Combining Transcriptome and Hormone-Targeted Metabolome Analyses to Dissect the Regulatory Mechanisms Underlying Wheat Peduncle Elongation
Abstract
1. Introduction
2. Results
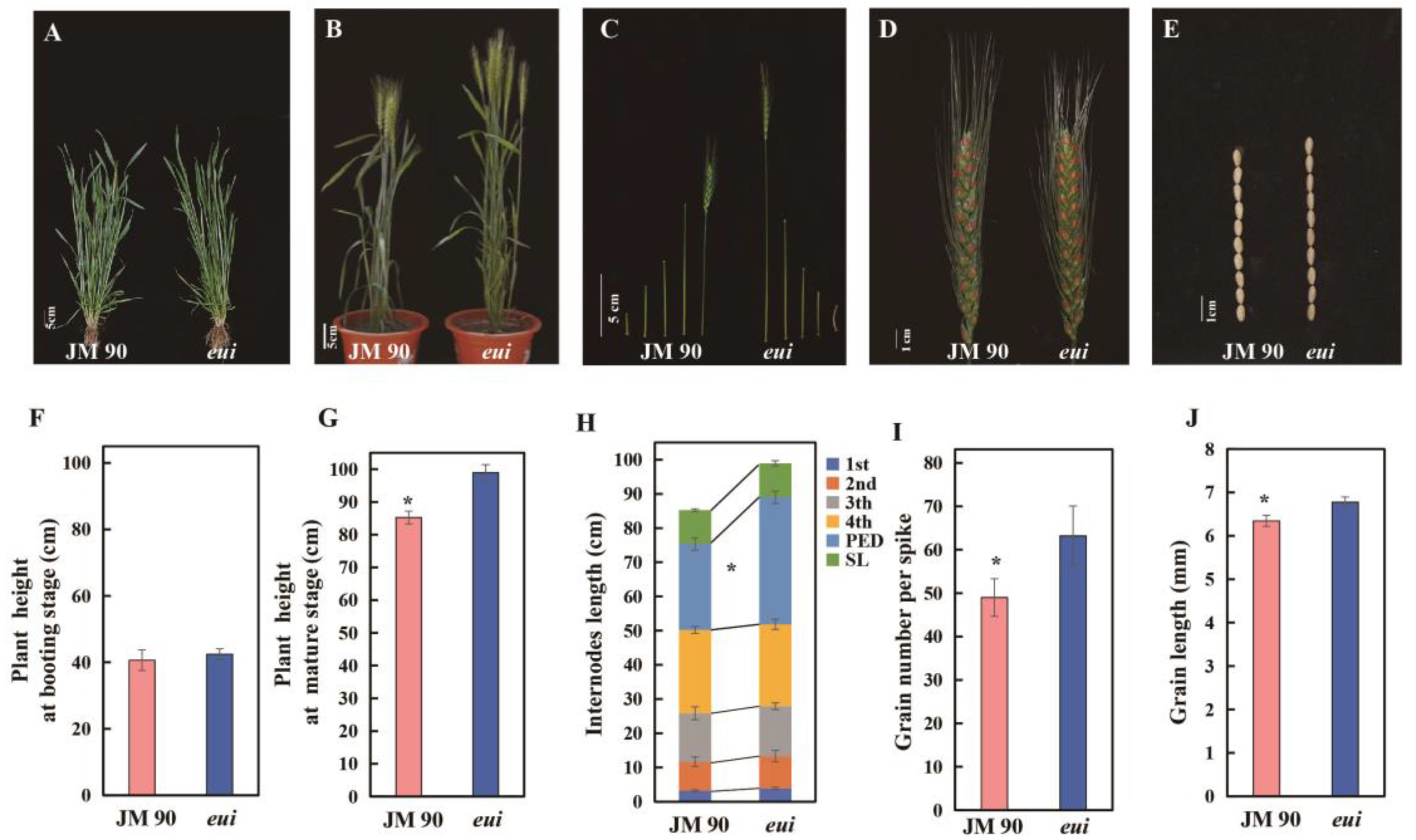
2.1. The Manifestations of the Elongated Uppermost Internode Mutant (eui)
2.2. Genetic Analysis of eui
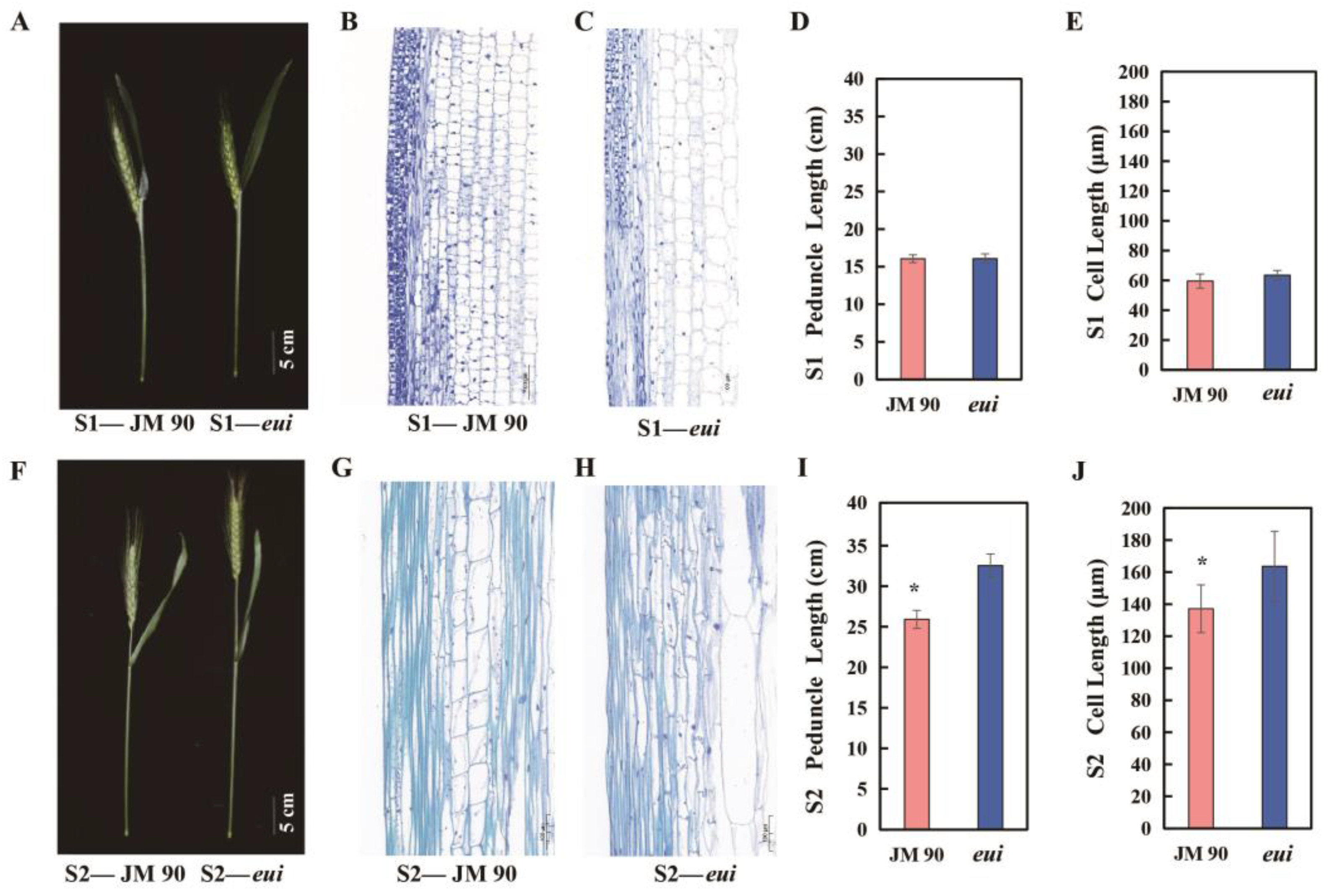
2.3. Observation of Cell Structure
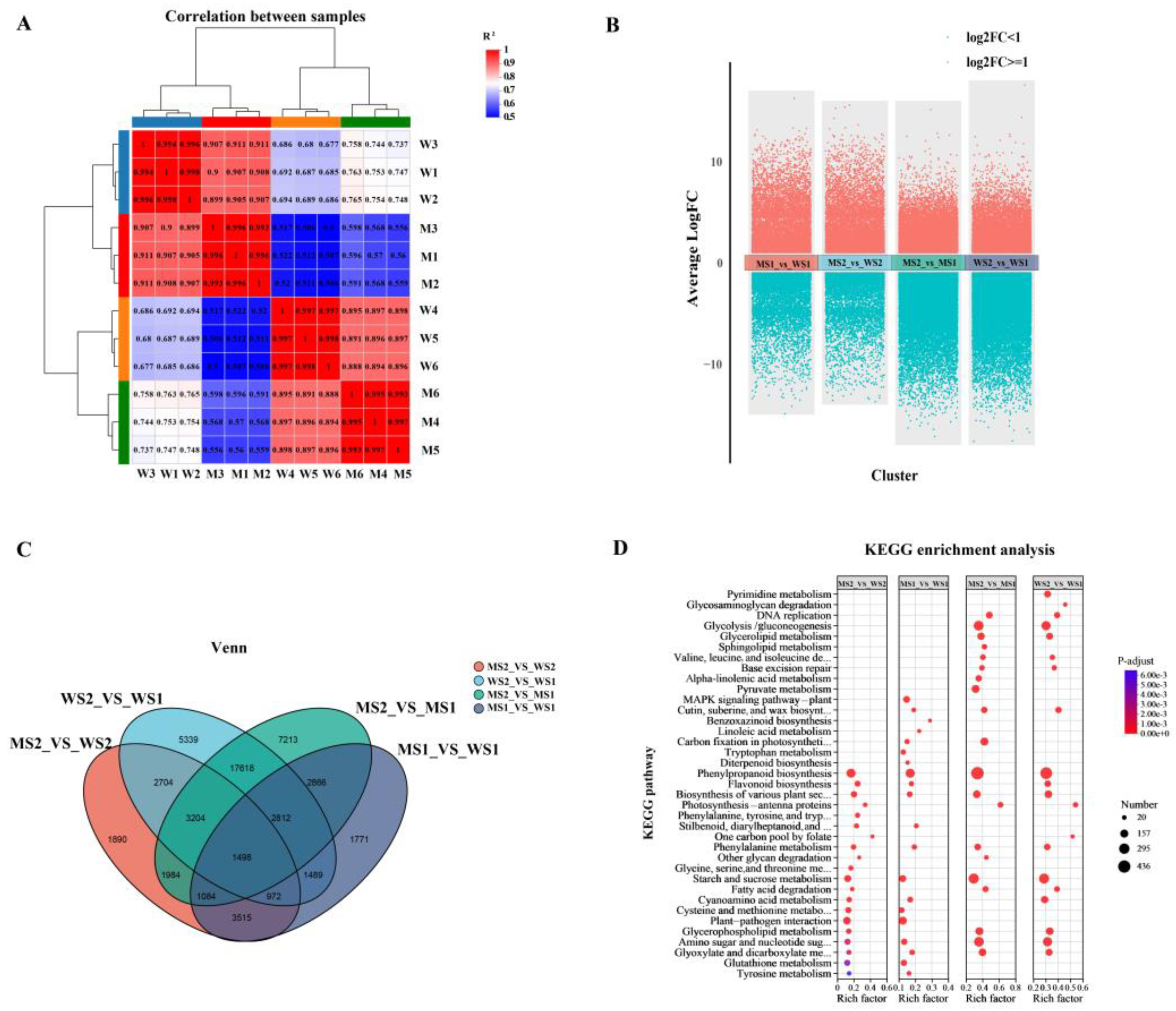
2.4. Dynamic Transcriptional Variations in eui and WT Wheat Peduncles
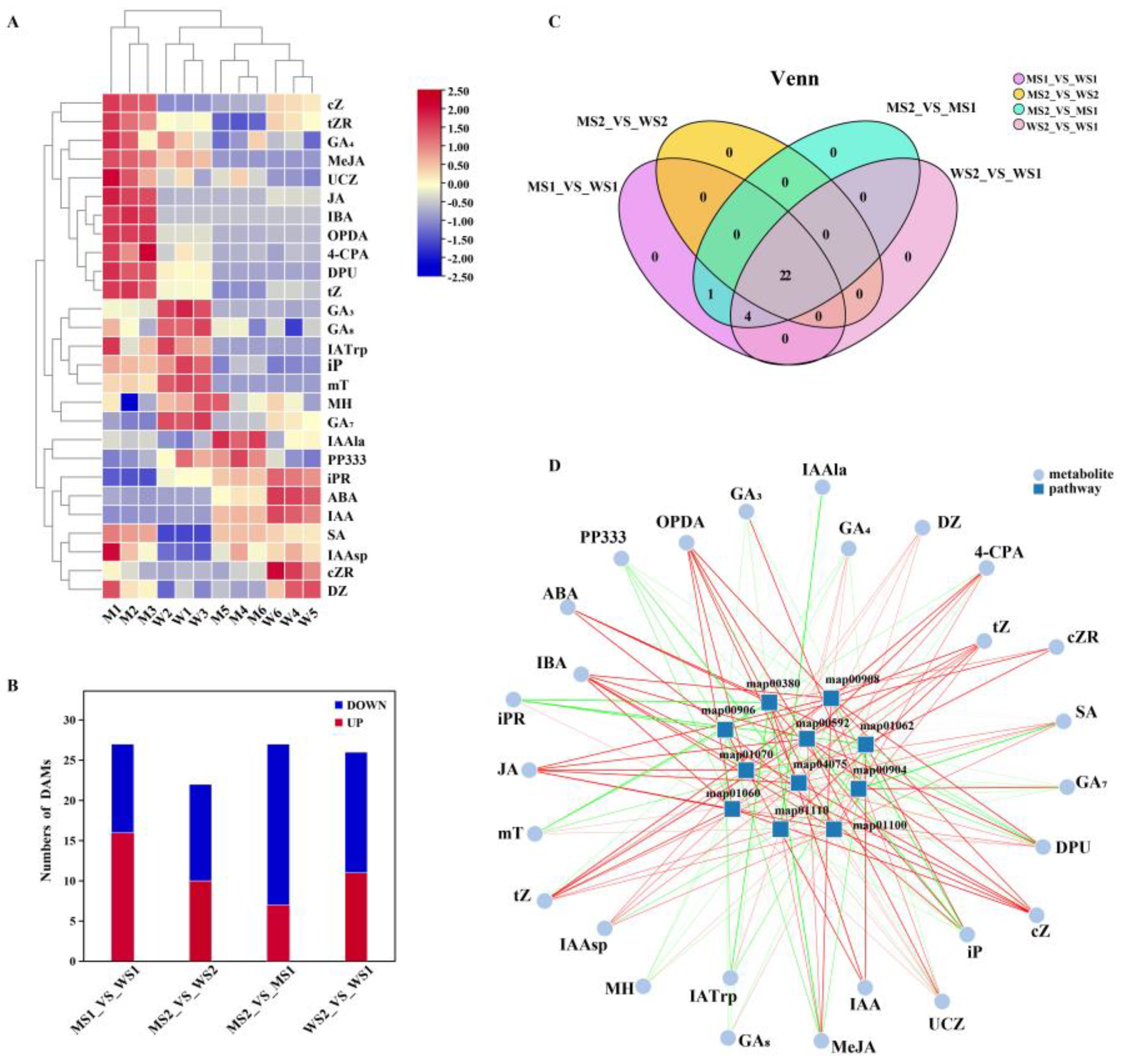
2.5. Dynamic Metabolomic Changes in eui and WT Peduncles
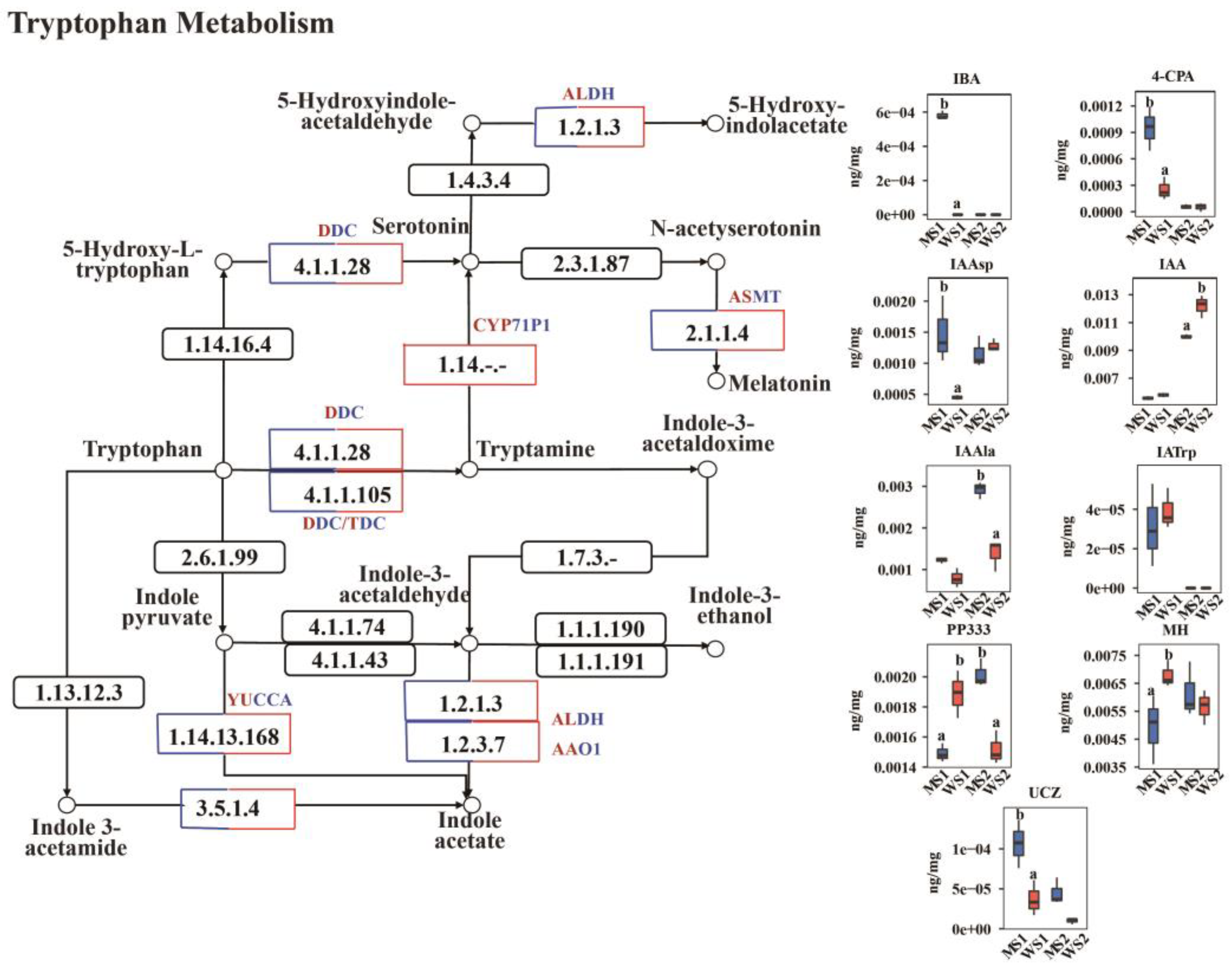
2.6. Differences in Detection of Auxin Between eui and WT
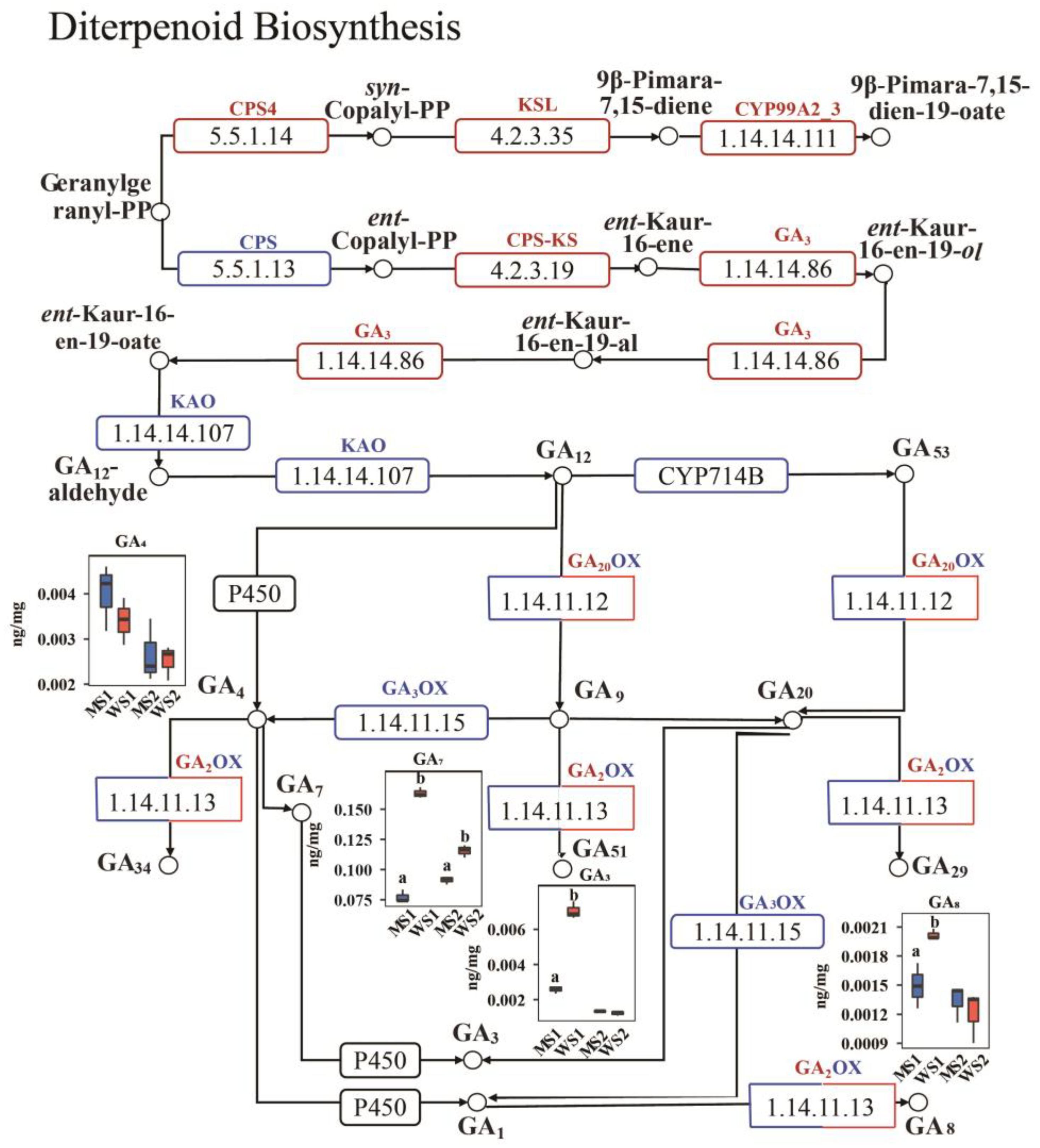
2.7. Differences in Detection of Gibberellin Between eui and WT
2.8. Differences in Detection of Other Hormones Between eui and WT
3. Discussion
3.1. eui Is a Mutant Causing Significant Peduncle Elongation and a Significant GNS Increase
3.2. eui Causes the Elongation of the Peduncle Through Cell Elongation
3.3. The Elongation of eui Plants’ Peduncle May Be Mainly Regulated by Auxin
4. Materials and Methods
4.1. Planting of Plant Materials and Measurement of Agronomic Traits
4.2. Cell Tissue Sections and Microscopic Observation
4.3. Sampling for RNA Sequencing and Targeted Metabolome Analysis
4.4. RNA-seq Analysis
4.5. Targeted Metabolome Analysis
4.6. Gene Expression Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Hadddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Tomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on Wheat Yield and Quality With Reduced Nitrogen Supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Murray, T.D.; McIntosh, R.A.; Zhou, Y. Breeding New Cultivars for Sustainable Wheat Production. Crop J. 2019, 7, 715–717. [Google Scholar] [CrossRef]
- Kong, L.G.; Wang, F.H.; Feng, B.; Li, S.D.; Si, J.S.; Zhang, B. The Structural and Photosynthetic Characteristics of the Exposed Peduncle of Wheat (Triticum aestivum L.): An Important Photosynthate Source for Grain-filling. BMC Plant Biol. 2010, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Gebbing, T. The Enclosed and Exposed Part of the Peduncle of Wheat (Triticum aestivum)—Spatial Separation of Fructan Storage. New Phytol. 2003, 159, 245–252. [Google Scholar] [CrossRef]
- Khatir, Z.; Thabet, S.G.; Alqahtani, M.D.; Schierenbeck, M.; Sehmisch, S.; Lantos, E.; Krebes, C.; Börner, A.; Alqudah, A.M. Discovery of New Genomic Regions and Candidate Genes Implicated in the Natural Variation of Barley Peduncle Length and Plant Height. Genet. Resour. Crop Evol. 2025, 72, 1741–1752. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Ellis, M.H.; Bonnett, D.G.; Condon, A.G.; Falk, D.; Richards, R.A. The Rht13 Dwarfing Gene Reduces Peduncle Length and Plant Height to Increase Grain Number and Yield of Wheat. Field Crops Res. 2011, 124, 323–331. [Google Scholar] [CrossRef]
- Li, C.; Tang, H.P.; Luo, W.; Zhang, X.M.; Mu, Y.; Deng, M.; Liu, Y.X.; Jiang, Q.T.; Chen, G.Y.; Wang, J.R.; et al. A Novel, Validated, and Plant Height-independent QTL for Spike Extension Length is Associated with Yield-related Traits in Wheat. Theor. Appl. Genet. 2020, 133, 3381–3393. [Google Scholar] [CrossRef]
- He, R.S.; Zhu, X.X.; Li, Q.Y.; Jiang, Y.M.; Yu, D.Y.; Sun, Y.L.; Liang, X.L.; Ni, Y.J.; Niu, J.S. Transcriptome Analysis for the Restrained Stem Development of the Wheat Mutant dms. Cienc Rural 2017, 47, e20170241. [Google Scholar] [CrossRef][Green Version]
- Xu, D.X.; Xie, Y.D.; Guo, H.J.; Zeng, W.W.; Xiong, H.C.; Zhao, L.S.; Gu, J.Y.; Zhao, S.R.; Ding, Y.P.; Liu, L.X. Transcriptome Analysis Reveals a Potential Role of Benzoxazinoid in Regulating Stem Elongation in the Wheat Mutant qd. Front. Genet. 2021, 12, 623861. [Google Scholar] [CrossRef]
- Miller, C.N.; Harper, A.L.; Trick, M.; Werner, P.; Waldron, K.; Bancroft, I. Elucidation of the Genetic Basis of Variation for Stem Strength Characteristics in Bread Wheat by Associative Transcriptomics. BMC Genom. 2016, 17, 500. [Google Scholar] [CrossRef] [PubMed]
- Calviño, M.; Bruggmann, R.; Messing, J. Characterization of the Small RNA Component of the Transcriptome from Grain and Sweet Sorghum Stems. BMC Genom. 2011, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.B.; Zhang, S.Q.; Hou, Q.L.; Gao, J.G.; Wang, H.X.; Chen, X.C.; Liao, X.Z.; Zhang, F.T.; Zhao, C.P.; Qin, Z.Q. Transcriptomic and Metabolomic Analysis Provides Insights into Lignin Biosynthesis and Accumulation and Differences in Lodging Resistance in Hybrid Wheat. J. Integr. Agric. 2024, 23, 1105–1117. [Google Scholar] [CrossRef]
- Yu, M.Z.; Wang, M.; Gyalpo, T.; Basang, Y.Z. Stem Lodging Resistance in Hulless Barley: Transcriptome and Metabolome Analysis of Lignin Biosynthesis Pathways in Contrasting Genotypes. Genomics 2021, 113, 935–943. [Google Scholar] [CrossRef]
- Karabulut, F.; Faizan, M.; Rasheed, D.; Ahmad, Z.; Unnisa, G.; Faraz, A. Integration of Plant Hormones in the Biological System as an Opportunity for Sustainable Crop Production; Springer: Singapore, 2024. [Google Scholar]
- Majda, M.; Robert, S. The Role of Auxin in Cell Wall Expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its Role in Plant Development: Structure, Signalling, Regulation and Response Mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Han, X.L.; Zhao, F.Y.; Wang, Z.L.; Che, X.; Cui, G.C. The Role of OsYUCCA2 in Auxin Synthesis and Promotion of Rice Growth and Development. Russ. J. Plant Physiol. 2020, 67, 1018–1027. [Google Scholar] [CrossRef]
- Zhang, S.N.; Wang, S.K.; Xu, Y.X.; Yu, C.L.; Shen, C.J.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y.H. The Auxin Response Factor, OsARF19, Controls Rice Leaf Angles through Positively Regulating OsGH3-5 and OsBRI 1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef]
- Sims, K.; Abedi-Samakush, F.; Szulc, N.; Macias Honti, M.G.; Mattsson, J. OsARF11 Promotes Growth, Meristem, Seed, and Vein Formation during Rice Plant Development. Int. J. Mol. Sci. 2021, 22, 4089. [Google Scholar] [CrossRef]
- Phillips, K.A.; Skirpan, A.L.; Liu, X.; Christensen, A.; Slewinski, T.L.; Hudson, C.; Barazesh, S. vanishing tassel2 Encodes a Grass-specific Tryptophan Aminotransferase Required for Vegetative and Reproductive Development in Maize. Plant Cell 2011, 23, 550–566. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.X.; Gao, Y.J.; Wang, Y.X.; Wu, H.Y.; Zhu, H.J.; Lu, X.D.; Ma, Q. An EMS-induced Allele of the brachytic2 Gene Can Reduce Plant Height in Maize. Plant Cell Rep. 2023, 42, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of Plant Hormone Response Pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef]
- Nagai, K.; Mori, Y.; Ishikawa, S.; Furuta, T.; Gamuyao, R.; Niimi, Y.; Hobo, T.; Fukuda, M.; Kojima, M.; Takebayashi, Y.; et al. Antagonistic Regulation of the Gibberellic Acid Response during Stem Growth in Rice. Nature 2020, 584, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhu, Y.Y.; Peng, Y.; Yan, D.Y.; Li, Q.; Wang, J.J.; Wang, L.Y.; He, Z.H. Gibberellin Homeostasis and Plant Height Control by EUI and a Role for Gibberellin in Root Gravity Responses in Rice. Cell Res. 2008, 18, 412–421. [Google Scholar] [CrossRef]
- Oikawa, T.; Koshioka, M.; Kojima, K.; Yoshida, H.; Kawata, M. A role of OsGA20ox1, Encoding an Isoform of Gibberellin 20-oxidase, for Regulation of Plant Stature in Rice. Plant Mol. Biol. 2004, 55, 687–700. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Q.W.; Xin, P.Y.; Yuan, J.; Ma, Y.H.; Wang, X.M.; Xu, J.F.; Peters, R.J.; Wang, G.D. CYP72A Enzymes Catalyse 13-hydrolyzation of Gibberellins. Nat. Plant 2019, 5, 1057–1065. [Google Scholar] [CrossRef]
- Niu, M.; Wang, H.R.; Yin, W.C.; Meng, W.J.; Xiao, Y.H.; Liu, D.P.; Zhang, X.X.; Dong, N.N.; Liu, J.H.; Yang, Y.Z.; et al. Rice DWARF AND LOW-TILLERING and the Homeodomain Protein OSH15 Interact to Regulate Internode Elongation Via Orchestrating Brassinosteroid Signaling and Metabolism. Plant Cell 2022, 34, 3754–3772. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Y.; Zhang, M.H.; Liu, X.; Li, H.; Jia, Z.J.; Wang, D.H.; Peng, T.; Hao, M.; Liu, D.C.; Jiang, B.; et al. Development and Identification of Four New Synthetic Hexaploid Wheat Lines with Solid Stems. Sci. Rep. 2022, 12, 4898. [Google Scholar] [CrossRef]
- Xu, D.G.; Jia, C.F.; Lyu, X.R.; Yang, T.Z.; Qin, H.M.; Wang, Y.L.; Hao, Q.L.; Liu, W.X.; Dai, X.H.; Zeng, J.B.; et al. In Silico Curation of QTL-rich Clusters and Candidate Gene Identification for Plant Height of Bread Wheat. Crop J. 2023, 11, 1480–1490. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, S.L.; Chen, G.Y.; Pu, Z.E.; Wei, Y.M.; Zheng, Y.L. QTLs for Uppermost Internode and Spike Length in Two Wheat RIL Populations and Their Affect Upon Plant Height at an Individual QTL Level. Euphytica 2014, 200, 95–108. [Google Scholar]
- Liu, Z.L.; Zhao, P.Z.; Lai, X.J.; Wang, X.M.; Ji, W.Q.; Xu, S.B. The Selection and Application of Peduncle Length QTL QPL_6D. 1 in Modern Wheat (Triticum aestivum L.) Breeding. Theor. Appl. Genet. 2023, 136, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Wei, A.L.; Zheng, D.M. Photosynthetic Characteristics of Non-leaf Organs of Winter Wheat Cultivars Differing in Ear Type and Their Relationship with Grain Mass Perear. Photosynthetica 2001, 39, 239–244. [Google Scholar] [CrossRef]
- Farooq, M.U.; Cheema, A.A.; Ishaaq, I.; Zhu, J. Correlation and Genetic Component Studies for Peduncle Length Affecting Grain Yield in Wheat. Int. J. Adv. Appl. Sci. 2018, 5, 67–75. [Google Scholar]
- Subedi, M.; Bagwell, J.W.; Ghimire, B.; Lopez, B.; Sapkota, S.; Babar, M.A.; Mergoum, M. Identifying Genomic Regions Associated with Key Agro-morphological Traits in Soft Red Winter Wheat Using Genome-wide Association Study. Crop Sci. 2024, 64, 2316–2335. [Google Scholar] [CrossRef]
- Pu, X.; Tang, Y.Y.; Zhang, M.H.; Li, T.; Qiu, X.B.; Wang, J.H.; Li, L.L.; Yang, Z.; Su, Y.; Zhang, H.L.; et al. Identification and Candidate Gene Mining of hvss1, a Novel Qualitative Locus on Chromosome 6h, Regulating the Uppermost Internode Elongation in Barley (Hordeum Vulgare L.). Theor. Appl. Genet. 2021, 134, 2481–2494. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, S.B.; Ji, L.; Zhu, H.B.; Yang, S.L.; Fang, X.J.; Yang, R.C. Fine Mapping and in Silico Isolation of the EUI1 Gene Controlling Upper Internode Elongation in Rice. Plant Mol. Biol. 2006, 60, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Yang, W.L.; Li, Y.F.; Shan, Q.Q.; Ye, X.B.; Wang, D.Z.; Yu, K.; Lu, W.W.; Xin, P.Y.; Pei, Z.; et al. A Wheat Dominant Dwarfing Line with Rht12, Which Reduces Stem Cell Length and Affects Gibberellic Acid Synthesis, is a 5AL Terminal Deletion Line. Plant J. 2019, 97, 887–900. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, S.; Luo, Y.; Li, F.; Tan, J.; Wang, B.; Zhao, Z.; Lin, H.; Zhang, T.; Liu, J.; et al. Rice OsUBR7 Modulates Plant Height by Regulating Histone H2B Monoubiquitination and Cell Proliferation. Plant Commun. 2022, 3, 16. [Google Scholar] [CrossRef]
- Wang, F.X.; Yu, Z.P.; Zhang, M.L.; Wang, M.L.; Lu, X.D.; Liu, X.; Li, Y.B.; Zhang, B.C.; Li, C.L.; Ding, Z.D. ZmTE1 Promotes Plant Height by Regulating Intercalary Meristem Formation and Internode Cell Elongation in Maize. Plant Biotechnol. J. 2022, 20, 526–537. [Google Scholar] [CrossRef]
- Hager, M.S.; Cook, J.P.; Bothner, B.; Weaver, D.K. Untargeted Metabolomic and Transcriptomic Analysis in Spring and Durum Wheat Reveals Potential Mechanisms Associated with the Early Stem Solidness Phenotype and Resistance to Wheat Stem Sawfly. Front. Plant Sci. 2025, 16, 1497732. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic Pathways of Hormones in Plants Metabolites. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nemoto, K. The Pathway of Auxin Biosynthesis in Plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Mateo-Bonmatí, E.; Ljung, K. Auxin Metabolism in Plants. Cold Spring Harb. Perspect. Biol. 2021, 13, a039867. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.Q.; Yin, H.R.; Liu, W.; Hu, X.; Li, D.Q.; Lan, C.X.; Gao, L.F.; He, Z.H.; Cui, F.; et al. The Pathway of Melatonin Biosynthesis in Common Wheat (Triticum aestivum). J. Pineal Res. 2023, 74, e12841. [Google Scholar] [CrossRef]
- Errum, A.; Rehman, N.; Khan, M.R.; Ali, G.M. Genome-wide Characterization and Expression Analysis of Pseudo-response Regulator Gene Family in Wheat. Mol. Biol. Rep. 2021, 48, 2411–2427. [Google Scholar] [CrossRef]
- Bage, S.A.; Barten, T.J.; Brown, A.N.; Crowley, J.H.; Deng, M.Q.; Fouquet, R.; Gomez, J.R.; Hatton, T.W.; Lamb, J.C.; Ledeaux, J.R.; et al. Genetic Characterization of Novel and CRISPR-Cas9 Gene Edited Maize brachytic 2 Alleles. Plant Gene 2020, 21, 100198. [Google Scholar] [CrossRef]
- Yin, C.X.; Gan, L.J.; Ng, D.N.; Zhou, X.; Xia, K. Decreased Panicle-derived Indole-3-acetic Acid Reduces Gibberellin A1 Level In the Uppermost Internode, Causing Panicle Enclosure in Male Sterile Rice Zhenshan 97A. J. Exp. Bot. 2007, 58, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Monna, L.; Kitazawa, N.; Yoshino, R.; Suzuki, J.; Masuda, H.; Maehara, Y.; Tanji, M.; Sato, M.; Nasu, S.; Minbe, Y. Positional Cloning of Rice Semi-dwarfing Gene, sd-1: Rice “Green Revolution Gene” Encodes a Mutant Enzyme Involved in Gibberellin Synthesis. DNA Res. 2022, 9, 11–17. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.C.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 Encodes a Soluble Receptor for Gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Luo, A.; Qian, Q.; Yin, H.; Liu, X.Q.; Yin, C.X.; Lan, Y.; Tang, J.Y.; Tang, Z.S.; Cao, S.Y.; Wang, X.J.; et al. Eui1, Encoding a Putative Cytochrome p450 Monooxygenase, Regulates Internode Elongation by Modulating Gibberellin Responses in Rice. Plant Cell Physiol. 2006, 47, 181–191. [Google Scholar] [CrossRef]
- Gao, S.P.; Fang, J.; Xu, F.; Wang, W.; Chu, C.C. Rice HOX12 Regulats Panicle Exsertion by Directly Modulating the Expression of ELONGATED UPPERMOST INTERNODE1. Plant Cell 2016, 28, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, J.; Cao, B.L.; Zhang, X.P.; Chen, Z.Y.; Dong, C.Q.; Liu, X.Q.; Zhang, Z.H.; Wang, W.X.; Chai, L.L.; et al. Reducing Brassinosteroid Signalling Enhances Grain Yield in Semi-dwarf Wheat. Nature 2023, 617, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.X.; Li, D.P.; Yang, R.Z.; Zhang, L.C.; Zhang, Y.W.; Liu, X.; Kong, X.Y.; Sun, J.Q. GSK3 Phosphorylates and Regulates the Green Revolution Protein Rht-B1b to Reduce Plant Height in Wheat. Plant Cell 2023, 35, 1970–1983. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Q.; Xu, Q.T.; Cao, Y.J.; Qian, K.; An, K.; Zhu, Y.; Hu, B.Z.; Zhao, H.F.; Kuai, B.K. Loss-of-Function Mutations in DET2 Gene Lead to an Enhanced Resistance to Oxidative Stress in Arabidopsis. Physiol. Plant. 2010, 123, 57–66. [Google Scholar] [CrossRef]
- He, G.H.; Liu, J.; Dong, H.X.; Sun, J.Q. The Blue-Light Receptor CRY1 Interacts with BZR1 and BIN2 to Modulate the Phosphorylation and Nuclear Function of BZR1 in Repres BR Signaling in Arabidopsis. Mol. Plant 2019, 12, 689–703. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-cytokinin Interactions in the Control of Shoot Branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Wolbang, C.M.; Chandler, P.M.; Smith, J.J.; Rose, J.J. Auxin from the Developing Inflorescence is Required for the Biosynthesis of Active Gibberellins in Barley Stems. Plant Physiol. 2004, 134, 769–776. [Google Scholar] [CrossRef]
- Meng, F.W.; Yang, C.; Cao, J.D.; Chen, H.; Pang, J.H.; Zhao, Q.Q.; Wang, Z.Y.; Fu, Z.Q.; Liu, J. A bHLH Transcription Activator Regulates Defense Signaling by Nucleo-cytosolic Trafficking in Rice. J. Integr. Plant Biol. 2020, 62, 1552–1573. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Luo, X.T.; Cai, B.D.; Chen, X.; Feng, Y.Q. Improved Methodology for Analysis of Multiple Phytohormones using Sequential Magnetic Solid-phase Extraction Coupled with Liquid Chromatography-tandem Mass Spectrometry. Anal. Chim. Acta 2017, 983, 112–120. [Google Scholar] [CrossRef]
- Han, C.; Shi, C.P.; Liu, L.M.; Han, J.C.; Yang, Q.Q.; Wang, Y.; Li, X.D.; Fu, W.Y.; Gao, H.; Huang, H.S.; et al. Majorbio Cloud 2024: Update Single-Cell and Multiomics Workflows. iMeta 2024, 3, e217. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Cross | Phenotype of F2 | Observed Count (O) | Expected Count (E) | (O-E)2/E | χ2 | Probable Ratio |
|---|---|---|---|---|---|---|
| AK58/eui | Mutation type | 476 | 508 | 2.01575 | 2.704 | 1 |
| Intermediate type | 1035 | 1016 | 0.35531 | 2 | ||
| Wild-type | 521 | 508 | 0.33268 | 1 | ||
| Sum | 2032 | 2032 | 2.70374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Chen, L.; Cao, Z.; Jin, X.; Guo, F.; Shi, Z.; Yang, J.; Lu, J.; Sun, D. Combining Transcriptome and Hormone-Targeted Metabolome Analyses to Dissect the Regulatory Mechanisms Underlying Wheat Peduncle Elongation. Plants 2025, 14, 1611. https://doi.org/10.3390/plants14111611
Hao H, Chen L, Cao Z, Jin X, Guo F, Shi Z, Yang J, Lu J, Sun D. Combining Transcriptome and Hormone-Targeted Metabolome Analyses to Dissect the Regulatory Mechanisms Underlying Wheat Peduncle Elongation. Plants. 2025; 14(11):1611. https://doi.org/10.3390/plants14111611
Chicago/Turabian StyleHao, Huifang, Lu Chen, Zhiyang Cao, Xiujuan Jin, Feng Guo, Zerui Shi, Jinwen Yang, Juan Lu, and Daizhen Sun. 2025. "Combining Transcriptome and Hormone-Targeted Metabolome Analyses to Dissect the Regulatory Mechanisms Underlying Wheat Peduncle Elongation" Plants 14, no. 11: 1611. https://doi.org/10.3390/plants14111611
APA StyleHao, H., Chen, L., Cao, Z., Jin, X., Guo, F., Shi, Z., Yang, J., Lu, J., & Sun, D. (2025). Combining Transcriptome and Hormone-Targeted Metabolome Analyses to Dissect the Regulatory Mechanisms Underlying Wheat Peduncle Elongation. Plants, 14(11), 1611. https://doi.org/10.3390/plants14111611





