Analysis of Spatial Suitable Habitats of Four Subspecies of Hippophae rhamnoides in China Based on the MaxEnt Model
Abstract
1. Introduction
2. Results
2.1. Model Accuracy Assessment and Contribution of Environmental Variables
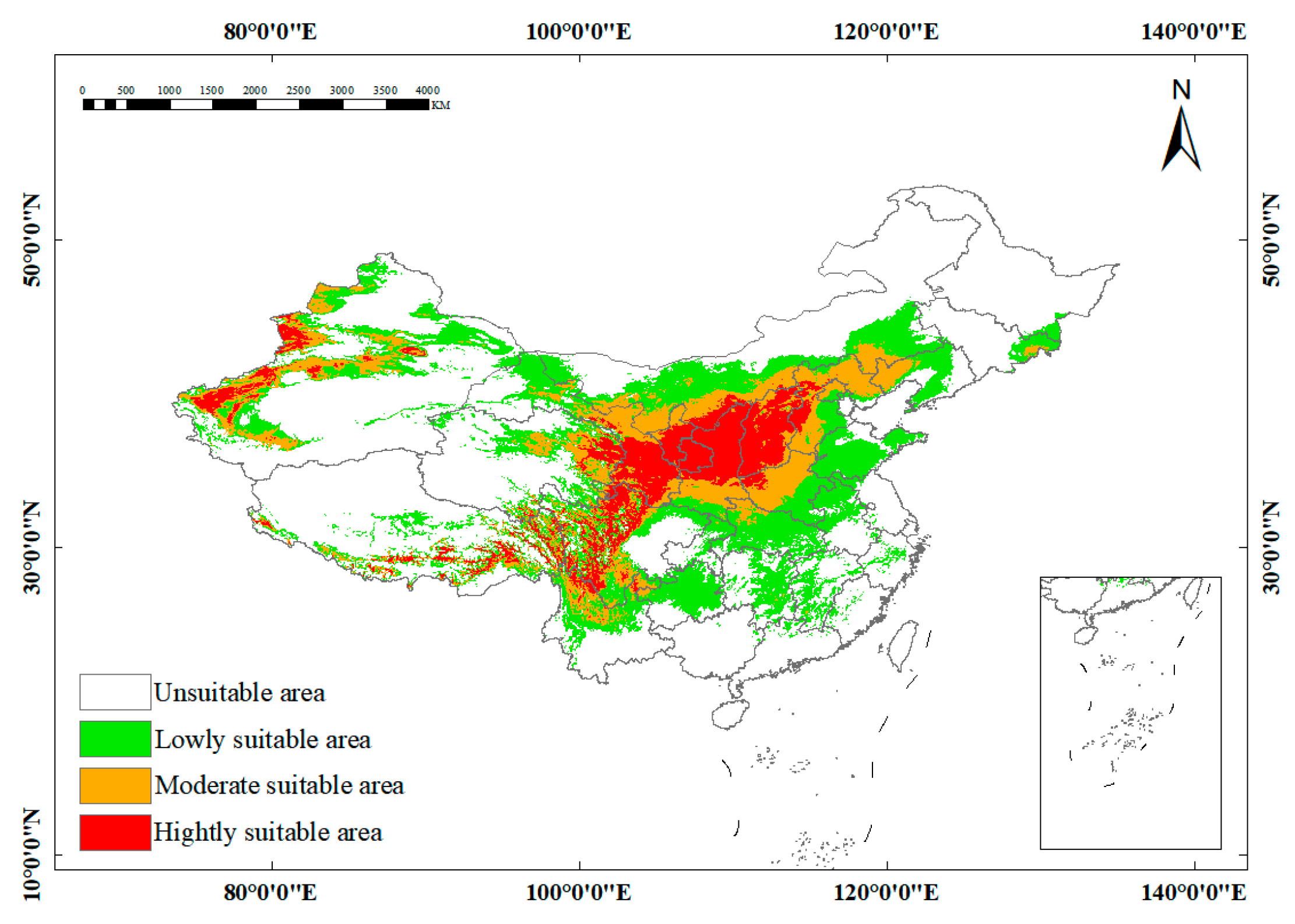
2.2. Current Distribution Analysis of Potentially Suitable Habitat
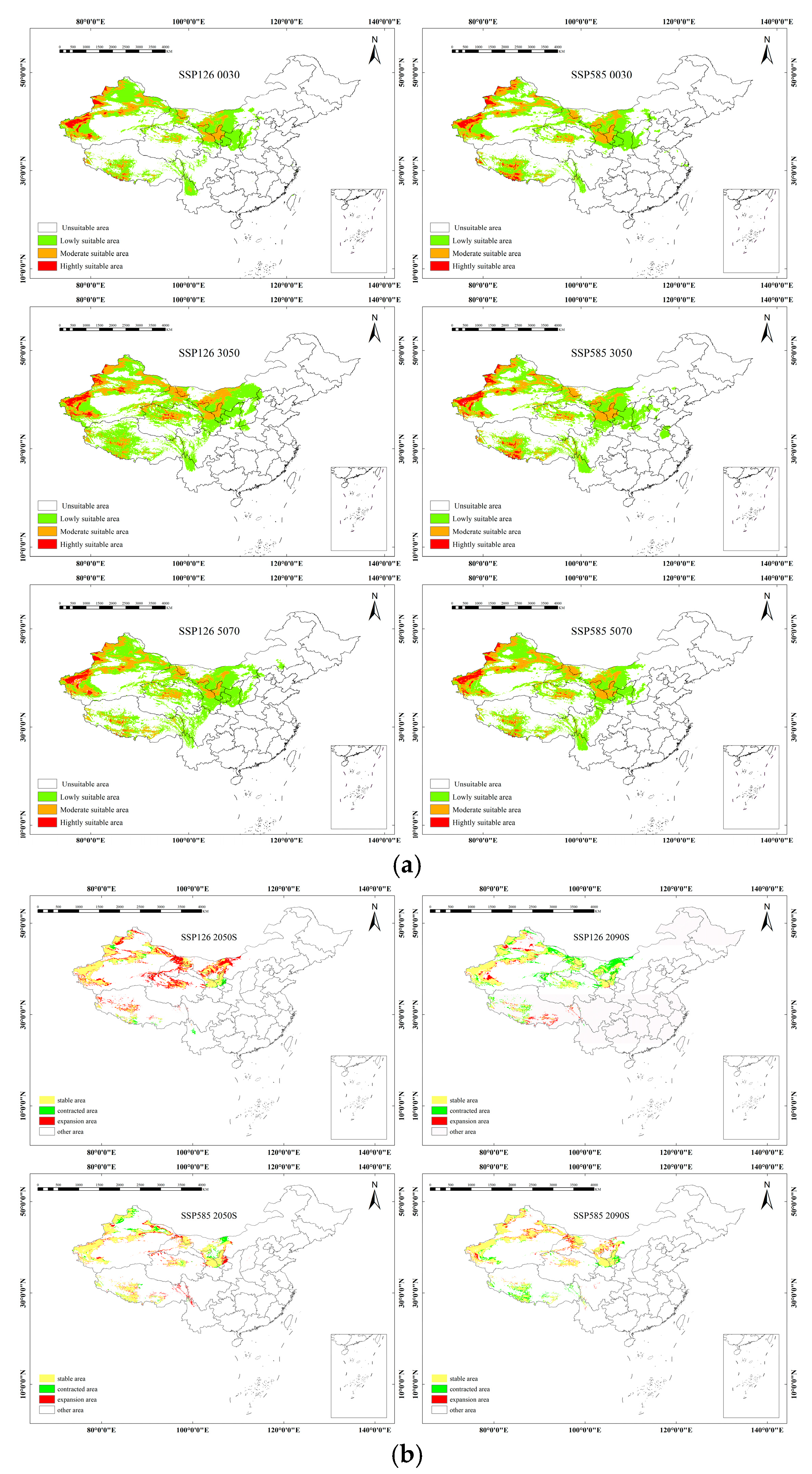
2.3. Future Potential Distribution Under Climate Change Scenarios
2.4. Migration of the Centroid of Suitable Habitats in Future Periods
3. Discussion
3.1. Effects of Environmental Factors on the Spatial Distribution of the Four Subspecies of Hippophae rhamnoides
3.1.1. Effects of Environmental Factors on the Spatial Distribution of sinensis
3.1.2. Effects of Environmental Factors on the Spatial Distribution of mongolica
3.1.3. Effects of Environmental Factors on the Spatial Distribution of yunnanensis
3.1.4. Effects of Environmental Factors on the Spatial Distribution of turkestanica
3.2. Potential Future Geographic Distribution of the Four Subspecies of Hippophae rhamnoides
3.2.1. Analysis of Potential Geographical Distribution Trends of sinensis
3.2.2. Analysis of Potential Geographical Distribution Trends of mongolica
3.2.3. Analysis of Potential Geographical Distribution Trends of yunnanensis
3.2.4. Analysis of Potential Geographical Distribution Trends of turkestanica
3.3. Migration Trend of the Center Point of Suitable Habitat for Sea Buckthorn Under Climate Change
4. Methods
4.1. Collection and Analysis of Data on the Distribution of Hippophae rhamnoides
4.1.1. Acquisition of Data Points on the Distribution of Hippophae rhamnoides in China
4.1.2. Environmental Data Collection and Analysis
4.2. Species Distribution Data
Processing with ENMTools
4.3. MaxEnt Modeling
4.4. Reliability Assessment of MaxEnt Modeling
4.5. Identification of Suitable Habitats and Center-of-Mass Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christaki, E. Hippophae rhamnoides L. (Sea Buckthorn): A Potential Source of Nutraceuticals. Food Public Health 2012, 2, 69–72. [Google Scholar] [CrossRef]
- Pundir, S.; Garg, P.; Dwivedi, A.; Ali, A.; Kapoor, V.K.; Kapoor, D.; Kulshrestha, S.; Lal, U.R.; Negi, P. Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: A review. J. Ethnopharmacol. 2020, 266, 113434. [Google Scholar] [CrossRef]
- Qinge, M.; Nengxin, H.; Huilian, H.; Xiaomei, F.; Zhongli, Z.; Jicheng, S.; Qinyuan, W.; Jie, C.; Guang, W.; Meining, Z.; et al. Hippophae rhamnoides L.: A comprehensive review on the botany, traditional uses, phytonutrients, health benefits, quality markers, and applications. J. Agric. Food Chem. 2023, 71, 12. [Google Scholar] [CrossRef]
- Hao, D.; Xiao, P. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef]
- Deren, Y. Discussion on Value Accounting of Hippophae rhamnoides L.; Inner Mongolia Forestry Science and Technology: Hohhot, China, 2002. [Google Scholar]
- Leeuw, J.; Njenga, M.; Wagner, B.; Iiyama, M. Treesilience: An Assessment of the Resilience Provided by Trees in the Drylands of Eastern Africa; FAO: Rome, Italy, 2014. [Google Scholar]
- Kitczak, T.; Jarnuszewski, G.; Łazar, E.; Malinowski, R. Sida hermaphrodita Cultivation on Light Soil—A Closer Look at Fertilization and Sowing Density. Agronomy 2022, 12, 2715. [Google Scholar] [CrossRef]
- Tian, L.; Wu, W.; Zhou, X.; Zhang, D.; Yu, Y.; Wang, H.; Wang, Q. The Ecosystem Effects of Sand-Binding Shrub Hippophae rhamnoides in Alpine Semi-Arid Desert in the Northeastern Qinghai–Tibet Plateau. Land 2019, 8, 183. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, J.; Wang, B.; Li, Y.; Li, Q. The Profiles and Tensile Strength on Straight Roots of Plants Withstand Transient Tensile Injured after Self-Repair. Sci. Rep. 2020, 10, 11468. [Google Scholar] [CrossRef]
- Ruan, C.; Li, D. Function and Benefit of Hippophae rhamnoides L. Improving Eco-Environment of Loess Plateau of China; Science Press: Beijing, China, 2002. [Google Scholar]
- Zhang, X.W.; Jiang, Y.M.; Bi, Y.; Liu, X.L.; Li, X.; Sun, T.; Chen, H.Y.; Li, J. Analysis of potential suitable distribution areas for Hippophae rhamnoides in China based on MaxEnt model. Acta Ecol. Sin. 2022, 42, 1420–1428. [Google Scholar]
- Cui, J.L. Potential Impacts of Climate Change on the Distribution of Three Commonly Used Medicinal Plants. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2015. [Google Scholar]
- Shahzad, K.; Alatalo, J.M.; Zhu, M.; Cao, L.; Hao, Y.; Dai, J. Geographic conditions impact the relationship between plant phenology and phylogeny. Sci. Total Environ. 2024, 945, 174083. [Google Scholar] [CrossRef]
- Chen, S.Q.; Dong, H.F.; Yue, Y.F.; Hao, Y.Y.; Liu, X.; Cao, X.Y.; Ma, J. Prediction of geographical distribution and dynamic changes of Hippophae rhamnoides in China under different climate scenarios. Arid Zone Res. 2024, 41, 1560–1571. [Google Scholar] [CrossRef]
- Yang, Y.L. Prediction of suitable distribution areas for Magnolia campbellii in Yunnan and Tibet based on MaxEnt model. Mod. Agric. Sci. Technol. 2024, 3, 98–102. (In Chinese) [Google Scholar]
- Rivera, Ó.R.D.; López-Quílez, A. Development and Comparison of Species Distribution Models for Forest Inventories. ISPRS Int. J. Geo-Inf. 2017, 6, 176. [Google Scholar] [CrossRef]
- Dewar, R.C. Maximum Entropy Production as an Inference Algorithm That Translates Physical Assumptions into Macroscopic Predictions: Don’t Shoot the Messenger. Entropy 2009, 11, 931–944. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, X.X.; Luo, Y.; Ma, Y.P.; Li, W.; Yang, L.X.; Liu, W.; Zhao, P.X.; Li, Z.H.; Ma, H. Selection of suitable tree species for Yunnan dry-hot valley based on MaxEnt model. Acta Ecol. Sin. 2024, 44, 3689–3707. [Google Scholar] [CrossRef]
- Wang, B.Y.; Dong, L.B.; Liu, Z.G. Prediction of potential distribution of three rare and endangered tree species in Xiaoxing ‘an Mountains under different climate scenarios. J. Cent. South Univ. For. Technol. 2024, 44, 29–40. [Google Scholar] [CrossRef]
- Tu, Y. Analysis of Potential Distribution Areas and Influencing Factors of Stipa Species in China Based on Species Distribution Models. Master’s Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Wang, H.; Zhi, F.; Zhang, G. Predicting Impacts of Climate Change on Suitable Distribution of Critically Endangered Tree Species Yulania Zenii (W. C. Cheng) D. L. Fu in China. Forests 2024, 15, 883. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Fan, W.-Y.; Wang, Z.-G. The Predictive Performance and Stability of Six Species Distribution Models. PLoS ONE 2014, 9, e112764. [Google Scholar] [CrossRef]
- Wang, W.Q.; Yang, B.; Li, X.W.; Liang, Y.L.; Li, J.Y. Impacts of future climate change on the potential geographical distribution of Hippophae rhamnoides in China. Chin. J. Appl. Ecol. 2024, 35, 2813–2821. [Google Scholar]
- Xie, J.Y.; He, X.H.; Zhu, L.; Hao, R.M. Potential geographical distribution of Hippophae neurocarpa in China under climate change. Prot. For. Sci. Technol. 2023, 1, 24–29. [Google Scholar] [CrossRef]
- Chen, X.L.; Lian, Y.S. Distribution pattern of Hippophae spp. and its cause. Hippophae 2007, 20, 1–5. (In Chinese) [Google Scholar]
- Ren, H.; Wen, Z.; Liu, Y.; Lin, Z.; Han, P.; Shi, H.; Wang, Z.; Su, T. Vegetation Response to Changes in Climate across Different Climate Zones in China. Ecol. Indic. 2023, 155, 110932. [Google Scholar] [CrossRef]
- Li, G.Y.; Sui, W. A Preliminary report on introduction of Russian thornless Hippophae varieties. Hippophae 1995, 8, 5. [Google Scholar]
- Pang, R.R.; Liu, M.Q.; Gao, L.S.; Li, S.J.; Han, X.Y. Study on probability distribution area dynamics of Xing’an larch forest based on species distribution model. J. Northwest Coll. For. 2023, 38, 1–9. [Google Scholar]
- Diao, S.F. Adaptive Differentiation and Molecular Mechanisms of Ascorbic Acid Synthesis in Hippophae rhamnoides and Hippophae mongolica. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2021. [Google Scholar]
- He, B.; Tuya, W.; Qinchaoketu, S.; Nanzad, L.; Yong, M.; Kesi, T.; Sun, C. Climate change characteristics of typical grassland in the Mongolian Plateau from 1978 to 2020. Sustainability 2022, 14, 16529. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Y.; Liu, X.; Yao, Y.; Qi, W. Relationship between the roots of Hippophae rhamnoides at different stump heights and the root microenvironment in feldspathic sandstone areas. PeerJ 2023, 11, e14819. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, L.; Li, Q.; Liu, K. Restoration strategies in the Heidaigou open-pit mine dump based on water sources and plant water utilization. Forests 2024, 15, 906. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, G.; Wang, J.; Liu, Y.; Gao, Y. Soil moisture distribution and time stability of aerially sown shrubland in the northeastern margin of Tengger Desert (China). Water 2023, 15, 3562. [Google Scholar] [CrossRef]
- Yang, Y.-Z.; Luo, M.-X.; Pang, L.-D.; Gao, R.-H.; Chang, J.-T.; Liao, P.-C. Parallel adaptation prompted core-periphery divergence of Ammopiptanthus mongolicus. Front. Plant Sci. 2022, 13, 956374. [Google Scholar] [CrossRef]
- Anonymous. Natural conditions, forest types, and stand structure of Hippophae distribution areas in Yunnan. J. West China For. Sci. 1989, 2, 6–15. (In Chinese) [Google Scholar]
- Chen, X.L.; Ma, R.J.; Sun, K. Study on germplasm resources and habitat types of Hippophae in China. Acta Bot. Boreali-Occident. Sin. 2003, 23, 5. [Google Scholar]
- Zhong, C.; Chen, Z.Y.; Wang, Y.; Liu, Y.Z. Advances in molecular-level research on the effects of UV-B radiation on plants. Chin. J. Ecol. 2009, 28, 9. [Google Scholar]
- Li, M.-Y.; Jiang, Y.-W.; Wang, W.-H.; Han, Y.-J.; Xu, G.-C. Dynamic analysis of potential habitats for invasive forest pests under climate change: A case study of Dendroctonus frontalis. J. Belg. For. Univ. 2009, 31, 64–69. [Google Scholar]
- Anonymous. Growth and development process of Hippophae in Yunnan and its basic patterns. J. West China For. Sci. 1989, 2, 6. (In Chinese) [Google Scholar]
- Chen, J.Z.; Xu, C.X.; Liang, L.F. Effects of low temperature on proteins and proline in banana leaves. J. South China Agric. Univ. 1999, 20, 54–58. [Google Scholar]
- Xu, C.X. Research progress on mechanisms for enhancing plant cold resistance. Acta Ecol. Sin. 2012, 32, 15. [Google Scholar]
- Rasmussen, C.R.; Thorup-Kristensen, K.; Dresbøll, D.B. Chicory Demonstrates Substantial Water Uptake from below 2 m Depth, but Still Did Not Escape Topsoil Drought. bioRxiv 2019, 494906. [Google Scholar] [CrossRef]
- Zhang, H.F. Prediction of potential suitable habitats for the endangered butterfly Teinopalpus aureus in China. J. Jinggangshan Univ. (Nat. Sci.) 2023, 44, 56–62. [Google Scholar]
- Pei, B.; Zhang, G.G.; Zhang, S.Y.; Wu, Q.; Xu, Z.Q.; Xu, P. Effects of soil drought stress on photosynthetic characteristics and antioxidant enzyme activities in Hippophae rhamnoides Linn. seedlings. Acta Ecol. Sin. 2013, 33, 1386–1396. [Google Scholar]
- Wang, R.X. Phylogeographic Study of Hippophae rhamnoides in China. Ph.D. Thesis, Northwest Normal University, Lanzhou, China, 2018. [Google Scholar]
- Yang, J.; Liu, Q.R.; Wang, X.T. Plant communities and soil nutrients in alpine Kobresia pygmaea meadows at different degradation stages on the Qinghai-Tibet Plateau. Chin. J. Appl. Ecol. 2020, 31, 6. [Google Scholar]
- Shi, H.X.; Hou, X.Y.; Shi, S.L.; Wu, X.H.; Li, P.; Yang, T.T. Relationships between primary productivity, diversity, and soil factors in alpine Kobresia pygmaea meadows. Pratacult. Sci. 2015, 24, 40–47. [Google Scholar]
- Zhang, L.; Ding, Y.H.; Wu, T.W.; Xin, X.G.; Zhang, Y.W.; Xu, Y. The 21st century annual mean surface air temperature change and the 2 °C warming threshold over the globe and China as projected by the CMIP5 models. Acta Meteorol. Sin. 2013, 71, 1047–1060. [Google Scholar]
- Yang, H.L.; Wang, B.K.; Du, Y.D.; Li, L. Projection analysis of climate change in Pearl River Basin under RCPs scenarios. J. Trop. Meteorol. 2014, 30, 8. (In Chinese) [Google Scholar]
- Shan, J.Y. Introduction to Hippophae varieties. China Seed Ind. 2009, 6, 73–75. (In Chinese) [Google Scholar] [CrossRef]
- Meirelles, M.; Carvalho, F.; Porteiro, J.; Henriques, D.; Navarro, P.; Vasconcelos, H. Climate change and impact on renewable energies in the Azores strategic visions for sustainability. Sustainability 2022, 14, 15174. [Google Scholar] [CrossRef]
- Zhou, B.T.; Qian, J. Interpretation of IPCC AR6 report: Changes in extreme weather and climate events. Adv. Clim. Change Res. 2021, 17, 713–718. (In Chinese) [Google Scholar]
- Xu, H.T. Impacts of Climate, Disturbance, and Forest Succession on Genetic Diversity of Thuja occidentalis. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2013. [Google Scholar]
- Yang, Z.; Liu, Y.; Han, H.; Zhao, X.; Chen, S.; Li, G.; Shi, S.; Feng, J. Physiological and transcriptome analyses reveal the response of Ammopiptanthus mongolicus to extreme seasonal temperature in plateau cold desert ecosystem. Sci. Rep. 2022, 12, 10630. [Google Scholar] [CrossRef]
- Du, Q.; Yan, X.F. Diversity and spatial distribution characteristics of vegetation types in Helan Mountain. J. Anhui Agric. Sci. 2010, 38, 3666–3667. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, X.H.; Wang, L.H.; Liang, X.R.; Wen, G.S.; Hong, Y. RAPD analysis of genetic diversity in natural populations of Juniperus sabina in Inner Mongolia. Arid Land Resour. Environ. 2005, z1, 6. (In Chinese) [Google Scholar] [CrossRef]
- Gan, X.L.; Chang, Y.P.; Jiang, Y.; Cao, F.F.; Zhao, C.Y.; Li, W.B. Impacts of climate change on the potential suitable habitat of Amygdalus mongolica in the Qilian Mountains. Acta Ecol. Sin. 2023, 43, 768–776. (In Chinese) [Google Scholar]
- Du, T.; Liu, J.; Dong, J.; Xie, H.; Wang, X.; Yang, X.; Yang, Y. Multifunctional coatings of nickel-titanium implant toward promote osseointegration after operation of bone tumor and clinical application: A review. Front. Bioeng. Biotechnol. 2024, 12, 1325707. [Google Scholar] [CrossRef]
- Seydi, S.T.; Arefi, H. A comparison of deep learning-based super-resolution frameworks for Sentinel-2 imagery in urban areas. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2023, 10, 1021–1026. [Google Scholar] [CrossRef]
- Feng, D.; Wang, G.; Wei, X.; Amankwah, S.O.Y.; Hu, Y.; Luo, Z.; Hagan, D.F.T.; Ullah, W. Merging and downscaling soil moisture data from CMIP6 projections using deep learning method. Front. Environ. Sci. 2022, 10, 847475. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, W.; Qian, L.; Yang, D.; Liu, X.; Wang, M. The effect of environmental factors on the diversity of crane flies (Tipulidae) in mountainous and non-mountainous regions of the Qinghai-Tibet Plateau and surrounding areas. Insects 2022, 13, 1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Liu, Z.; Han, Y.; Xie, Q. Comparative chloroplast genome analysis of four Hippophae rhamnoides subspecies and its phylogenetic analysis. Genet. Resour. Crop Evol. 2024, 71, 2557–2571. [Google Scholar] [CrossRef]
- Wang, T.Y. Genetic Differentiation Between Hippophae rhamnoides and Hippophae neurocarpa in China. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2021. [Google Scholar]
- Cheng, K.; Sun, K.; Wen, H.Y.; Zhang, M.; Jia, D.R.; Liu, J.Q. Phylogeographic differentiation and genetic relationships between Hippophae gyantsensis and Hippophae neurocarpa. J. Plant Ecol. 2009, 33, 11. (In Chinese) [Google Scholar]
- Zhang, M.T. Editorial and publishing conference for the Comprehensive Scientific Expedition Series of the Qinghai-Tibet Plateau (Hengduan Mountains section) held in Beijing. Nat. Resour. 1985, 3, 45. (In Chinese) [Google Scholar]
- John, C.; Post, E. Seasonality, Niche Management and Vertical Migration in Landscapes of Relief. Ecography 2022, 2022, e05774. [Google Scholar] [CrossRef]
- Xiang, R.; Steger, C.R.; Li, S.; Pellissier, L.; Sørland, S.L.; Willett, S.D.; Schär, C. Assessing the Regional Climate Response to Different Hengduan Mountains Geometries with a High-Resolution Regional Climate Model. J. Geophys. Res. Atmos. 2024, 129, e2023JD040208. [Google Scholar] [CrossRef]
- Cao, Z.L.; Wang, X.L.; Li, G.Q. Effects of three soil types on seedling emergence and growth of Hippophae rhamnoides subsp. sinensis in the Central Yunnan Plateau. Seed 2020, 39, 139–143. [Google Scholar] [CrossRef]
- Trujano-Ortega, M.; Ávalos-Hernández, O.; Callaghan, C.J.; García-Vázquez, U.O.; Luis-Martínez, A.; Llorente-Bousquets, J. Challenges for organismic taxonomical revisions in the age of phylogenomic: A response to Zhang et al. Zootaxa 2020, 4838, 436–440. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, Y.P.; Li, W.H.; Wang, Y.Y. Spatial distribution characteristics of fine roots of Populus euphratica under different groundwater depths in arid areas. J. Plant Sci. 2018, 36, 9. (In Chinese) [Google Scholar]
- Sun, W.; Shu, J.; Gu, Y.; Morigengaowa; Du, X.; Liu, B.; Yan, Y. Conservation genomics analysis revealed the endangered mechanism of Adiantum nelumboides. Biodivers. Sci. 2022, 30, 21508. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.F.; Wang, C.Q. Evaluation of future temperature and precipitation trends in the Yellow River Basin under SSP scenarios. J. Hydrol. 2024, 44, 51–59. (In Chinese) [Google Scholar] [CrossRef]
- Li, J. Study on Groundwater Level Dynamics and Prediction Models in Yaoba Oasis, Alxa. Ph.D. Thesis, Chang’an University, Xi’an, China, 2023. [Google Scholar]
- Liu, Q.; Liu, Y.; Niu, J.; Gui, D.; Hu, B.X. Prediction of the irrigation area carrying capacity in the Tarim River Basin under climate change. Agriculture 2022, 12, 657. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.H.; Wang, P.L.; Yu, H.P.; Yue, P.; Liu, X.Y.; Lin, J.J.; Duan, X.Y.; Zhu, B.; Yan, X.Y. Research progress and prospects of climate warming and humidification in Northwest China. Chin. Sci. Bull. 2023, 68, 1814–1828. (In Chinese) [Google Scholar] [CrossRef]
- Jin, L.Y.; Fu, J.L.; Chen, F.H. Regional differences in precipitation changes over Northwest China in recent 44 years and their response to global warming. Sci. Geogr. Sin. 2005, 25, 567–572. [Google Scholar]
- Zhai, P.M.; Wang, C.C.; Li, W. Observational study on changes in extreme precipitation events. Adv. Clim. Change Res. 2007, 3, 5. [Google Scholar]
- Liu, G.-Z.; Zhao, K.; Zhang, S.-Q.; Liang, Y.-M.; Yue, Y.-J.; Liu, G.-H.; Qin, F.-C. Biomass allocation and allometric relationship of Salix gordejevii branches in sandy habitats heterogeneity in Northern China. Sustainability 2024, 16, 5483. [Google Scholar] [CrossRef]
- Chang, H.; Liu, T.; Wang, D.W.; Ji, X.R. Potential distribution of Haloxylon ammodendron in arid Northwest China under climate change. J. Desert Res. 2019, 39, 1–12. (In Chinese) [Google Scholar]
- Zhao, X.J. Photosynthetic Eco-Physiological Responses of Hippophae Populations and Species to Enhanced UV-B Radiation. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2008. [Google Scholar]
- Chen, Y.; Chen, Y.; Xu, C.; Ye, Z.; Li, Z.; Zhu, C.; Ma, X. Effects of ecological water conveyance on groundwater dynamics and riparian vegetation in the lower reaches of Tarim River, China. Hydrol. Process. 2010, 24, 170–177. [Google Scholar] [CrossRef]
- Yan, Z.L. Study on Dynamic Changes of Ecological Environment and Ecological Water Demand in Tarim River Basin Based on RS and GIS. Ph.D. Thesis, Xi’an University of Technology, Xi’an, China, 2008. [Google Scholar]
- Deng, M.J. Remote sensing monitoring and evaluation of ecological water transfer and vegetation restoration in the lower reaches of Tarim River. J. Glaciol. Geocryol. 2007, 29, 7. [Google Scholar]
- Yan, J.; Xia, J.; Jiang, Y.; Xia, J. The hydrological characteristics of runoff and its response to climatic change in Tarim River Basin. Resour. Sci. 2007, 29, 45–52. [Google Scholar]
- Jeong, Y.S.; Lee, D.-S.; Lee, D.-Y.; Park, Y.-S. Predicting potential occurrence of Adelges tsugae (Homoptera: Adelgidae) on a global scale under climate change scenarios using maximum entropy model. Glob. Ecol. Conserv. 2024, 50, e02861. [Google Scholar] [CrossRef]
- Li, S.P.; Quan, W.J.; Wang, Z.; Chen, Y.Z.; Su, T.; Yan, P.C. Evaluation of BCC-CSM2-MR global climate model simulations for precipitation and temperature over East Asia. J. Arid Meteorol. 2023, 41, 984–996. (In Chinese) [Google Scholar]
- Zhao, J.L.; Droma, L.; Wang, W.; Wang, H.D.; Wang, W.; Shen, J.P. Analysis of climate change impacts on the suitable habitat of highland barley in the Tibetan Plateau based on MaxEnt model. Chin. J. Eco-Agric. Advance online publication. 2024. (In Chinese) [Google Scholar] [CrossRef]
- Shin, M.-S.; Yoon, H. Bioclimatic Variables in South Korea under Climate Change SSP Scenario. Geo Data 2021, 3, 57–65. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, W.; Li, Y.; Huang, C.; Zhang, X.; Peñuelas, J.; Zhang, Y.; Gentine, P.; Li, Z.; Wang, X.; et al. Increasing Meteorological Drought under Climate Change Reduces Terrestrial Ecosystem Productivity and Carbon Storage. One Earth 2023, 6, 1326–1339. [Google Scholar] [CrossRef]
- Wang, B.-X.; Zhu, L.; Ma, G.; Najar-Rodriguez, A.; Zhang, J.-P.; Zhang, F.; Avila, G.A.; Ma, C.-S. Correction: Wang et al. Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios. Biology 2023, 12, 1045. [Google Scholar] [CrossRef]
- Liao, W.; Cao, S.; Jiang, Y.; Shao, W.; Zhao, L.; Yan, C. Predicting Conservation Status of Testudoformes under Climate Change Using Habitat Models. Animals 2024, 14, 2300. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, W.; Zhao, H.; Xian, X.; Jing, K.; Yang, N.; Lu, X.; Liu, W. Potential Global Distribution of Invasive Alien Species, Anthonomus Grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model. Agriculture 2022, 12, 1759. [Google Scholar] [CrossRef]
- Li, Y.; Ding, C. Effects of sample size, sample accuracy and environmental variables on predictive performance of MaxEnt model. Pol. Acad. Sci. 2016, 64, 303–312. [Google Scholar] [CrossRef]
- Du, B.Y. Analysis of Potential Distribution Changes for Larix gmelinii and Quercus mongolica in the Greater Khingan Mountains Under Climate Change Using MaxEnt Model. Master’s Thesis, Harbin Normal University, Harbin, China, 2024. [Google Scholar]
- Wang, H.Y.; Ma, W.Q.; Jing, Z.X.; Jiang, D.Q.; Peng, Z.; Xu, Y.; Zhang, Y.; Kang, C.Z. Ecological suitability and quality zoning of Atractylodes based on MaxEnt model and GIS. World Chin. Med. 2023, 18, 1847–1856. (In Chinese) [Google Scholar]
- Guo, X.D.; Xi, S.Y.; Yang, L.P.; Ji, J.; Ma, X.H.; Jin, L. Prediction of Potential Suitable Areas for Epimedium in China Based on Optimized MaxEnt Model. Chin. J. Inf. Tradit. Chin. Med. 2024, 31, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Ying, J.L. Prediction of Climate Change Impacts on the Potential Distribution of Endemic viburnum Species in China Using MaxEnt Model. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2024. [Google Scholar]
- Xue, X.; Meng, C.; Cen, Y.; Xiao, J.; Li, X.; Chen, W. Modulation of Hydrothermal Conditions on the Inhibiting and Promoting Effects of Cumulative Drought on Vegetation Productivity in Southwest China. Ecol. Indic. 2024, 169, 112924. [Google Scholar] [CrossRef]
- Somantri, L.; Noviandi, I.E.; Fahrezi, A.A.; Arrafi, M. Administrative Unit Proliferation Through Spatial Interaction Approach: Case Study of Lembang City Region. J. Reg. City Plan. 2023, 34, 137–155. [Google Scholar] [CrossRef]
- Pei, Y.; Lu, B.; Song, Y.; Yang, Y.; Feng, X.; Shen, W. Collaborative Ecological Flow Decision Making under the Bengbu Sluice Based on Ecological-Economic Objectives. Water 2022, 14, 4133. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Su, M.; Gong, W.; Liu, Y.; Yang, J.; Huang, Y.; Zhao, C. Modeling the Potential Distribution of the Energy Tree Species Triadica sebifera in Response to Climate Change in China. Sci. Rep. 2024, 14, 1220. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, S.; Huang, T.; Liu, J.; Yue, J. Estimation of Potential Suitable Habitats for the Relict Plant Euptelea pleiosperma in China via Comparison of Three Niche Models. Sustainability 2023, 15, 11035. [Google Scholar] [CrossRef]






| Ranking | Environment Variable | Full Name (In English) | Contribution Rate (%) |
|---|---|---|---|
| 1 | bio11 | Mean Temperature of Coldest Quarter | 20.0% |
| 2 | Altitude | Elevation | 18.7% |
| 3 | bio6 | Min Temperature of Coldest Month | 11.6% |
| 4 | bio12 | Annual Precipitation | 9.2% |
| 5 | bio2 | Mean Diurnal Range (Mean of Monthly Max–Min Temperature) | 7.8% |
| 6 | bio15 | Precipitation Seasonality (Coefficient of Variation) | 4.4% |
| 7 | bio17 | Precipitation of Driest Quarter | 4.4% |
| 8 | bio4 | Temperature Seasonality (Coefficient of Variation) | 4.4% |
| 9 | bio7 | Annual Temperature Range (Max Temperature of Warmest Month − Min of Coldest) | 4.0% |
| 10 | bio14 | Precipitation of Driest Month | 4.0% |
| Subspecies | Dominant Environmental Factors | Full Name (In English) | Suitable Range |
|---|---|---|---|
| sinensis | bio12 | Annual Precipitation | 506.38–1052.38 mm |
| bio14 | Precipitation of Driest Month | 1.08–5.24 mm | |
| bio11 | Mean Temperature of Coldest Quarter | −6.24–3.59 °C | |
| mongolica | bio13 | Wettest monthly precipitation | 31.78–33.61 mm |
| Altitude | Elevation | 741.91–2222.73 m | |
| bio17 | Precipitation of Driest Quarter | 15.75–20.17 mm | |
| yunnanensis | Altitude | Elevation | 2673.62–4018.12 m |
| bio4 | Temperature Seasonality | 532.92–642.78 | |
| bio11 | Mean Temperature of Coldest Quarter | −2.99–5.09 °C | |
| turkestanica | bio13 | Wettest monthly precipitation | 40.05–40.84 mm |
| bio2 | Mean Diurnal Range (Mean of Monthly Max–Min Temperature) | 12.41–14.04 °C | |
| bio6 | Min Temperature of Coldest Month | −16.79–−8.39 °C |
| Subspecies | Total Suitable Habitat Area | Percentage of China’s Land Area | Main Distribution Regions | Highly Suitable (Proportion) | Moderately Suitable (Proportion) | Low-Suitability (Proportion) |
|---|---|---|---|---|---|---|
| sinensis | 3.0142 × 106 km2 | 31% | Central China (Qinghai–Tibet–Sichuan border regions); scattered in Ningxia, Inner Mongolia, Hebei, Liaoning | 0.6203 × 106 km2 (21%) | 0.6656 × 106 km2 (22%) | 1.753 × 106 km2 (58%) |
| mongolica | 3.0220 × 106 km2 | 31% | Northwestern China (Xinjiang, Inner Mongolia, Gansu); scattered in Shanxi, Tibet, Hebei | 0.3434 × 106 km2 (11%) | 1.5712 × 106 km2 (52%) | 1.1321 × 106 km2 (37%) |
| yunnanensis | 0.9555 × 106 km2 | 10% | Narrow belt along Tibet–Sichuan–Yunnan–Qinghai borders | 0.1704 × 106 km2 (18%) | 0.2508 × 106 km2 (26%) | 0.5589 × 106 km2 (58%) |
| turkestanica | 2.7403 × 106 km2 | 29% | Xinjiang (Kashgar, Yining, Hotan); Tibet, Ningxia, Gansu, Qinghai, Inner Mongolia | 0.2999 × 106 km2 (11%) | 0.8193 × 106 km2 (30%) | 1.6457 × 106 km2 (60%) |
| Subspecies | Climate Scenario | Habitat Area Change (×104 km2) | Key Time Period | Direction and Distance of Centroid Migration |
|---|---|---|---|---|
| sinensis | SSP1–2.6 | −37.41 (2021–2060) → +29.52 (2061–2080) | 2021–2060 | Northeast migration, accumulated about 120 km |
| SSP5–8.5 | +2.48 (2021–2060) → −17.63 (2061–2080) | 2061–2080 | Swinging from northeast to southwest, generally stable in Southern Gansu | |
| mongolica | SSP1–2.6 | −61.54 (2041–2060) | 2041–2060 | Swinging from northeast to southwest, generally stable in central and Southern Xinjiang |
| SSP5–8.5 | −38.82 (2061–2080) | 2061–2080 | Continuously migrating southwest and ultimately staying in the northwest of Xinjiang | |
| yunnanensis | SSP1–2.6 | −8.57 (2041–2060) → −13.16 (2061–2080) | 2041–2080 | Short distance migration from southeast to southwest, overall stable |
| SSP5–8.5 | +16.98 (2041–2060) → Widespread reduction (2061–2080) | 2041–2060 | Short distance migration from southwest to southeast | |
| turkestanica | SSP1–2.6 | −26.58 (2061–2080) | 2061–2080 | Swing from northeast to southwest, stay in Tarim Basin |
| SSP5–8.5 | +33.97 (2041–2060) → subsequent reduction | 2041–2060 | Continuous northwest migration, staying in Tarim Basin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Ma, F.; Ding, J.; Niu, P.; Luo, C.; Wang, M.; Jiang, P. Analysis of Spatial Suitable Habitats of Four Subspecies of Hippophae rhamnoides in China Based on the MaxEnt Model. Plants 2025, 14, 1682. https://doi.org/10.3390/plants14111682
He M, Ma F, Ding J, Niu P, Luo C, Wang M, Jiang P. Analysis of Spatial Suitable Habitats of Four Subspecies of Hippophae rhamnoides in China Based on the MaxEnt Model. Plants. 2025; 14(11):1682. https://doi.org/10.3390/plants14111682
Chicago/Turabian StyleHe, Mengyao, Fanyan Ma, Junjie Ding, Panxin Niu, Cunkai Luo, Mei Wang, and Ping Jiang. 2025. "Analysis of Spatial Suitable Habitats of Four Subspecies of Hippophae rhamnoides in China Based on the MaxEnt Model" Plants 14, no. 11: 1682. https://doi.org/10.3390/plants14111682
APA StyleHe, M., Ma, F., Ding, J., Niu, P., Luo, C., Wang, M., & Jiang, P. (2025). Analysis of Spatial Suitable Habitats of Four Subspecies of Hippophae rhamnoides in China Based on the MaxEnt Model. Plants, 14(11), 1682. https://doi.org/10.3390/plants14111682




