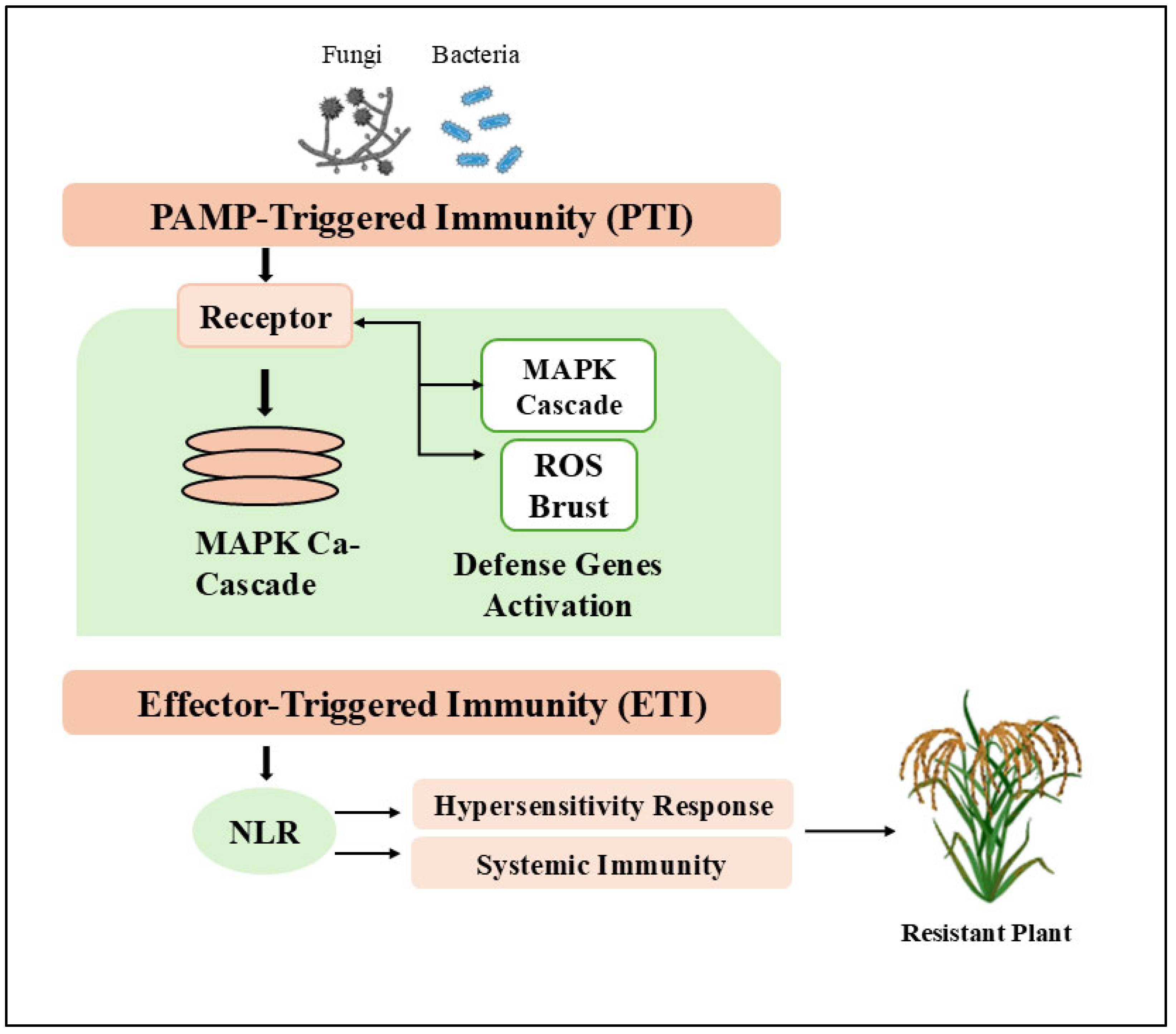
4. MAPK Signaling in Rice Immunity: Key Roles in Defense Against Xoo
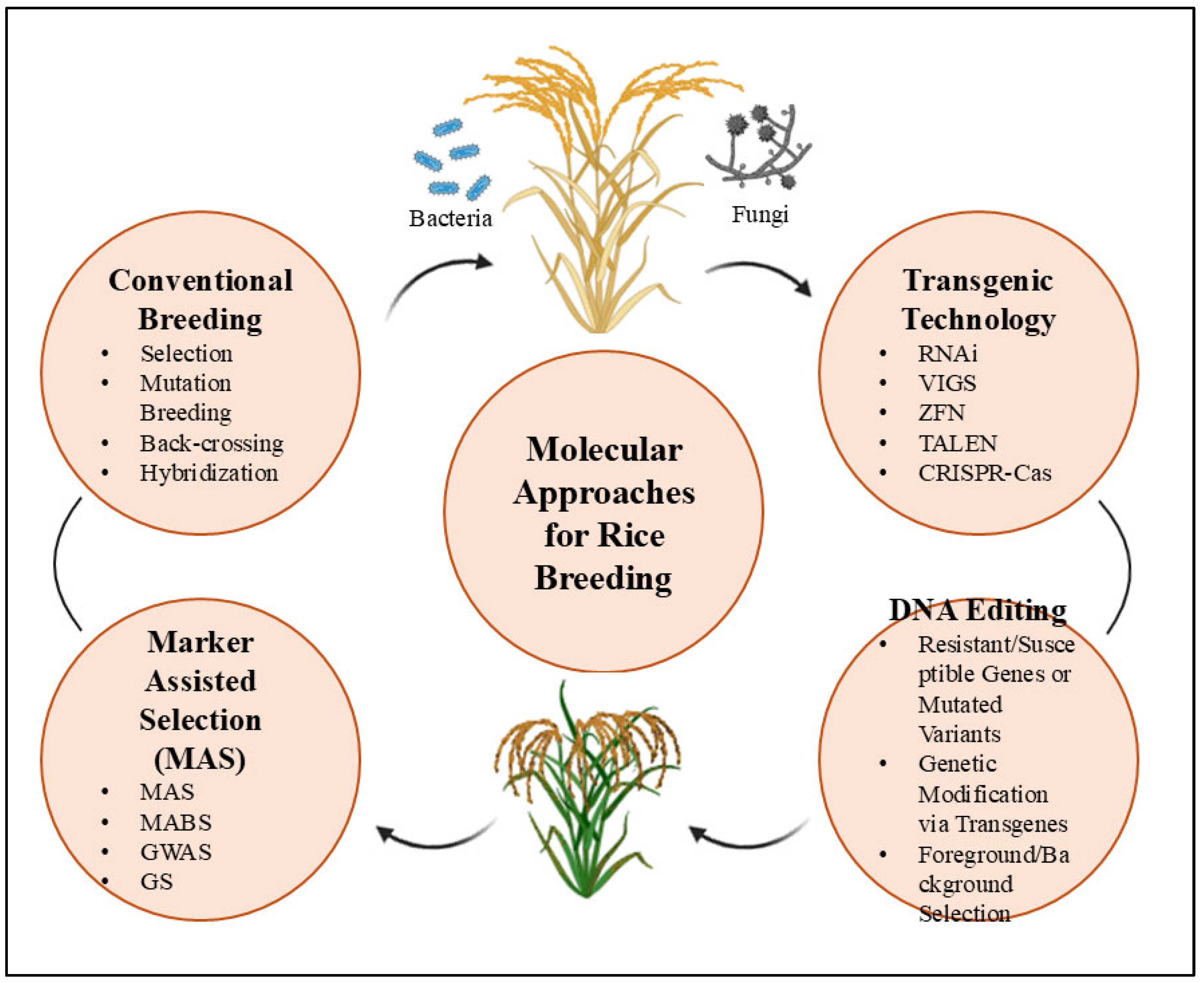
5. Conventional Breeding for Disease-Resistant Rice: Challenges and Advances
5.1. Marker-Assisted Selection in Rice Breeding: Successes, Challenges, and Future Directions
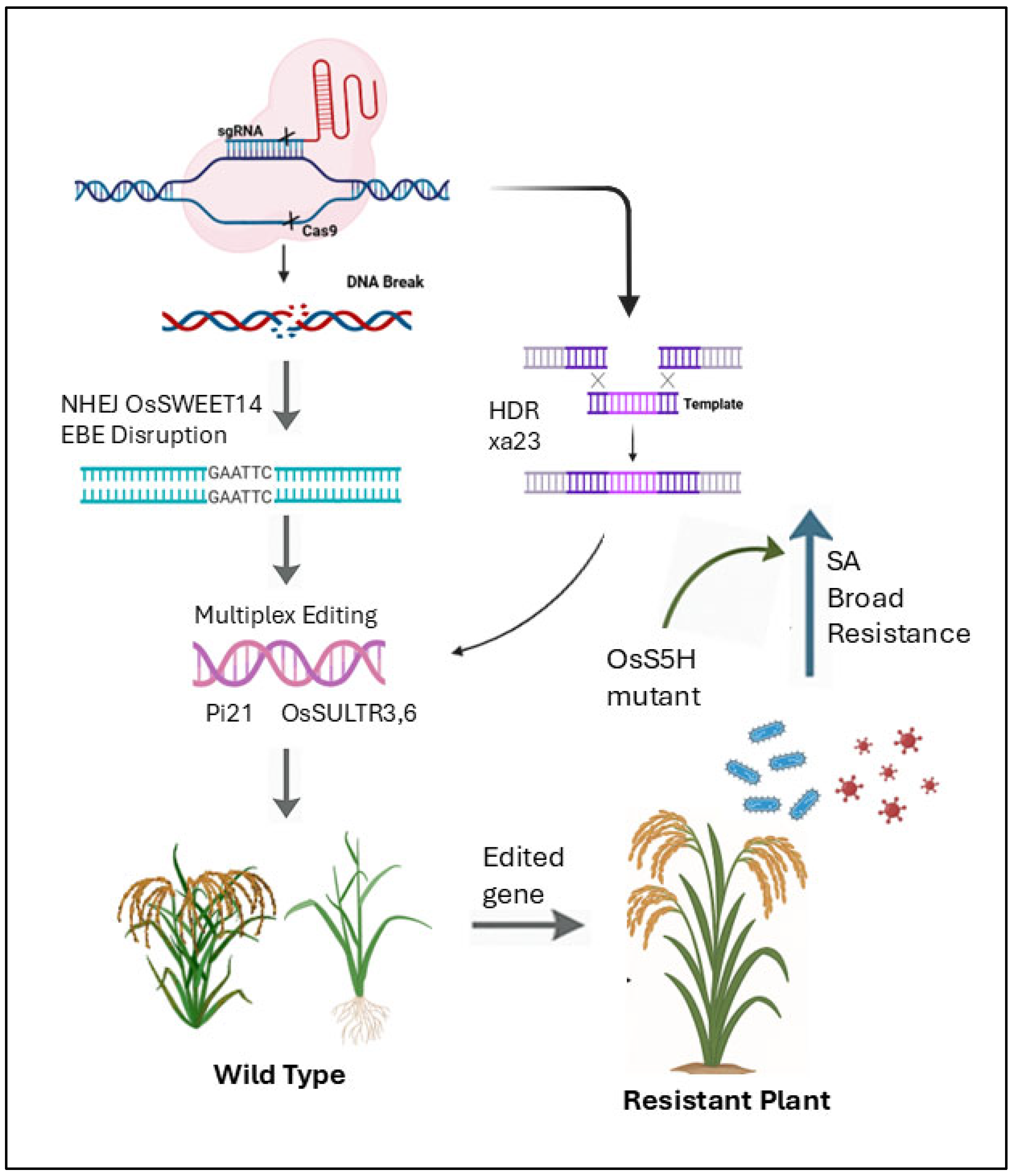
5.2. CRISPR/Cas9: A Revolutionary Tool for Enhancing Disease Resistance in Rice
6. Development and Impact of Disease-Resistant Rice Varieties
7. Environmental Impact of Disease-Resistant Rice Varieties
8. The Future of Disease-Resistant Rice: Challenges and Opportunities
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Younas, M.U.; Wang, G.; Du, H.; Zhang, Y.; Ahmad, I.; Rajput, N.; Li, M.; Feng, Z.; Hu, K.; Khan, N.U. Approaches to reduce rice blast disease using knowledge from host resistance and pathogen pathogenicity. Int. J. Mol. Sci. 2023, 24, 4985. [Google Scholar] [CrossRef] [PubMed]
- Bag, M.K.; Raghu, S.; Banerjee, A.; Prabhukarthikeyan, S.R.; Baite, M.S.; Yadav, M. Durable resistance of rice to major and emerging diseases: Current status. Open Agric. J. 2023, 17. [Google Scholar] [CrossRef]
- Younas, M.U.; Qasim, M.; Ahmad, I.; Feng, Z.; Iqbal, R.; Jiang, X.; Zuo, S. Exploring the molecular mechanisms of rice blast resistance and advances in breeding for disease tolerance. Mol. Biol. Rep. 2024, 51, 1093. [Google Scholar] [CrossRef]
- Rajput, N.; Younas, M.U.; Qasim, M.; Memon, S.P.; Memon, S.; El-Rahman, M.A.; Aghayeva, S.; Ercisli, S.; Iqbal, R.; Zuo, S. Understanding rice blast: Investigating biotechnological methods to speed up the development of robust rice cultivars. Genet. Resour. Crop Evol. 2024, 72, 1333–1352. [Google Scholar] [CrossRef]
- Younas, M.U.; Ahmad, I.; Qasim, M.; Ijaz, Z.; Rajput, N.; Parveen Memon, S.; UL Zaman, W.; Jiang, X.; Zhang, Y.; Zuo, S. Progress in the management of Rice Blast Disease: The role of Avirulence and Resistance genes through gene-for-gene interactions. Agronomy 2024, 14, 163. [Google Scholar] [CrossRef]
- Gülmez, B. Advancements in rice disease detection through convolutional neural networks: A comprehensive review. Heliyon 2024, 10, e33328. [Google Scholar] [CrossRef]
- Karam, N.; Kumar, P.; Anand, Y.R.; Choudhury, D. Management of Rice Blast—A Review of the Strategies Available. Biopestic. Int. 2023, 19. [Google Scholar] [CrossRef]
- Sabar, M.; Sana-e-Mustafa, M.I.; Khan, R.A.R.; Fatima, R.; Saher, H.; Shahzadi, F.; Javed, H.M.; Zafar, S.A.; Siddique, S.; Saleem, M.U. Sheath Blight and Bacterial Blight Resistance in Rice: Mechanisms, Progress and Future Perspectives for Sustainable Rice Production. Plant Bull. 2024, 3, 102–112. [Google Scholar] [CrossRef]
- Islam, M.R.; Jannat, R.; Protic, I.A.; Happy, M.N.A.; Samin, S.I.; Mita, M.M.; Bashar, S.; Masud, M.M.; Islam, M.H.; Uddin, M.N. First report of bacterial panicle blight in rice caused by Burkholderia gladioli in Bangladesh. Plant Dis. 2023, 107, 2837. [Google Scholar] [CrossRef]
- Nalley, L.L.; Tsiboe, F.; Durand-Morat, A.; Shew, A.; Thoma, G. Economic and Environmental Impact of Rice Blast Pathogen (Magnaporthe oryzae) Alleviation in the United States. PLoS ONE 2016, 11, e0167295. [Google Scholar] [CrossRef]
- Mew, T.W.; Vera Cruz, C.M.; Medalla, E.S. Changes in Race Frequency of Xanthomonas oryzae pv. oryzae in Response to Rice Cultivars Planted in the Philippines. Plant Dis. 1992, 76, 1029–1032. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Rafii, M.Y.; Mahmud, T.M.M.; Azizi, P.; Osman, M.; Taheri, S. Molecular Breeding Strategies and Challenges Towards Improvement of Blast Disease Resistance in Rice Crop. Front. Plant Sci. 2015, 6, 886. [Google Scholar] [CrossRef]
- Shrivastava, V.K.; Pradhan, M.K.; Minz, S.; Thakur, M.P. Rice plant disease classification using transfer learning of deep convolution neural network. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, 42, 631–635. [Google Scholar] [CrossRef]
- Khakimov, A.; Salakhutdinov, I.; Omolikov, A.; Utaganov, S. Traditional and current-prospective methods of agricultural plant diseases detection: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1043, 012002. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R.P.; Jena, D.; Singh, V.; Rout, D.; Arsode, P.B.; Choudhary, M.; Singh, P.; Chahar, S.; Samantaray, S. Marker-Assisted improvement for durable bacterial blight resistance in aromatic rice cultivar HUR 917 popular in Eastern parts of India. Plants 2023, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, Y.; He, P.; Shan, L. Unlocking nature’s defense: Plant pattern recognition receptors as guardians against pathogenic threats. Mol. Plant-Microbe Interact. 2024, 37, 73–83. [Google Scholar] [CrossRef]
- Giulietti, S.; Bigini, V.; Savatin, D.V. ROS and RNS production, subcellular localization, and signaling triggered by immunogenic danger signals. J. Exp. Bot. 2024, 75, 4512–4534. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Ijaz, U.; Zhao, C.; Shabala, S.; Zhou, M. Molecular Basis of Plant–Pathogen Interactions in the Agricultural Context. Biology 2024, 13, 421. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, Y.; He, T.; Chen, T.; Pan, Y.; Zhou, D.; Li, X.; Lu, Y.; Wu, Q.; Wang, L. Integrated transcriptome and metabolome analysis unveil the response mechanism in wild rice (Zizania latifolia griseb.) against sheath rot infection. Front. Genet. 2023, 14, 1163464. [Google Scholar] [CrossRef]
- Leung, H.; Zhu, Y.; Revilla-Molina, I.; Fan, J.X.; Chen, H.; Pangga, I.; Cruz, C.V.; Mew, T.W. Using genetic diversity to achieve sustainable rice disease management. Plant Dis. 2003, 87, 1156–1169. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Deng, Y.; Yang, X.; Li, G.; Li, Q.; Zhou, H.; Chen, Z.; Guo, X.; Su, Y.; Luo, Y. Association analysis of rice resistance genes and blast fungal avirulence genes for effective breeding resistance cultivars. Front. Microbiol. 2022, 13, 1007492. [Google Scholar] [CrossRef]
- Gilbert, G.; Parker, I. The Evolutionary Ecology of Plant Disease; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Younas, M.U.; Qasim, M.; Ahmad, I.; Feng, Z.; Iqbal, R.; Abro, T.F.; Ahmad, S.; Jiang, X.; Rajput, N.; Zuo, S. Integrated Approaches for Enhancing Magnaporthe oryzae Resistance: Mechanisms and Breeding Strategies. Plant Mol. Biol. Rep. 2025. [Google Scholar] [CrossRef]
- Naidu, B.N.; Madhavilatha, L.; Maraskole, S.K.; Panotra, N.; Singh, O.B.; Kumar, A.; Devi, O.R.; Laishram, B.; Anbarasan, S. Enhancing the Genetic Understanding of Rice for Strategic Breeding of High Yielding and Superior Quality Varieties. J. Adv. Biol. Biotechnol. 2024, 27, 1252–1261. [Google Scholar] [CrossRef]
- Deng, Z.; Qin, P.; Liu, K.; Jiang, N.; Yan, T.; Zhang, X.; Fu, C.; He, G.; Wang, K.; Yang, Y. The development of multi-resistant rice restorer lines and hybrid varieties by pyramiding resistance genes against blast and brown planthopper. Agronomy 2024, 14, 878. [Google Scholar] [CrossRef]
- Qu, S.; Liu, G.; Zhou, B.; Bellizzi, M.; Zeng, L.; Dai, L.; Han, B.; Wang, G.-L. The Broad-Spectrum Blast Resistance Gene Pi9 Encodes a Nucleotide-Binding Site–Leucine-Rich Repeat Protein and Is a Member of a Multigene Family in Rice. Genetics 2006, 172, 1901–1914. [Google Scholar] [CrossRef]
- Ramalingam, J.; Raveendra, C.; Savitha, P.; Vidya, V.; Chaithra, T.L.; Velprabakaran, S.; Saraswathi, R.; Chandrababu, R. Gene Pyramiding for Achieving Enhanced Resistance to Bacterial Blight, Blast, and Sheath Blight Diseases in Rice. Front. Plant Sci. 2020, 11, 591457. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.-S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-Spectrum Resistance to Bacterial Blight in Rice Using Genome Editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-Based Control of Fusarium graminearum Infections Through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Wang, Y.; Yao, G.; Ma, T.; Sun, X.; Zhang, Y.; Su, N.; Tan, X.; Abbas, H.M.K.; Ji, S. A Critical Review of Conventional and Modern Approaches to Develop Herbicide-Resistance in Rice. Physiol. Plant. 2024, 176, e14254. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Goto, S.; Inoue, H.; Suwazono, H.; Takatsuji, H.; Mori, M. Improvement of Broad-Spectrum Disease-Resistant Rice by the Overexpression of BSR1 via a Moderate-Strength Constitutive Promoter and a Pathogen-Inducible Promoter. Plants 2024, 13, 1138. [Google Scholar] [CrossRef]
- Gong, Q.; Sha, G.; Han, X.; Guo, Z.; Yang, L.; Yang, W.; Tan, R.; Chen, G.; Li, Y.; Shen, X. Mutation of phosphatidate phosphohydrolase genes confers broad-spectrum disease resistance in plants. bioRxiv 2024. [Google Scholar] [CrossRef]
- Devanna, B.N.; Sucharita, S.; Sunitha, N.; Anilkumar, C.; Singh, P.K.; Pramesh, D.; Samantaray, S.; Behera, L.; Katara, J.L.; Parameswaran, C. Refinement of rice blast disease resistance QTLs and gene networks through meta-QTL analysis. Sci. Rep. 2024, 14, 16458. [Google Scholar] [CrossRef]
- Okello, M.; Ssemakula, M.O.; Lamo, J.; Onaga, G.; Odong, T.L.; Geoffrey, T.; Tukamuhabwa, P.; Mukasa, S.B.; Wasswa, P.; Ogwal, J. Genome-wide association mapping in rice MAGIC indica panel detects QTLs and genes for broad-spectrum resistance breeding against African bacterial blight. Oryza 2024, 61, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J.-M. Identification of MicroRNAs Involved in Pathogen-Associated Molecular Pattern-Triggered Plant Innate Immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Shao, H.; Tang, X. Recent Advances in Utilizing Transcription Factors to Improve Plant Abiotic Stress Tolerance by Transgenic Technology. Front. Plant Sci. 2016, 7, 67. [Google Scholar] [CrossRef]
- Lai, K.Y.; Hussin, N.A.; Mohamad, N.K.; Ten, H.Y.; San Lai, L.; San Yeo, F.K. Qualitative resistance of Sarawak rice landraces against Pyricularia oryzae. Borneo J. Resour. Sci. Technol. 2019, 9, 115–118. [Google Scholar]
- Zuanetti, D.A.; Soler, J.M.P.; Krieger, J.E.; Milan, L.A. Bayesian diagnostic analysis for quantitative trait loci mapping. Stat. Methods Med. Res. 2020, 29, 2238–2249. [Google Scholar] [CrossRef]
- Wisser, R.J.; Sun, Q.; Hulbert, S.H.; Kresovich, S.; Nelson, R.J. Identification and characterization of regions of the rice genome associated with broad-spectrum, quantitative disease resistance. Genetics 2005, 169, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, M.; Wang, X.; Xu, Z.; Wu, K.; Sun, Q.; Du, H.; Younas, M.U.; Zhang, Y.; Feng, Z. Identification of Elite R-gene combinations against blast disease in Geng rice varieties. Int. J. Mol. Sci. 2023, 24, 3984. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, J. Regulatory role of receptor-like cytoplasmic kinases in early immune signaling events in plants. FEMS Microbiol. Rev. 2020, 44, 845–856. [Google Scholar] [CrossRef]
- Dalio, R.J.; Paschoal, D.; Arena, G.D.; Magalhães, D.M.; Oliveira, T.S.; Merfa, M.V.; Maximo, H.J.; Machado, M.A. Hypersensitive response: From NLR pathogen recognition to cell death response. Ann. Appl. Biol. 2021, 178, 268–280. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, Z.; Liu, X.; Yin, D.; Li, D.; Zhao, X.; Li, X.; Li, S.; Chen, R.; Lu, L. The OsSPK1–OsRac1–RAI1 defense signaling pathway is shared by two distantly related NLR proteins in rice blast resistance. Plant Physiol. 2021, 187, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Katagiri, S.; Kanamori, H.; Mukai, Y.; Sasaki, T.; Matsumoto, T.; Wu, J. Evolutionary dynamics and impacts of chromosome regions carrying R-gene clusters in rice. Sci. Rep. 2020, 10, 872. [Google Scholar] [CrossRef]
- Riangwong, K.; Aesomnuk, W.; Sonsom, Y.; Siangliw, M.; Unartngam, J.; Toojinda, T.; Wanchana, S.; Arikit, S. QTL-seq identifies genomic regions associated with resistance to dirty panicle disease in rice. Agronomy 2023, 13, 1905. [Google Scholar] [CrossRef]
- Michel, S.; Löschenberger, F.; Ametz, C.; Bürstmayr, H. Toward combining qualitative race-specific and quantitative race-nonspecific disease resistance by genomic selection. Theor. Appl. Genet. 2023, 136, 79. [Google Scholar] [CrossRef]
- Song, Z.; Zheng, J.; Zhao, Y.; Yin, J.; Zheng, D.; Hu, H.; Liu, H.; Sun, M.; Ruan, L.; Liu, F. Population genomics and pathotypic evaluation of the bacterial leaf blight pathogen of rice reveals rapid evolutionary dynamics of a plant pathogen. Front. Cell. Infect. Microbiol. 2023, 13, 1183416. [Google Scholar] [CrossRef]
- Mondal, K.K.; Kalaivanan, N. T3SS mediated transcriptional reprogramming of rice by the virulent Indian race 4 of Xanthomonas oryzae pv. oryzae. ORYZA-Int. J. Rice 2023, 60, 249–259. [Google Scholar]
- Wongsa, T.; Chankaew, S.; Monkham, T.; Sanitchon, J. Broad-Spectrum Resistance and Monogenic Inheritance of Bacterial Blight Resistance in an Indigenous Upland Rice Germplasm ULR207. Agronomy 2024, 14, 898. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Li, H.; Song, C.; Xie, W.; Zuo, S.; Zhou, X.; Zhou, C.; Ji, Z.; Zhou, H. Genome editing of a dominant resistance gene for broad-spectrum resistance to bacterial diseases in rice without growth penalty. Plant Biotechnol. J. 2023, 22, 529. [Google Scholar] [CrossRef]
- Gou, M.; Balint-Kurti, P.; Xu, M.; Yang, Q. Quantitative disease resistance: Multifaceted players in plant defense. J. Integr. Plant Biol. 2023, 65, 594–610. [Google Scholar] [CrossRef]
- Langlands-Perry, C.; Pitarch, A.; Lapalu, N.; Cuenin, M.; Bergez, C.; Noly, A.; Amezrou, R.; Gélisse, S.; Barrachina, C.; Parrinello, H. Quantitative and qualitative plant-pathogen interactions call upon similar pathogenicity genes with a spectrum of effects. Front. Plant Sci. 2023, 14, 1128546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, Y.; Shi, H.; Qiu, J.; Ding, X.; Kou, Y. Recent progress in rice broad-spectrum disease resistance. Int. J. Mol. Sci. 2021, 22, 11658. [Google Scholar] [CrossRef]
- Silva, A.; Montoya, M.E.; Quintero, C.; Cuasquer, J.; Tohme, J.; Graterol, E.; Cruz, M.; Lorieux, M. Genetic bases of resistance to the rice hoja blanca disease deciphered by a quantitative trait locus approach. G3 Genes Genomes Genet. 2023, 13, jkad223. [Google Scholar] [CrossRef]
- Neelam, K.; Kumar, K.; Kaur, A.; Kishore, A.; Kaur, P.; Babbar, A.; Kaur, G.; Kamboj, I.; Lore, J.S.; Vikal, Y. High-resolution mapping of the quantitative trait locus (QTLs) conferring resistance to false smut disease in rice. J. Appl. Genet. 2022, 63, 35–45. [Google Scholar] [CrossRef]
- Inoue, H.; Hayashi, N. The panicle blast resistance mechanism of qPbm11 in the rice cultivar Miyazaki-mochi is independent from that of Pb1. Jpn. Agric. Res. Q. JARQ 2019, 53, 289–293. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, D.; Zhang, C.-S.; Lu, J.-L.; Chen, T.-J.; Xie, J.-P.; Zhou, Y.-L. Genome-wide association analysis of the genetic basis for sheath blight resistance in rice. Rice 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Fu, R.; Zhao, L.; Chen, C.; Wang, J.; Lu, D. Conjunctive analysis of bsa-seq and ssr markers unveil the candidate genes for resistance to rice false smut. Biomolecules 2024, 14, 79. [Google Scholar] [CrossRef]
- Hiremath, S.S.; Bhatia, D.; Jain, J.; Hunjan, M.S.; Kaur, R.; Zaidi, N.W.; Singh, U.S.; Zhou, B.; Lore, J.S. Identification of potential donors and QTLs for resistance to false smut in a subset of rice diversity panel. Eur. J. Plant Pathol. 2021, 159, 461–470. [Google Scholar] [CrossRef]
- Zhao, D.-D.; Chung, H.; Jang, Y.-H.; Farooq, M.; Choi, S.Y.; Du, X.-X.; Kim, K.-M. Analysis of rice blast fungus genetic diversity and identification of a novel blast resistance OsDRq12 gene. Phytopathology 2024, 114, 1917–1925. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Jiang, X.; Zhang, S.; Yang, W.; Dong, J.; Yang, T.; Ma, Y.; Zhou, L.; Chen, J. Pangenome-wide association study and transcriptome analysis reveal a novel QTL and candidate genes controlling both panicle and leaf blast resistance in rice. Rice 2024, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.S.; Nadarajah, K. A meta-analysis of quantitative trait loci associated with multiple disease resistance in rice (Oryza sativa L.). Plants 2020, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-M.; Qiu, D.-Y.; Shen, X.-L.; Li, X.-H.; Wang, S.-P. Isolation and manipulation of quantitative trait loci for disease resistance in rice using a candidate gene approach. Mol. Plant 2008, 1, 786–793. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S. Toward an understanding of the molecular basis of quantitative disease resistance in rice. J. Biotechnol. 2012, 159, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zhang, Y.; Yin, Y.; Li, G.; Zhang, G.; Wang, H.; Chen, Z.; Pan, X. Fine-mapping of qSB-9 TQ, a gene conferring major quantitative resistance to rice sheath blight. Mol. Breed. 2014, 34, 2191–2203. [Google Scholar] [CrossRef]
- Hossain, M.K.; Islam, M.R.; Sundaram, R.M.; Bhuiyan, M.A.R.; Wickneswari, R. Introgression of the QTL qSB11-1TT conferring sheath blight resistance in rice (Oryza sativa) into an elite variety, UKMRC 2, and evaluation of its backcross-derived plants. Front. Plant Sci. 2023, 13, 981345. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, S.; Rioux, R.; Singh, P.; Jia, M.H.; Jia, Y.; Mysore, K.S. Analysis of Differentially Expressed Rice Genes Reveals the ATP-Binding Cassette Transporters as Candidate Genes Against the Sheath Blight Pathogen, Rhizoctonia solani. PhytoFrontiers™ 2022, 2, 105–115. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Z.; Zhang, B.; Guan, H.; Zheng, Y.; Lan, T.; Zhang, J.; Qin, M.; Wu, W. Transcriptome analysis of xa5-mediated resistance to bacterial leaf streak in rice (Oryza sativa L.). Sci. Rep. 2020, 10, 19439. [Google Scholar] [CrossRef]
- Park, J.-R.; Lee, C.-M.; Ji, H.; Baek, M.-K.; Seo, J.; Jeong, O.-Y.; Park, H.-S. Characterization and QTL Mapping of a Major Field Resistance Locus for Bacterial Blight in Rice. Plants 2022, 11, 1404. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Tomita, A.; Yoshida, T.; Nakamura, M.; Suzuki, T.; Ikeda, A.; Kato, T.; Nakajima, Y.; Tanimoto, R.; Tani, T. Characterization of Six Partial Resistance Genes and One Quantitative Trait Locus to Blast Disease Using Near Isogenic Lines with a Susceptible Genetic Background of Indica Group Rice (Oryza sativa). PhytoFrontiers™ 2022, 2, 230–241. [Google Scholar] [CrossRef]
- Yasuda, N.; Mitsunaga, T.; Hayashi, K.; Koizumi, S.; Fujita, Y. Effects of pyramiding quantitative resistance genes pi21, Pi34, and Pi35 on rice leaf blast disease. Plant Dis. 2015, 99, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N. From gene-for-gene to resistosomes: Flor’s enduring legacy. Mol. Plant-Microbe Interact. 2023, 36, 461–467. [Google Scholar] [CrossRef]
- Terauchi, R.; Fujisaki, K.; Shimizu, M.; Oikawa, K.; Takeda, T.; Takagi, H.; Abe, A.; Okuyama, Y.; Yoshida, K.; Saitoh, H. Using genomics tools to understand plant resistance against pathogens: A case study of Magnaporthe-rice interactions. Appl. Plant Biotechnol. Improv. Resist. Biot. Stress 2020, 8, 181–188. [Google Scholar] [CrossRef]
- Jia, Y.; McAdams, S.A.; Bryan, G.T.; Hershey, H.P.; Valent, B. Direct Interaction of Resistance Gene and Avirulence Gene Products Confers Rice Blast Resistance. EMBO J. 2000, 19, 4004–4014. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Wu, J.; Lu, G.; Hu, Y.; Zhang, X.; Zhang, Z.; Wang, H.; Wang, S. The Magnaporthe oryzae Avirulence Gene AvrPiz-t Encodes a Predicted Secreted Protein that Triggers Piz-t–Mediated Resistance in Rice. Mol. Plant-Microbe Interact. 2009, 22, 411–420. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Feng, A.; Su, J.; Wang, W.; Feng, J.; Chen, B.; Zhang, M.; Yang, J.; Zeng, L. Xa7, a small orphan gene harboring promoter trap for AvrXa7, leads to the durable resistance to Xanthomonas oryzae pv. oryzae. Rice 2021, 14, 48. [Google Scholar] [CrossRef]
- Rathore, J.S.; Ghosh, C. Pathogen-associated molecular patterns and their perception in plants. Mol. Asp. Plant-Pathog. Interact. 2018, 79–113. [Google Scholar] [CrossRef]
- Thakur, A.; Verma, S.; Reddy, V.P.; Sharma, D. Hypersensitive responses in plants. Agric. Rev. 2019, 40, 113–120. [Google Scholar] [CrossRef]
- He, L.; Liu, P.; Mei, L.; Luo, H.; Ban, T.; Chen, X.; Ma, B. Disease resistance features of the executor R gene Xa7 reveal novel insights into the interaction between rice and Xanthomonas oryzae pv. oryzae. Front. Plant Sci. 2024, 15, 1365989. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zhao, W.; Zhang, X.; Han, Y.; Zou, L.; Chen, G. Identification of an avirulence gene, a vrxa5, from the rice pathogen Xanthomonas oryzae pv. oryzae. Sci. China Life Sci. 2010, 53, 1440–1449. [Google Scholar] [CrossRef]
- Ji, C.; Ji, Z.; Liu, B.; Cheng, H.; Liu, H.; Liu, S.; Yang, B.; Chen, G. Xa1 allelic R genes activate rice blight resistance suppressed by interfering TAL effectors. Plant Commun. 2020, 1, 100087. [Google Scholar] [CrossRef]
- Kaur, A.; Rana, R.; Bansal, K.; Patel, H.K.; Sonti, R.V.; Patil, P.B. Insights into the diversity of transcription activator-like effectors in Indian pathotype strains of Xanthomonas oryzae pv. oryzae. Phytopathology 2023, 113, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Char, S.N.; Park, S.; Yang, B. Interaction of rice and Xanthomonas TAL effectors. Rice Genom. Genet. Breed. 2018, 375–391. [Google Scholar]
- Wu, X.; Li, Y.; Zou, L.; Chen, G. Gene-for-gene relationships between rice and diverse avrBs3/pthA avirulence genes in Xanthomonas oryzae pv. oryzae. Plant Pathol. 2007, 56, 26–34. [Google Scholar] [CrossRef]
- Vikal, Y.; Bhatia, D. Genetics and genomics of Bacterial Blight Resistance. Chapter 2017, 10, 175–213. [Google Scholar]
- Wilson, R.A.; Talbot, N.J. Under Pressure: Investigating the Biology of Plant Infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Talbot, N.J. On the Trail of a Cereal Killer: Investigating the Biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef]
- Han, J.; Wang, X.; Wang, F.; Zhao, Z.; Li, G.; Zhu, X.; Su, J.; Chen, L. The fungal effector Avr-Pita suppresses innate immunity by increasing COX activity in rice mitochondria. Rice 2021, 14, 12. [Google Scholar] [CrossRef]
- Li, J.; He, C.; Dong, C.; Lu, L.; He, C.; Bi, Y.; Shi, Z.; Fan, H.; Shi, J.; Wang, K. Diversity and Evolution of the Avirulence Gene AvrPi54 in Yunnan Rice Fields. Agronomy 2024, 14, 454. [Google Scholar] [CrossRef]
- Ma, L.; van den Burg, H.A.; Cornelissen, B.J.; Takken, F.L. Molecular basis of effector recognition by plant NB-LRR proteins. Mol. Plant Immun. 2012, 23–40. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.; Osakina, A.; Olsen, K.M.; Huang, Y.; Jia, M.H.; Ponniah, S.; Pedrozo, R.; Nicolli, C.; Edwards, J.D. Receptor-ligand interactions in plant inmate immunity revealed by AlphaFold protein structure prediction. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sugihara, Y.; Abe, Y.; Takagi, H.; Abe, A.; Shimizu, M.; Ito, K.; Kanzaki, E.; Oikawa, K.; Kourelis, J.; Langner, T. Disentangling the complex gene interaction networks between rice and the blast fungus identifies a new pathogen effector. PLoS Biol. 2023, 21, e3001945. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Bao, J.; Chen, X.; Xie, J.; Liu, Y.; Chen, H.; Zheng, H.; Tang, W.; Wang, Z. Large-scale genome scanning within exonic regions revealed the contributions of selective sweep prone genes to host divergence and adaptation in Magnaporthe oryzae species complex. Microorganisms 2021, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, S.; Zhou, X.; Zhao, C.; Guo, L.; Zhang, J.; Liu, F.; Huo, Q.; Zhao, W.; Guo, Z. Phosphorylation and ubiquitination of OsWRKY31 are integral to OsMKK10-2-mediated defense responses in rice. Plant Cell 2023, 35, 2391–2412. [Google Scholar] [CrossRef]
- Taj, G.; Giri, P.; Tasleem, M.; Kumar, A. MAPK signaling cascades and transcriptional reprogramming in plant–pathogen interactions. In Approaches to Plant Stress and Their Management; Springer: Berlin/Heidelberg, Germany, 2014; pp. 297–316. [Google Scholar]
- Jiang, R.; Zhou, S.; Da, X.; Yan, P.; Wang, K.; Xu, J.; Mo, X. OsMKK6 regulates disease resistance in rice. Int. J. Mol. Sci. 2023, 24, 12678. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Sasakura-Shimoda, F.; Suetsugu, M.; Selvaraj, M.G.; Hayashi, N.; Yamazaki, M.; Ishitani, M.; Shimono, M.; Sugano, S.; Matsushita, A. Development of disease-resistant rice by optimized expression of WRKY45. Plant Biotechnol. J. 2015, 13, 753–765. [Google Scholar] [CrossRef]
- Li, S.; Khoso, M.A.; Xu, H.; Zhang, C.; Liu, Z.; Wagan, S.; Dinislam, K.; Liu, L. WRKY Transcription Factors (TFs) as Key Regulators of Plant Resilience to Environmental Stresses: Current Perspective. Agronomy 2024, 14, 2421. [Google Scholar] [CrossRef]
- Zhou, X.; Lei, Z.; An, P. Post-Translational Modification of WRKY Transcription Factors. Plants 2024, 13, 2040. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Bateman, N.; Kariyat, R.R. Rice physical defenses and their role against insect herbivores. Planta 2024, 259, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Chen, Y.; Huang, J.; Liang, W. Genome-Wide Identification of MKK Gene Family and Response to Hormone and Abiotic Stress in Rice. Plants 2024, 13, 2922. [Google Scholar] [CrossRef]
- Nadim, M.; Islam, M.; Hoque, M.; Hasan, M.; Uddin, M. Development of blast-resistant rice varieties through marker-assisted selection: Development of blast-resistant rice varieties. Bangladesh J. Agric. 2024, 49, 41–51. [Google Scholar] [CrossRef]
- Shanmugam, A.; Suresh, R.; Ramanathan, A.; Anandhi, P.; Pushpa, R.; Sassikumar, D. Characterization of Traditional Rice Varieties for Leaf Blast Resistant Genes Pi5, Pi54, Pi9 and Pi2 using Gene Specific Markers. Res. Biot. 2023, 5, 158–161. [Google Scholar] [CrossRef]
- Tang, L.; Song, J.; Cui, Y.; Fan, H.; Wang, J. Detection and Evaluation of Blast Resistance Genes in Backbone Indica Rice Varieties from South China. Plants 2024, 13, 2134. [Google Scholar] [CrossRef]
- Bian, Z.; Cao, D.-P.; Zhuang, W.-S.; Zhang, S.-W.; Liu, Q.-Q.; Zhang, L. Revelation of rice molecular design breeding: The blend of tradition and modernity. Yi Chuan Hered. 2023, 45, 718–740. [Google Scholar]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.; Prasanna, B. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef] [PubMed]
- Ofori, A.D.; Zheng, T.; Titriku, J.K.; Appiah, C.; Xiang, X.; Kandhro, A.G.; Ahmed, M.I.; Zheng, A. The Role of Genetic Resistance in Rice Disease Management. Int. J. Mol. Sci. 2025, 26, 956. [Google Scholar] [CrossRef]
- Mondal, S.; Rutkoski, J.E.; Velu, G.; Singh, P.K.; Crespo-Herrera, L.A.; Guzman, C.; Bhavani, S.; Lan, C.; He, X.; Singh, R.P. Harnessing diversity in wheat to enhance grain yield, climate resilience, disease and insect pest resistance and nutrition through conventional and modern breeding approaches. Front. Plant Sci. 2016, 7, 991. [Google Scholar] [CrossRef]
- Sabar, M.; Mustafa, S.E.; Ijaz, M.; Khan, R.A.R.; Shahzadi, F.; Saher, H.; Javed, H.M.; Zafar, S.A.; Saleem, M.U.; Siddique, S. Rice Breeding for Yield Improvement through Traditional and Modern Genetic Tools. Eur. J. Ecol. Biol. Agric. 2024, 1, 14–19. [Google Scholar] [CrossRef]
- Thulasinathan, T.; Ayyenar, B.; Kambale, R.; Manickam, S.; Chellappan, G.; Shanmugavel, P.; Narayanan, M.B.; Swaminathan, M.; Muthurajan, R. Marker assisted introgression of resistance genes and phenotypic evaluation enabled identification of durable and broad-spectrum blast resistance in elite rice cultivar, CO 51. Genes 2023, 14, 719. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Recent advances in rice varietal development for durable resistance to biotic and abiotic stresses through marker-assisted gene pyramiding. Sustainability 2021, 13, 10806. [Google Scholar] [CrossRef]
- Punniakotti, E.; Kousik, M.; Chaitra, K.; Harika, G.; Kumar, T.D.; Mastanbee, S.; Vivek, G.; Rekha, G.; Aleena, D.; Sinha, P. International Journal of Current Microbiology and Applied Sciences. Int. J. Curr. Microbiol. Appl. Sci. 2023, 12, 275–282. [Google Scholar]
- Mapari, A.R.; Mehandi, S. Enhancing Crop Resilience: Advances and Challenges in Marker-Assisted Selection for Disease Resistance. J. Adv. Biol. Biotechnol. 2024, 27, 569–580. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, R.K.; Zhao, K. Genome editing in rice: Recent advances, challenges, and future implications. Front. Plant Sci. 2018, 9, 1361. [Google Scholar] [CrossRef]
- Mthiyane, P.; Aycan, M.; Mitsui, T. Strategic advancements in rice cultivation: Combating heat stress through genetic innovation and sustainable practices—A review. Stresses 2024, 4, 452–480. [Google Scholar] [CrossRef]
- Acosta-Soto, A.F.; López-Díaz, D.; Esquivel-Ramírez, J.; Mora-Soriano, J.; Lazalde-Medina, B. Fundamentals of CRISPR-Cas9: Gene-editing technology and basic. GSC Adv. Res. Rev 2024, 20, 42–49. [Google Scholar] [CrossRef]
- Bhuyan, S.J.; Kumar, M.; Ramrao Devde, P.; Rai, A.C.; Mishra, A.K.; Singh, P.K.; Siddique, K.H. Progress in gene editing tools, implications and success in plants: A review. Front. Genome Ed. 2023, 5, 1272678. [Google Scholar] [CrossRef]
- Rifhani, N.F.; Apriana, A.; Sisharmini, A.; Santoso, T.J.; Trijatmiko, K.R.; Slamet-Loedin, I.H.; Yunus, A. Construction of the CRISPR/Cas9 module and genetic transformation of aromatic rice cv. Mentik Wangi for developing bacterial leaf blight resistance. Biodiversitas J. Biol. Divers. 2023, 24. [Google Scholar] [CrossRef]
- Schepler-Luu, V.; Sciallano, C.; Stiebner, M.; Ji, C.; Boulard, G.; Diallo, A.; Auguy, F.; Char, S.N.; Arra, Y.; Schenstnyi, K. Genome editing of an African elite rice variety confers resistance against endemic and emerging Xanthomonas oryzae pv. oryzae strains. Elife 2023, 12, e84864. [Google Scholar] [CrossRef]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Yasmin, S.; Arif, M.; Ejaz, K.; Mansoor, S. Precise CRISPR-Cas9 mediated genome editing in super basmati rice for resistance against bacterial blight by targeting the major susceptibility gene. Front. Plant Sci. 2020, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.-F.; Li, L.; Piao, R.-H.; Wu, S.; Song, A.; Gao, M.; Jin, Y.-M. CRISPR/Cas9-mediated knockout of Bsr-d1 enhances the blast resistance of rice in Northeast China. Plant Cell Rep. 2024, 43, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Y.; Yao, W.; Yin, Z.; Wang, Y.; Huang, Z.; Zhou, J.Q.; Liu, J.; Lu, X.; Wang, F. CRISPR/Cas9-mediated simultaneous mutation of three salicylic acid 5-hydroxylase (OsS5H) genes confers broad-spectrum disease resistance in rice. Plant Biotechnol. J. 2023, 21, 1873–1886. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-Efficiency TALEN-Based Gene Editing Produces Disease-Resistant Rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of Genome Editing to Rice Genetic Improvement. J. Integr. Plant Biol. 2021, 63, 312–328. [Google Scholar] [CrossRef]
- Kozub, N.; Sozinova, O.; Sozinov, I.; Karelov, A.; Janse, L.; Mishchenko, L.; Borzykh, O.; Blume, Y. Advances in durable resistance to diseases in staple food crops: A review. Open Agric. J. 2022, 17. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, A.; Singh, A.; Pandey, G.C.; Srivastava, G. An overview of symbiotic and pathogenic interactions at the fungi-plant interface under environmental constraints. Front. Fungal Biol. 2024, 5, 1363460. [Google Scholar] [CrossRef]
- Pandian, B.A.; Joel, J.; Nachimuthu, V.V.; Swaminathan, M.; Govintharaj, P.; Tannidi, S.; Sabariappan, R. Marker-aided selection and validation of various Pi Pi gene combinations for rice blast resistance in elite rice variety ADT 43. J. Genet. 2018, 97, 945–952. [Google Scholar] [CrossRef]
- Sagar, V.; Dhawan, G.; Gopala Krishnan, S.; Vinod, K.; Ellur, R.K.; Mondal, K.K.; Rathour, R.; Prakash, G.; Nagarajan, M.; Bhowmick, P.K. Marker assisted introgression of genes governing resistance to bacterial blight and blast diseases into an elite Basmati rice variety, ‘Pusa Basmati 1509’. Euphytica 2020, 216, 16. [Google Scholar] [CrossRef]
- He, Z.; Xin, Y.; Wang, C.; Yang, H.; Xu, Z.; Cheng, J.; Li, Z.; Ye, C.; Yin, H.; Xie, Z. Genomics-Assisted improvement of super high-yield hybrid rice variety “super 1000” for resistance to bacterial blight and blast diseases. Front. Plant Sci. 2022, 13, 881244. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Dong, X.; Ali, J.; Mou, T. Introgression of bacterial blight (BB) resistance genes Xa7 and Xa21 into popular restorer line and their hybrids by molecular marker-assisted backcross (MABC) selection scheme. Afr. J. Biotechnol. 2012, 11, 8225–8233. [Google Scholar]
- Jiang, G.; Xu, C.; Tu, J.; Li, X.; He, Y.; Zhang, Q. Pyramiding of insect-and disease-resistance genes into an elite indica, cytoplasm male sterile restorer line of rice,‘Minghui 63’. Plant Breed. 2004, 123, 112–116. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Vishnupriya, M.R.; Biradar, S.K.; Laha, G.S.; Reddy, G.A.; Rani, N.S.; Sarma, N.P.; Sonti, R.V. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica 2008, 160, 411–422. [Google Scholar] [CrossRef]
- Arshad, H.M.I.; Sahi, S.T.; Atiq, M.; Wakil, W. Appraisal of resistant genes and gene pyramid lines of rice against indigenous pathotypes of Xanthomonas oryzae pv. oryzae in Punjab, Pakistan. Pak. J. Agric. Sci. 2016, 53, 365–370. [Google Scholar]
- Oliveira-Garcia, E.; Budot, B.O.; Manangkil, J.; Lana, F.D.; Angira, B.; Famoso, A.; Jia, Y. An efficient method for screening rice breeding lines against races of Magnaporthe oryzae. Plant Dis. 2024, 108, 1179–1187. [Google Scholar] [CrossRef]
- Tahir, R.; Afzal, F.; Jamil, H.; Razzaq, M.; Khan, M. Physiological Impacts of Pesticidal Contamination: Challenge to Sustainable Agriculture and Biodegradation Methods. Pak. J. Agric. Agric. Eng. Vet. Sci. 2024, 40, 24–37. [Google Scholar] [CrossRef]
- Onorati, F.; Tornambé, A.; Paina, A.; Maggi, C.; Sesta, G.; Berducci, M.T.; Bellucci, M.; Rivella, E.; D’Antoni, S. Ecotoxicological and chemical approach to assessing environmental effects from pesticide use in organic and conventional rice paddies. Water 2022, 14, 4136. [Google Scholar] [CrossRef]
- Boeraeve, F.; Hatt, S. Integrating agroecological practices to manage pests while combining organic and conservation agriculture. Concept Ecostacking: Tech. Appl. 2024, 163–190. [Google Scholar] [CrossRef]
- Saba, N.; Balwan, W.K. Genetic Pollution: A Safe or Risky Bet. Sch. Acad. J. Biosci 2023, 4, 159–162. [Google Scholar] [CrossRef]
- Mangosongo, H.M.; Mneney, E.E.; Wanjala, B. Gene Flow Between the Wild Rice Species (Oryza longistaminata) and Two Varieties of Cultivated Rice (Oryza sativa) in Kilombero District, Tanzania. Tanzan. J. Sci. 2023, 49, 943–953. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Wang, Z.; Lu, B.-R. Non-random transmission of parental alleles into crop-wild and crop-weed hybrid lineages separated by a transgene and neutral identifiers in rice. Sci. Rep. 2017, 7, 10436. [Google Scholar] [CrossRef] [PubMed]
| Disease | Pathogen | Symptoms | Region and Year | Economic Impact | References |
|---|---|---|---|---|---|
| Rice Blast | Magnaporthe oryzae | Leaf lesions, neck rot, panicle blast | Mid-South USA, 2016 | Annual producer gains of USD 69.34 million with blast-resistant rice adoption. | [10] |
| Bacterial Blight | Xanthomonas oryzae pv. oryzae | Water-soaked lesions, wilting, yellowing of leaves | India, 1980s | Yield losses up to 30% in the Punjab region. | [11] |
| Sheath Blight | Rhizoctonia solani | Lesions on leaf sheaths, lodging, reduced grain quality | India (Uttar Pradesh), 2015 | Yield losses ranged between 14.3% and 39.7% across surveyed districts. | [12] |
| Gene/QTLs | Pathogen | Role | References |
|---|---|---|---|
| qSB-9 | R. solani | Decreases the severity of sheath blight infection | [68] |
| qSBR11 | R. solani | Promotes sheath blight resistance | [69] |
| hb9-2 | R. solani | Imparts partial resistance to sheath blight | [70] |
| qBlsr5a | Xoo | Increases host resistance to bacterial leaf streak | [71] |
| qSBR11-1 | Xoo | Provides durable resistance across bacterial blight races | [72] |
| Pi21 | M. oryzae | Offers partial resistance to M. oryzae | [73] |
| Pi35 | M. oryzae | Provides partial resistance to M. oryzae | [74] |
| Resistant Genes | Variety | Disease | References |
|---|---|---|---|
| Pi-1, Pi-2, Pi-33 | C101A51 | Rice blast | [130] |
| Pi-2, Pi-54 | Puta Basmati 1509 | Rice blast | [131] |
| Pi9 | IRBL9-W | Rice blast | [30] |
| Xa23, Pi9 | Super 1000 | Bacterial blight, rice blast | [132] |
| Xa21 | IR72 | Bacterial blight | [133] |
| Xa21, Xa23 | Minghui 63 | Bacterial blight | [134] |
| X4, X5, X13, X21 | IR36 | Bacterial blight | [89] |
| Xa21, xa13, Xa5 | Samba Mahsuri | Bacterial blight | [135] |
| Xa21 | IRBB21 | Bacterial blight | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younas, M.U.; Rao, B.; Qasim, M.; Ahmad, I.; Wang, G.; Sun, Q.; Xuan, X.; Iqbal, R.; Feng, Z.; Zuo, S.; et al. Molecular Insights into Rice Immunity: Unveiling Mechanisms and Innovative Approaches to Combat Major Pathogens. Plants 2025, 14, 1694. https://doi.org/10.3390/plants14111694
Younas MU, Rao B, Qasim M, Ahmad I, Wang G, Sun Q, Xuan X, Iqbal R, Feng Z, Zuo S, et al. Molecular Insights into Rice Immunity: Unveiling Mechanisms and Innovative Approaches to Combat Major Pathogens. Plants. 2025; 14(11):1694. https://doi.org/10.3390/plants14111694
Chicago/Turabian StyleYounas, Muhammad Usama, Bisma Rao, Muhammad Qasim, Irshad Ahmad, Guangda Wang, Quanyi Sun, Xiongyi Xuan, Rashid Iqbal, Zhiming Feng, Shimin Zuo, and et al. 2025. "Molecular Insights into Rice Immunity: Unveiling Mechanisms and Innovative Approaches to Combat Major Pathogens" Plants 14, no. 11: 1694. https://doi.org/10.3390/plants14111694
APA StyleYounas, M. U., Rao, B., Qasim, M., Ahmad, I., Wang, G., Sun, Q., Xuan, X., Iqbal, R., Feng, Z., Zuo, S., & Lackner, M. (2025). Molecular Insights into Rice Immunity: Unveiling Mechanisms and Innovative Approaches to Combat Major Pathogens. Plants, 14(11), 1694. https://doi.org/10.3390/plants14111694










