Response of Calcium-Dependent Protein Kinase Genes’ Expression in ‘Feizixiao’ Litchi Pulp to Foliar Nutrient Treatment of Calcium–Magnesium Mixed Solution and Their Regulation of Sugar Transformation
Abstract
1. Introduction
2. Results
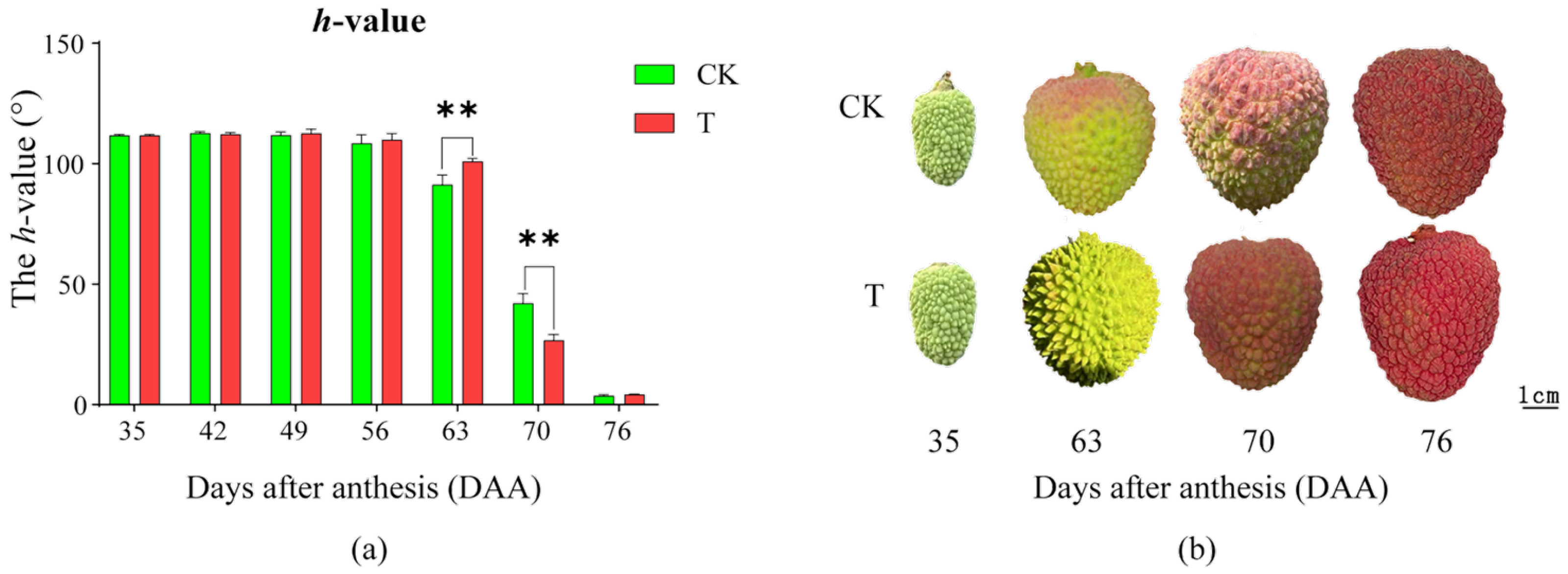
2.1. Effects of the Treatment on Peel Coloration
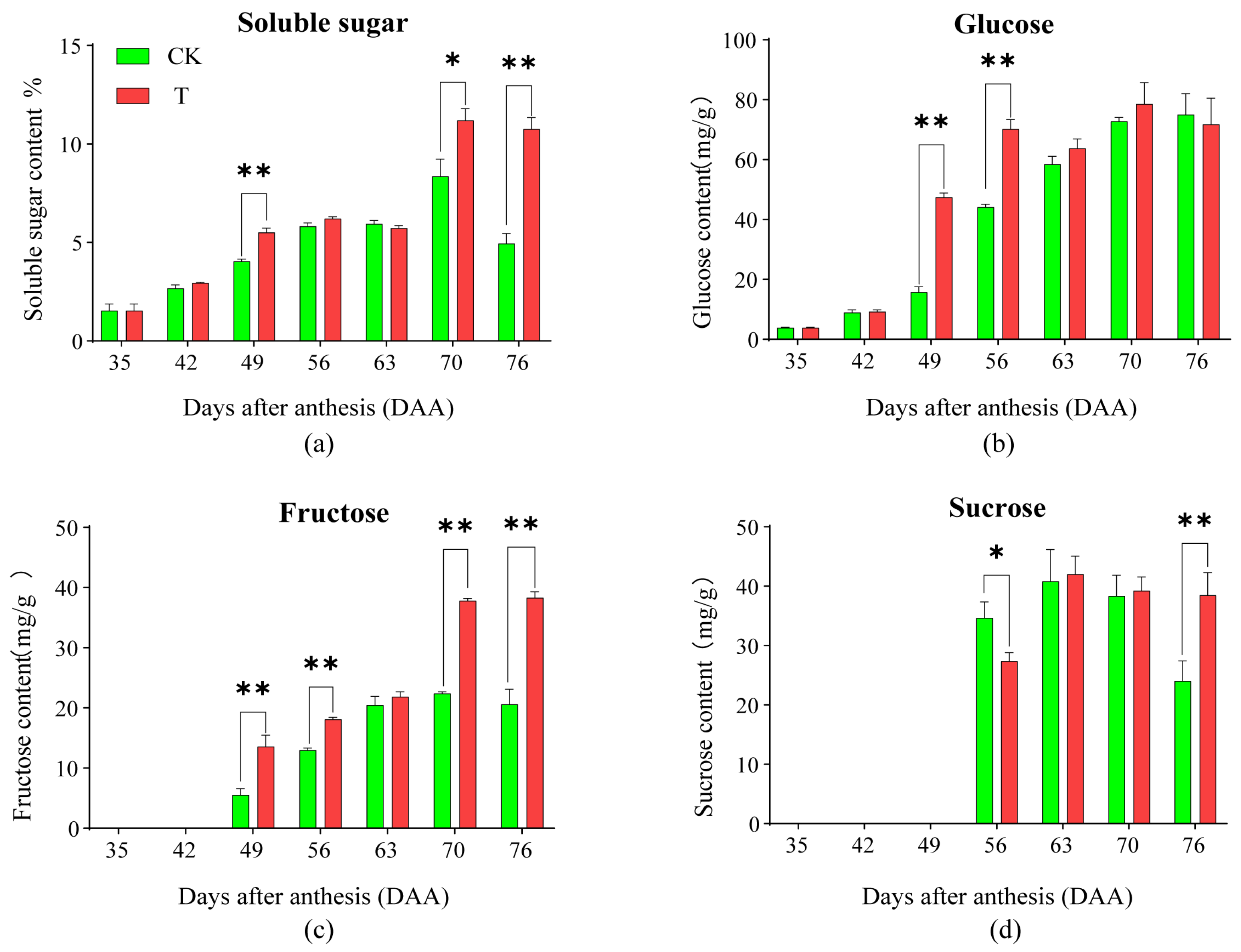
2.2. Effects of the Treatment on the Total of Soluble Sugar and the Contents of Sugar Components in Pulp
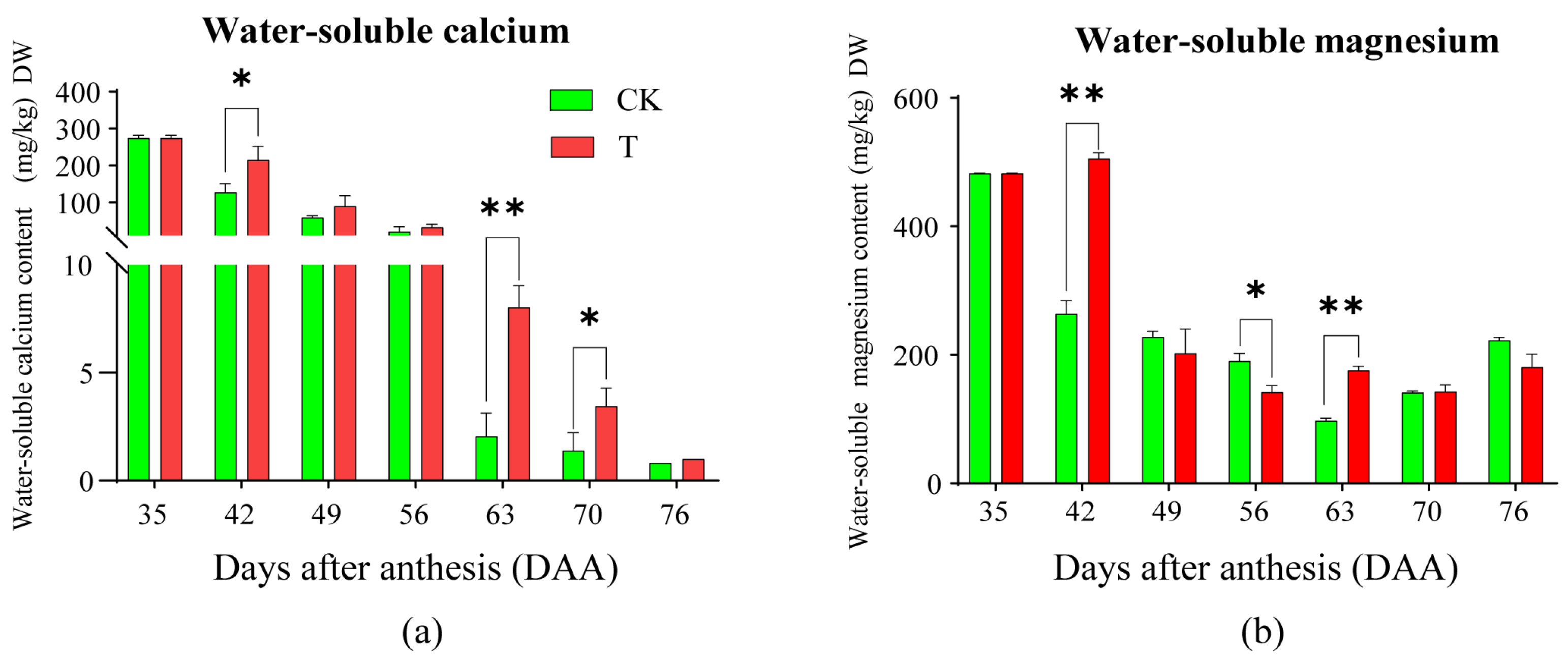
2.3. Effects of the Treatment on Water-Soluble Calcium and Magnesium in Pulp
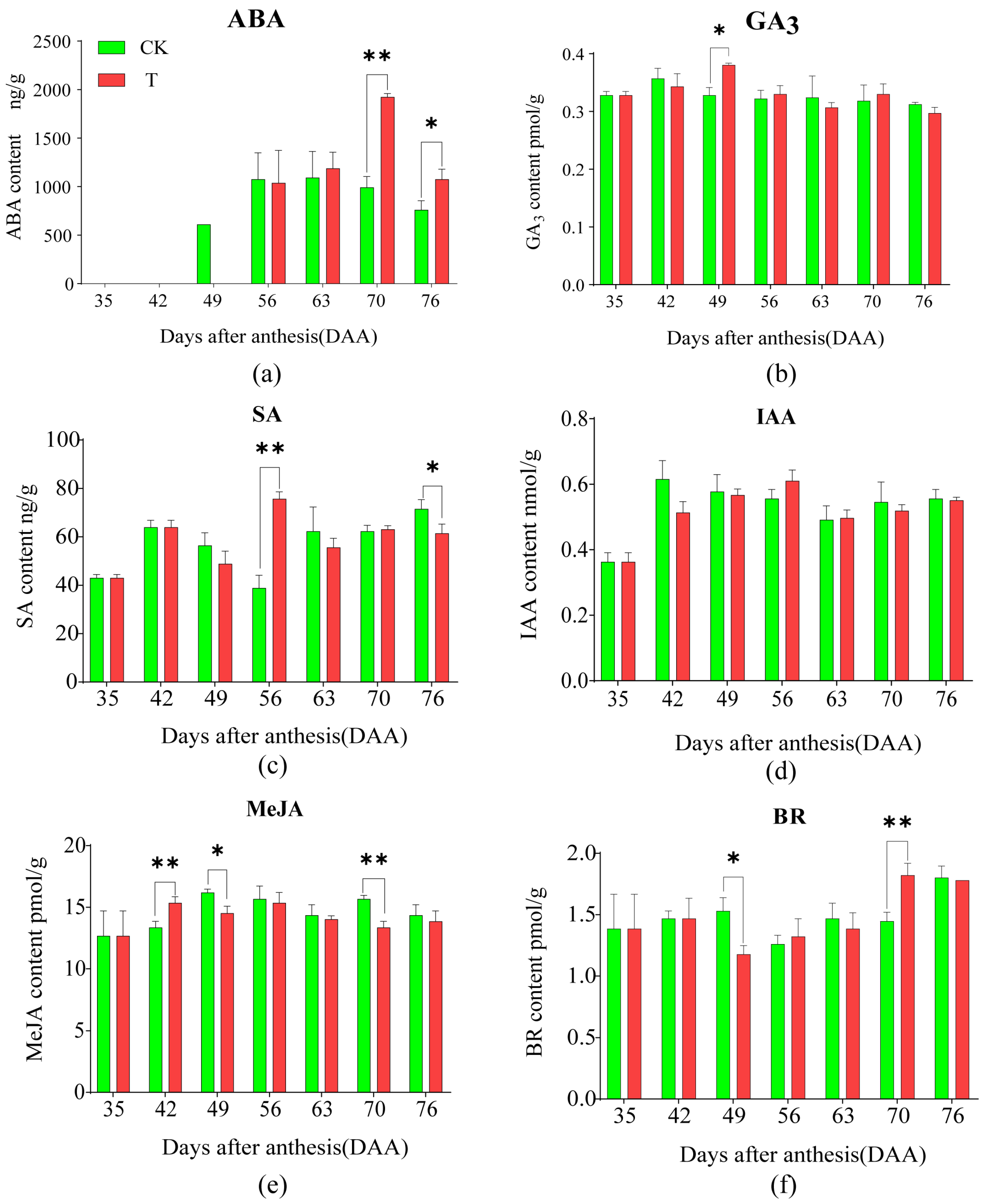
2.4. Effects of the Treatment on the Content of Seven Plant Hormones in Pulp
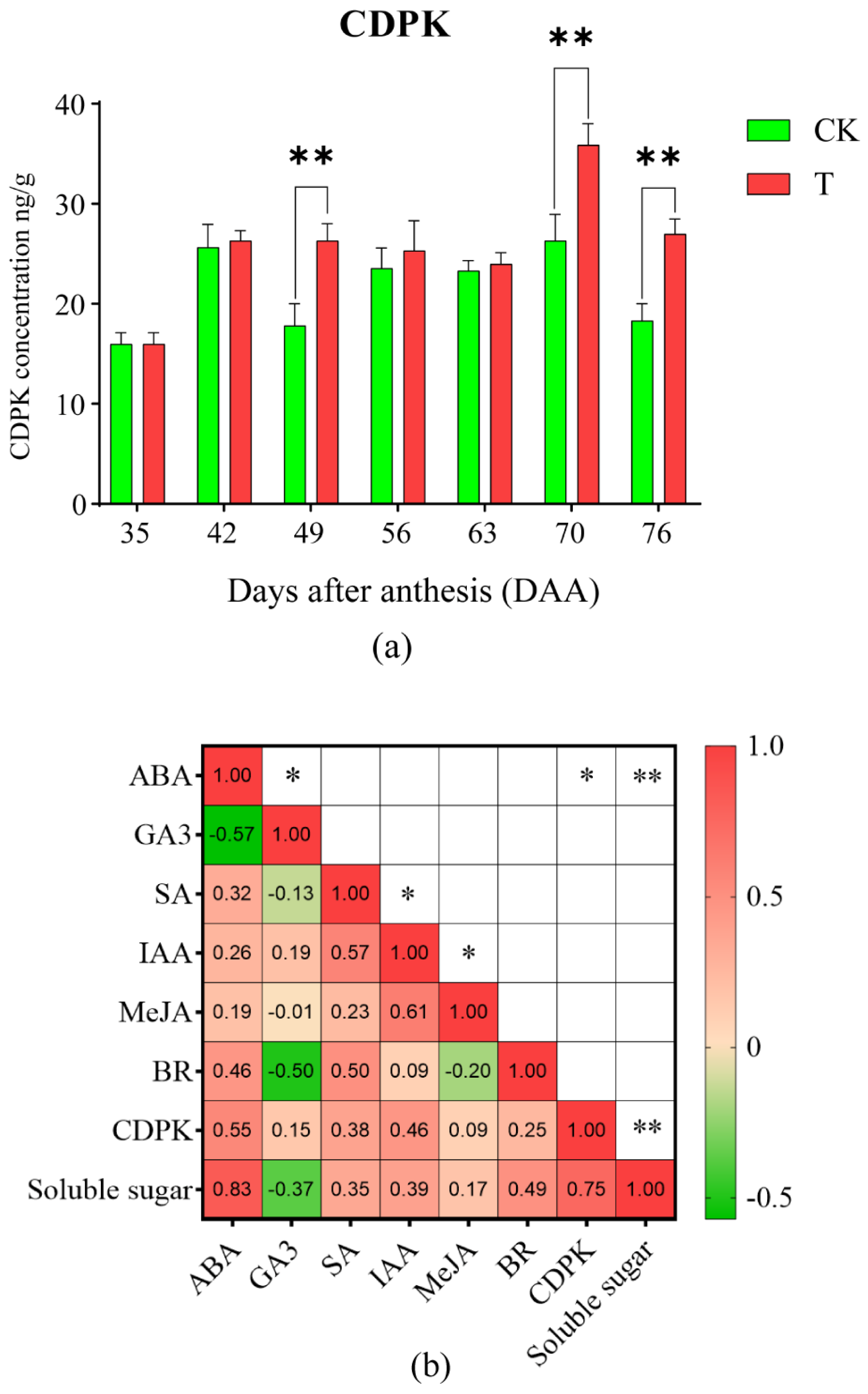
2.5. Effects of the Treatment on the Concentration of CDPK in Litchi Pulp
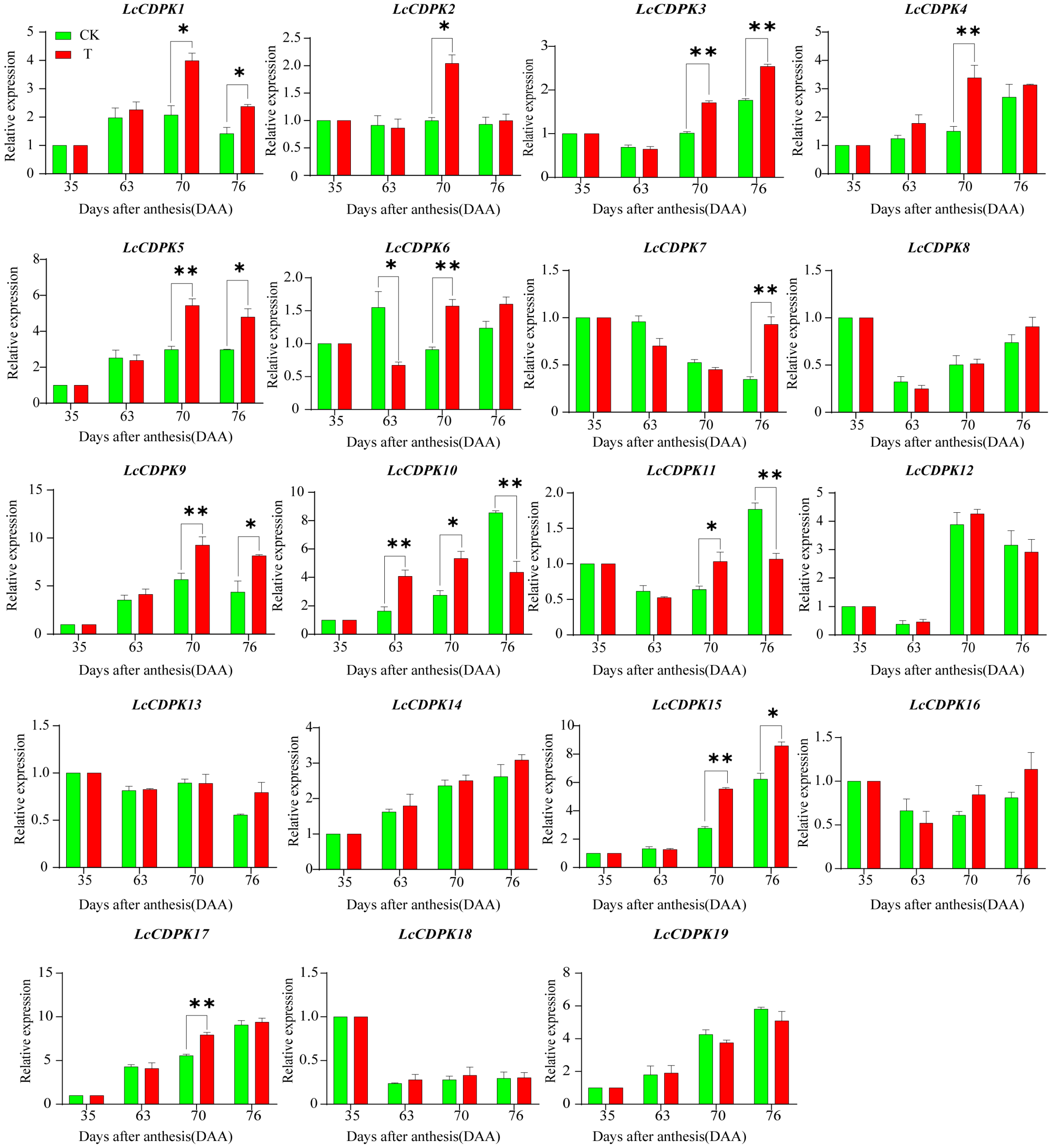
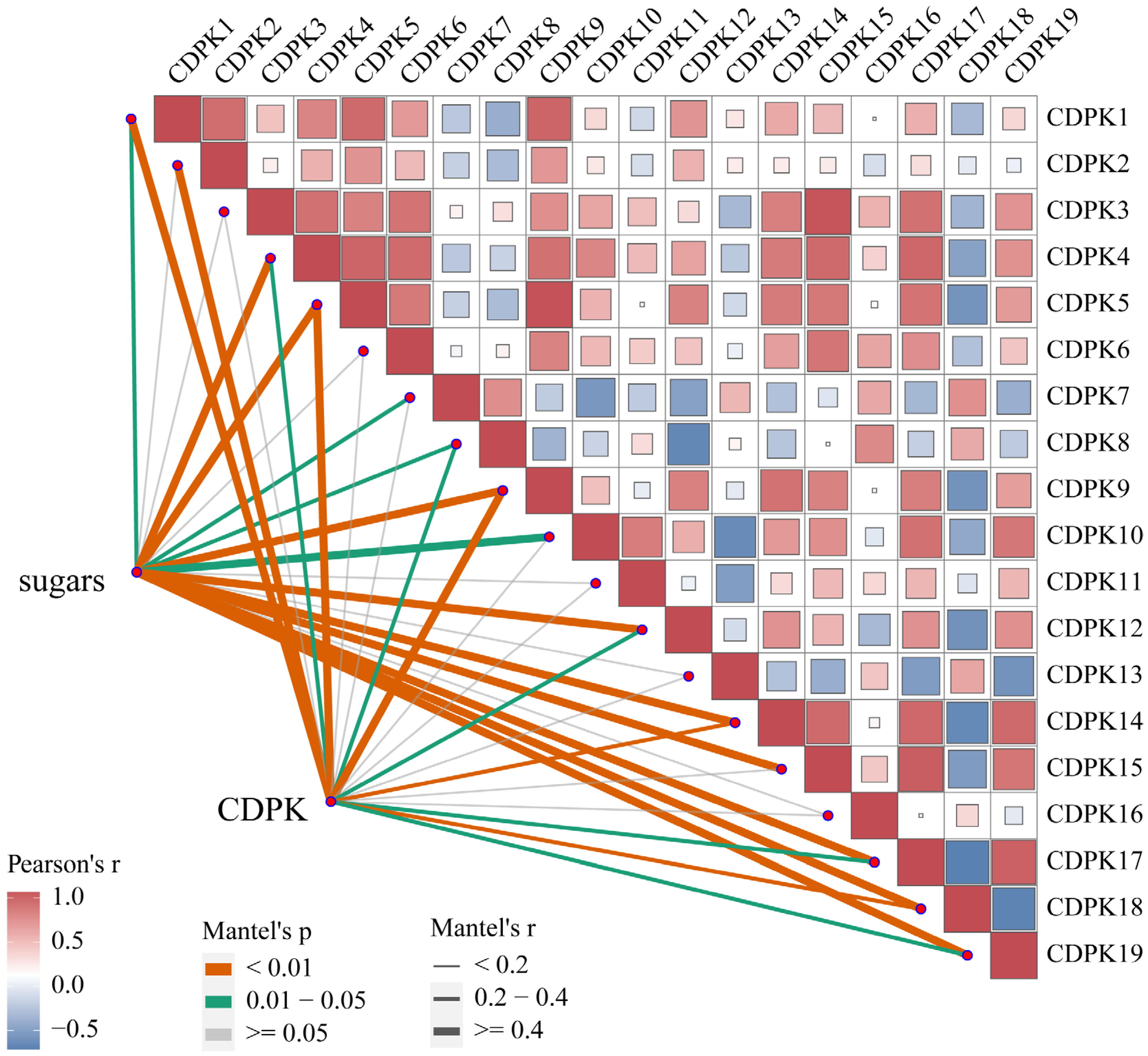
2.6. ‘Feizixiao’ Litchi CDPK Gene Family RT-qPCR Analysis
3. Discussion
3.1. The Treatment to Mitigate the ‘Feiziao’ Litchi Pulp “Sugar Receding” Phenomenon
3.2. Synergistic Regulation of Pulp Sugar Conversion by Foliar Calcium–Magnesium Nutrition
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design, Field Treatment, and Sampling Methods
4.3. Determination Methods of Physiological and Biochemical Indexes
4.3.1. Peel Color
4.3.2. Determination of Sugar Content in Pulps
4.3.3. Water-Soluble Calcium and Magnesium
4.3.4. Determination of Plant Hormones Content and CDPK Concentration
4.4. Real-Time Quantitative PCR Verification
4.5. Statistical Analysis Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDPK | calcium-dependent protein kinases |
| Ca2+ | water-soluble calcium |
| Mg2+ | water-soluble magnesium |
| CK | control group |
| T | treatment group |
| DAA | days after anthesis |
| ABA | abscisic acid |
| EMP-TCA | Embden–Meyerhof–Parnas-tricarboxylic acid |
| PPP | pentose phosphate pathway |
| CP | cytochrome respiration pathway |
| AP | cyanide-resistant respiratory pathways |
| HK | hexokinase |
| Ser/Thr | serine/threonine |
| CAM | calmodulin-like |
| SPS | sucrose-phosphate synthase |
| h | hue angle |
| HPLC | high-performance liquid chromatography |
| ICP-OES | inductively coupled plasma optical emission spectrometry |
| BR | brassinosteroids |
| SA | salicylic acid |
| IAA | auxins |
| GA3 | gibberellins |
| MeJA | methyl jasmonate |
| ELISA | enzyme-linked immunosorbent assay |
| PBS | phosphate-buffered saline |
| SS | sucrose synthase |
| SOX | sorbitol oxidase |
| RT-qPCR | real-time quantitative PCR |
References
- Ni, Y.Y.; Wu, S.F. Lychee Cultivation; Agriculture Press: Beijing, China, 1990; Volume 1, pp. 7–8. (In Chinese) [Google Scholar]
- Zhang, R.; Han, L.T.; Wang, J.; Zhou, K.B. Comparison of Characteristics of Skin Coloring and Sugar and Acid Accumulation in Flesh of Two Litchi Varieties. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2014, 43, 374–378. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.C.; Huang, X.M.; Huang, H.B. A Study on the Causative Factors Retarding Pigmentation in the Fruit of ‘Feizixiao’ Litchi. Acta Hortic. Sin. 2002, 29, 408–412. (In Chinese) [Google Scholar]
- Peng, J.J.; Du, J.J.; Ma, W.Q.; Chen, T.T.; Shui, X.; Liao, H.Z.; Lin, X.K.; Zhou, K.B. Transcriptomics-based Analysis of the Causes of Sugar Receding in Feizixiao Litchi (Litchi chinensis Sonn.) pulp. Front. Plant Sci. 2022, 13, 1083753. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Du, J.J.; Ma, W.Q.; Chen, T.T.; Shui, X.; Zhou, K.B. Effect of Calcium and Magnesium Foliar Fertilizer on Sugar Contents and Sugar Metabolizing Enzyme Activities in ‘Feizixiao’ Litchi Pulp. J. Trop. Biol. 2024, 15, 217–223. [Google Scholar] [CrossRef]
- Sajjad, M.; Tahir, H.; Ma, W.; Shaopu, S.; Farooq, M.A.; Zeeshan Ul Haq, M.; Sajad, S.; Zhou, K. Effects of Foliar Ca and Mg Nutrients on the Respiration of ‘Feizixiao’ Litchi Pulp and Identification of Differential Expression Genes Associated with Respiration. Agronomy 2024, 14, 1347. [Google Scholar] [CrossRef]
- Shi, S.; Du, J.; Peng, J.; Zhou, K.; Ma, W. The Effects of Mixed Foliar Nutrients of Calcium and Magnesium on the Major Bypass Respiratory Pathways in the Pulp of ‘Feizixiao’ Litchi. Horticulturae 2024, 10, 248. [Google Scholar] [CrossRef]
- Florencia, M.P.; David, P.; Carmen, M. Non-Climacteric Fruit Development and Ripening Regulation. The Phytohormones Show. J. Exp. Bot. 2023, 74, 6237–6253. [Google Scholar] [CrossRef]
- Himmelbach, A.; Yang, Y.; Grill, E. Relay and Control of Abscisic Acid Signaling. Curr. Opin. Plant Biol. 2023, 6, 470–479. [Google Scholar] [CrossRef]
- Fan, L.M.; Zhao, Z.X.; Assmann, S.M. Guard Cells: A Dynamic Signaling Model. Curr. Opin. Plant Biol. 2004, 7, 537–546. [Google Scholar] [CrossRef]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium Signaling Through Protein Kinases. The Arabidopsis Calcium-Dependent Protein Kinase Gene Family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef]
- Yip Delormel, T.; Boudsocq, M. Properties and Functions of Calcium-Dependent Protein Kinases and Their Relatives in Arabidopsis Thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2(-Delta Delta C(T)). Method Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Edel, H.K.; Kudla, J. Integration of Calcium and Aba Signaling. Curr. Opin. Plant Biol. 2016, 33, 83–91. [Google Scholar] [CrossRef]
- Li, J.M. Purification, Sequencing and Subcellular Localization of an Abscisicacid-Activated Calcium-Dependent Protein Kinase Firom Grape Berry; China Agricultural University: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Toroser, D.; Huber, S.C. Protein Phosphorylation as a Mechanism for Osmotic-Stress Activation of Sucrose-Phosphate Synthase in Spinach Leaves. Plant Physiol. 1997, 114, 947–955. [Google Scholar] [CrossRef]
- Shui, X.; Wang, W.; Ma, W.; Yang, C.; Zhou, K. Mechanism by Which High Foliar Calcium Contents Inhibit Sugar Accumulation in Feizixiao Lychee Pulp. Horticulturae 2022, 8, 1044. [Google Scholar] [CrossRef]
- He, Z.; Shang, X.; Jin, X.; Wang, X.; Xing, Y. Calcium and Magnesium Regulation of Kernel Sugar Content in Maize: Role of Endogenous Hormones and Antioxidant Enzymes. Int. J. Mol. Sci. 2024, 26, 200. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of Spraying Calcium Fertilizer on Photosynthesis, Mineral Content, Sugar–Acid Metabolism and Fruit Quality of Fuji Apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]
- Zhu, C.; Jing, B.; Lin, T.; Li, X.; Zhang, M.; Zhou, Y.; Yu, J.; Hu, Z. Phosphorylation of Sugar Transporter Tst2 by Protein Kinase Cpk27 Enhances Drought Tolerance in Tomato. Plant Physiol. 2024, 195, 1005–1024. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, K.; Wang, Y.C. Analysis of the Calcium-Dependent Protein Kinase Rtcdpk16 Response to Abiotic Stress In Reaumuria Trigyna. Acta Prataculturae Sin. 2023, 32, 97–109. (In Chinese) [Google Scholar] [CrossRef]
- Liese, A.; Romeis, T. Biochemical Regulation of in Vivo Function of Plant Calcium-Dependent Protein Kinases (CDPK). Biochim. Biophys. Acta 2013, 1833, 1582–1589. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.J.; Li, X.D.; Chen, Y.M. The Functional Roles of Calcium-Dependent Protein Kinases in Plant Growth and Stress Response. Chin. Sci. Bull. 2024, 69, 1082–1095. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, L.; Cassan-Wang, H.; Zhao, Y.; Bao, Y.; Hou, Y.; Liu, Y.; Wu, Z.; Bouzayen, M.; Zheng, Y.; Jin, P. Calcium-Dependent Protein Kinase Ppcdpk29-Mediated Ca2+-ROS Signal and Pphsfa2a Phosphorylation Regulate Postharvest Chilling Tolerance of Peach Fruit. Plant Biotechnol. 2025, 27. Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Wang, L.; Liu, X.; Yu, Z.; Liu, J.; Wu, W.; Ding, L.; Xia, C.; Zhang, L.; et al. Tabzip60 is Involved in the Regulation of Aba Synthesis-Mediated Salt Tolerance Through Interacting with Tacdpk30 In Wheat (Triticum aestivum L.). Planta 2023, 257, 107. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Xu, J.; Hendrich, C.; Pandey, S.S.; Yu, Q.; Gmitter, F.G., Jr.; Wang, N. Seasonal Transcriptome Profiling of Susceptible and Tolerant Citrus Cultivars to Citrus Huanglongbing. Phytopathology 2023, 113, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Zhao, W.Y. Experiment Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007; pp. 101–115. (In Chinese) [Google Scholar]
- Wang, J.; Wang, Q.; Xiang, W.S. Application of Chromatography to the Analysis of Carbohydrates. Chin. J. Anal. Chem. 2001, 29, 222–227. (In Chinese) [Google Scholar]
- Liu, H.L.; Yan, J.; Jiang, Y.H.; Shi, F.C.; Chen, J.Z.; Cai, C.H.; Ou, L.X. Identification of LcCDPKs and Analysis of Their Expression Patterns in Re-Sponse to Downy Mildew Stresses in Lychee. J. Fruit Sci. 2023, 40, 442–456. (In Chinese) [Google Scholar] [CrossRef]
| Primer Name | Gene ID | Sequence (5′-3′) | Primer Name | Gene ID | Sequence (5′-3′) |
|---|---|---|---|---|---|
| Actin_F | DQ990337.1 | TTGGATTCTGGTGATGGTGTG | LcCDPK10_F | LITCHI015851 | GGAACTCTGGACTGTGACGA |
| Actin_R | DQ990337.1 | CAGCAAGGTCCAACCGAAG | LcCDPK10_R | LITCHI015851 | CATCGTTGGGGCTGGAAATT |
| LcCDPK1_F | LITCHI001674 | GAGGAGGAGGAGGAGGAAGA | LcCDPK11_F | LITCHI019005 | GTGTGGAGAAAGGGAGTGGA |
| LcCDPK1_R | LITCHI001674 | TCTTGGCTTTGGGGATGACT | LcCDPK11_R | LITCHI019005 | ATCGAATAGCTCCCCACCTG |
| LcCDPK2_F | LITCHI002195 | TTTGATGCGGTGTTGAAGGG | LcCDPK12_F | LITCHI019021 | ATGTGGCCCCAGAAGTGTTA |
| LcCDPK2_R | LITCHI002195 | TCTGTCAGGAGCAACACCAT | LcCDPK12_R | LITCHI019021 | GTCAACCGCTTCTTGGGATC |
| LcCDPK3_F | LITCHI006474 | CAAGGCCAATGGAGATCGTG | LcCDPK13_F | LITCHI022010 | AAAACGAGCATCTGGAGCAC |
| LcCDPK3_R | LITCHI006474 | AGCAATTCACCTCCCTCACA | LcCDPK13_R | LITCHI022010 | GCGCAAATTCCCCGAAAATG |
| LcCDPK4_F | LITCHI006812 | ACATGCTCGTTGTGCCTATG | LcCDPK14_F | LITCHI022230 | CACACACGCATCACTGACAA |
| LcCDPK4_R | LITCHI006812 | TTTGCAAGGACTCGGCTAGA | LcCDPK14_R | LITCHI022230 | TATCATCACCTCACGTCGCA |
| LcCDPK5_F | LITCHI007334 | GGCAAGAAACTGGGTCAAGG | LcCDPK15_F | LITCHI023733 | TCGGTAGAGGGCAATTTGGT |
| LcCDPK5_R | LITCHI007334 | ACTCTCCTCCCTCACAAAGC | LcCDPK15_R | LITCHI023733 | CGCACAACTCCATCACCAAA |
| LcCDPK6_F | LITCHI008906 | GCGGAATTATGGCCCAGAAG | LcCDPK16_F | LITCHI023923 | ATGGACACTGGCAAGAGAGG |
| LcCDPK6_R | LITCHI008906 | GGGATCAGGGTCAAGCATCT | LcCDPK16_R | LITCHI023923 | GGTGAACTGAAACCGCAACA |
| LcCDPK7_F | LITCHI010062 | CAACATGCACCAAAACAGCC | LcCDPK17_F | LITCHI027028 | ACTTGGTGTTGGAGTTGTGC |
| LcCDPK7_R | LITCHI010062 | TTCCCAGTTGCCTTCTCAGT | LcCDPK17_R | LITCHI027028 | CTCCGGCTTCAAGTCCCTAT |
| LcCDPK8_F | LITCHI011639 | GTGGTGCAGATCAAAGGGAC | LcCDPK18_F | LITCHI027050 | ATTGTTGGGGTTGTGGAAGC |
| LcCDPK8_R | LITCHI011639 | ATGCATGACCCCAAGAGAGT | LcCDPK18_R | LITCHI027050 | GCTGCCAACCACATCAGAAA |
| LcCDPK9_F | LITCHI014137 | CAACAGGGAGGAAGTTTGCC | LcCDPK19_F | LITCHI029637 | GCTCCTGAAGTATTGCGTCG |
| LcCDPK9_R | LITCHI014137 | CCAGCACACAACTCCATCAC | LcCDPK19_R | LITCHI029637 | TTCCTGACTAGCTCCTTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Wei, L.; Shi, S.; Gao, Y.; Jiang, C.; Sajjad, M.; Zhou, K. Response of Calcium-Dependent Protein Kinase Genes’ Expression in ‘Feizixiao’ Litchi Pulp to Foliar Nutrient Treatment of Calcium–Magnesium Mixed Solution and Their Regulation of Sugar Transformation. Plants 2025, 14, 1583. https://doi.org/10.3390/plants14111583
Jiao J, Wei L, Shi S, Gao Y, Jiang C, Sajjad M, Zhou K. Response of Calcium-Dependent Protein Kinase Genes’ Expression in ‘Feizixiao’ Litchi Pulp to Foliar Nutrient Treatment of Calcium–Magnesium Mixed Solution and Their Regulation of Sugar Transformation. Plants. 2025; 14(11):1583. https://doi.org/10.3390/plants14111583
Chicago/Turabian StyleJiao, Jiabing, Ling Wei, Shaopu Shi, Yijia Gao, Chenyu Jiang, Muhammad Sajjad, and Kaibing Zhou. 2025. "Response of Calcium-Dependent Protein Kinase Genes’ Expression in ‘Feizixiao’ Litchi Pulp to Foliar Nutrient Treatment of Calcium–Magnesium Mixed Solution and Their Regulation of Sugar Transformation" Plants 14, no. 11: 1583. https://doi.org/10.3390/plants14111583
APA StyleJiao, J., Wei, L., Shi, S., Gao, Y., Jiang, C., Sajjad, M., & Zhou, K. (2025). Response of Calcium-Dependent Protein Kinase Genes’ Expression in ‘Feizixiao’ Litchi Pulp to Foliar Nutrient Treatment of Calcium–Magnesium Mixed Solution and Their Regulation of Sugar Transformation. Plants, 14(11), 1583. https://doi.org/10.3390/plants14111583






