Alfalfa Photosynthesis Under Partial Root-Zone Drying: Diurnal Patterns and Its Non-Stomatal Limitations
Abstract
1. Introduction
2. Results
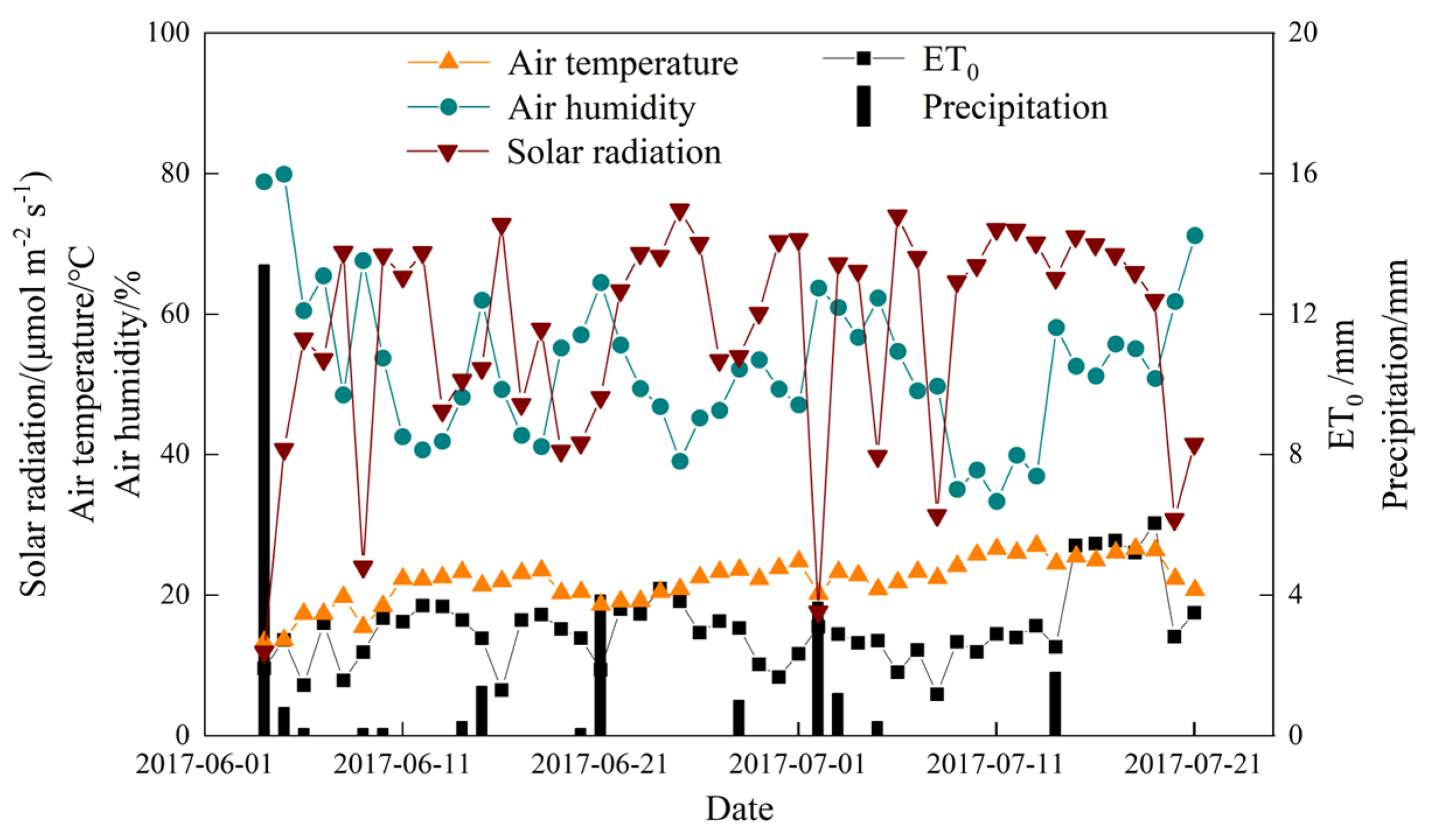
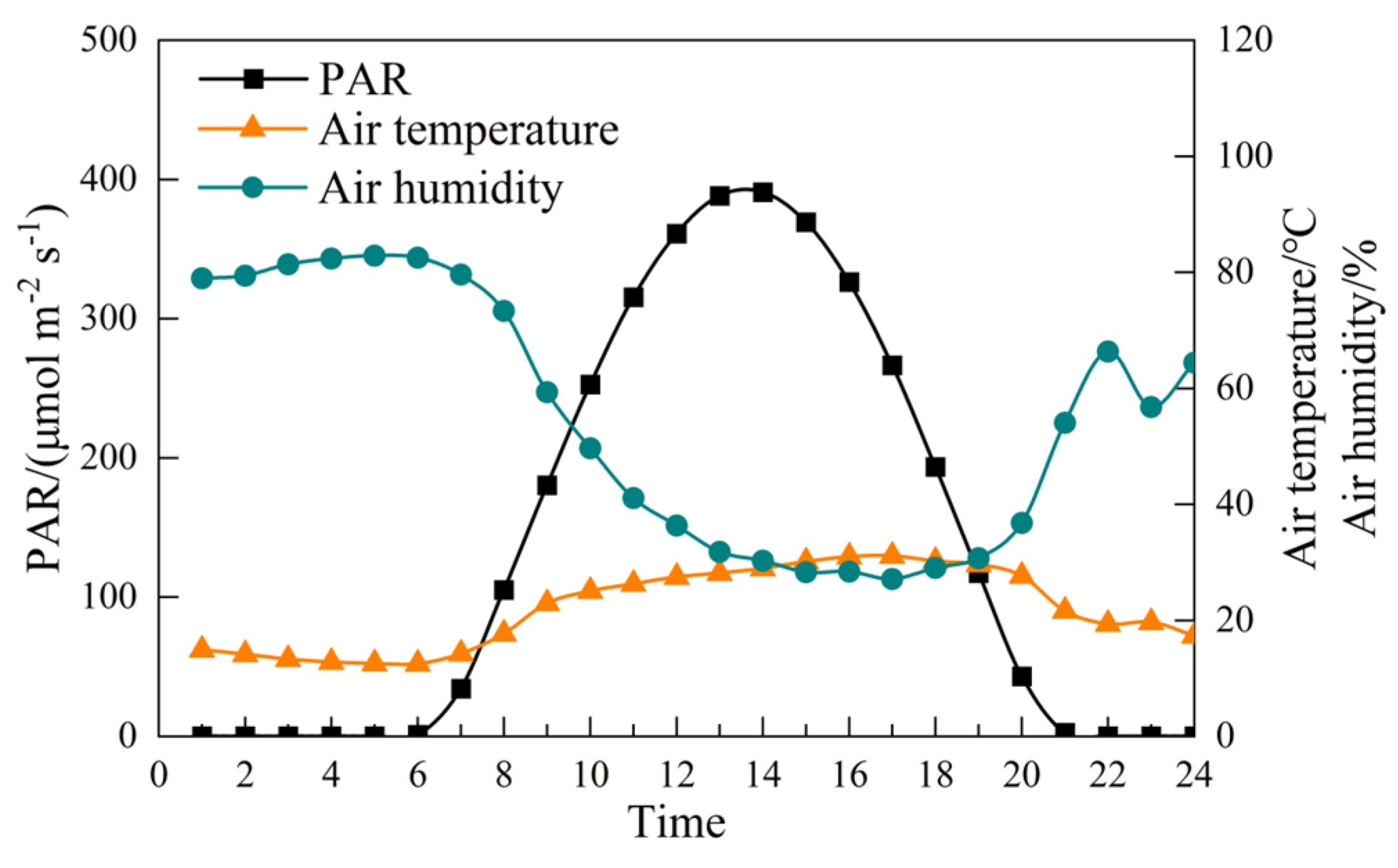
2.1. Meteorological Data
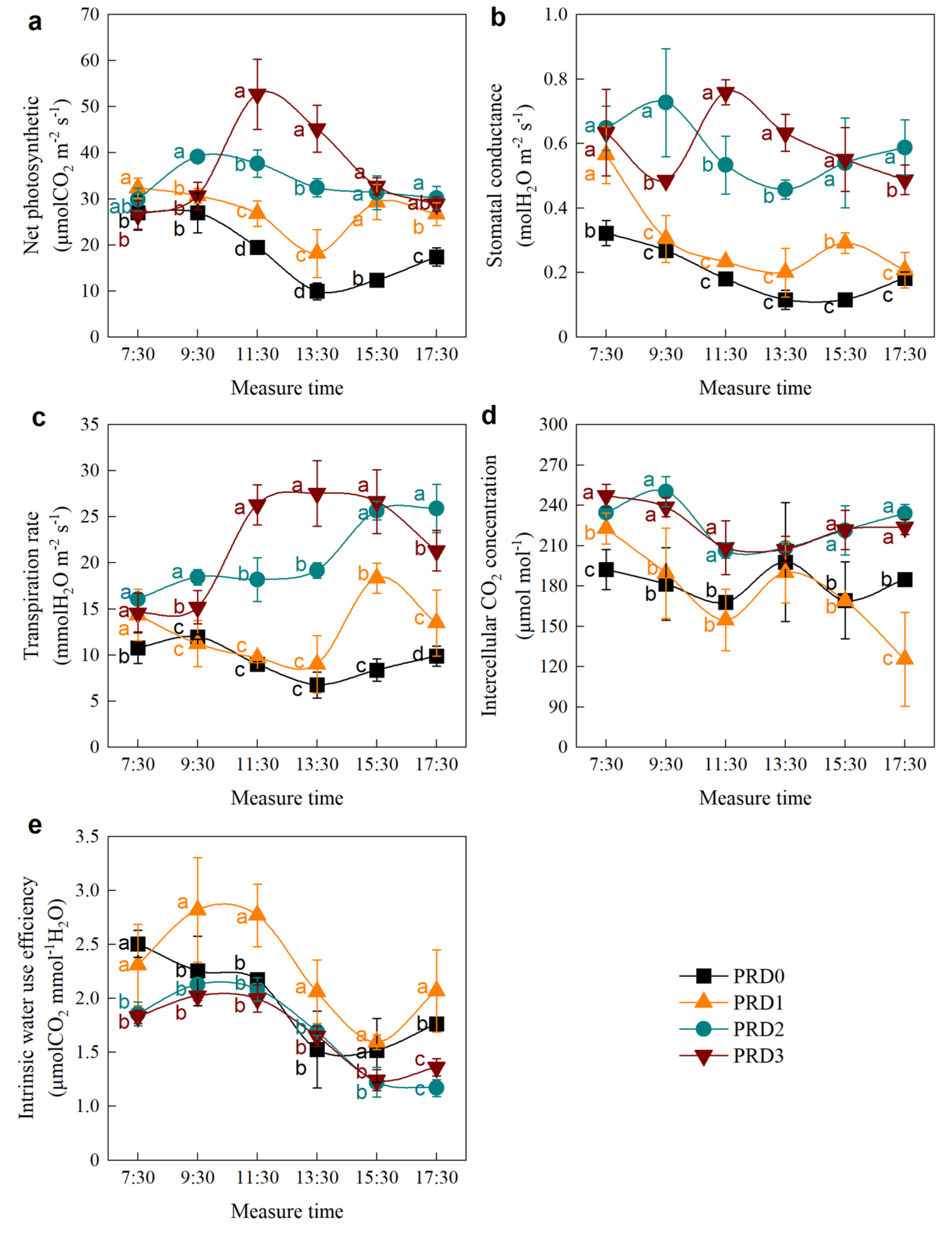
2.2. Photosynthesis
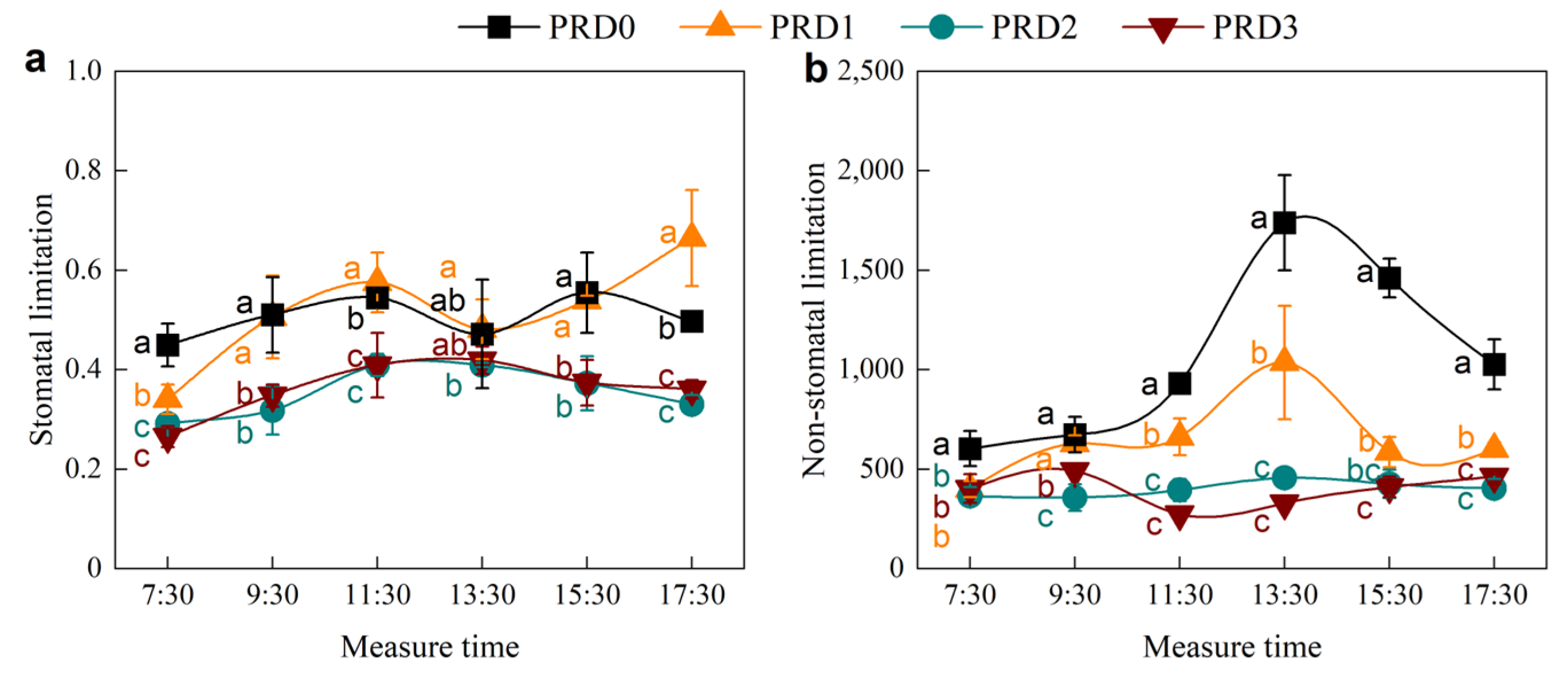
2.3. Stomatal and Non-Stomatal Limitation
2.4. Soil Moisture
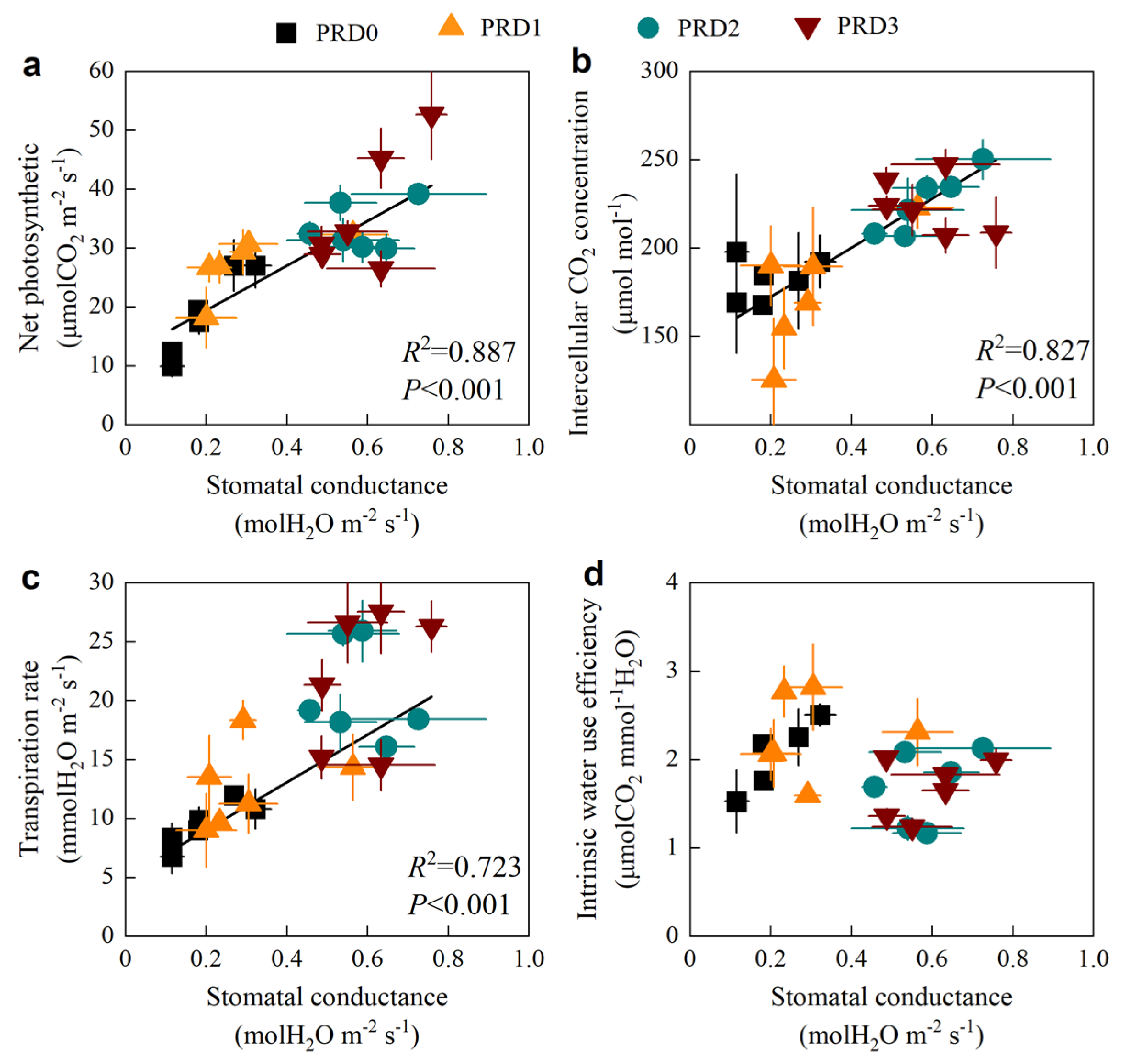
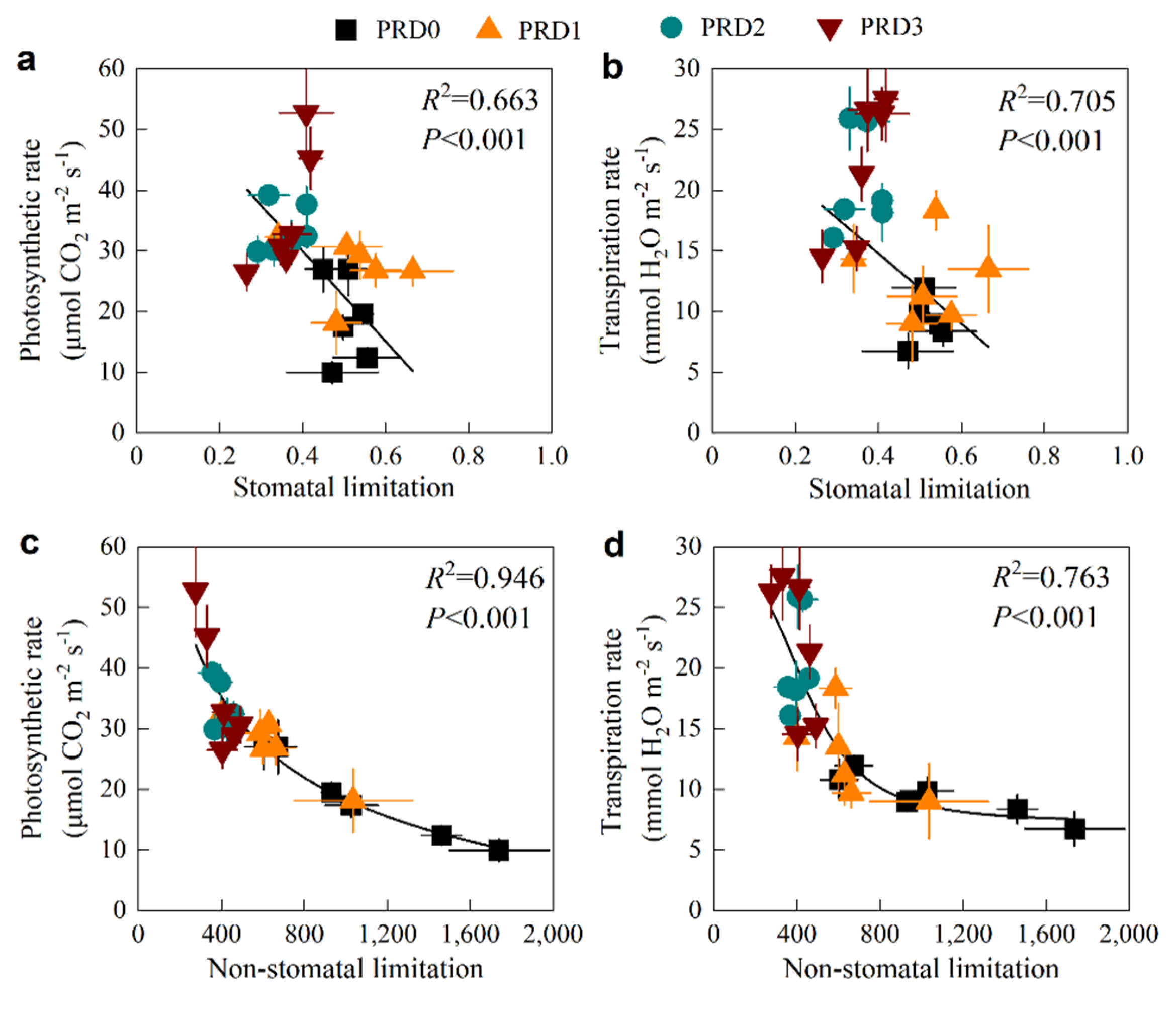
2.5. Relationship Between Soil Moisture and Photosynthetic Parameters
2.6. Hay Yield and Water Productivity
3. Discussion
4. Materials and Methods
4.1. Experimental Location
4.2. Subsurface Drip Irrigation Design for Partial Root-Zone Drying
4.3. Experimental Design
4.4. Site Management
4.5. Measurements
4.5.1. Soil Moisture
4.5.2. Alfalfa Photosynthetic Characteristics
4.5.3. Hay Yield and Water Productivity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holbrook, N.M.; Shashidhar, V.R.; James, R.A.; Munns, R. Stomatal Control in Tomato with ABA-deficient Roots: Response of Grafted Plants to Soil Drying. J. Exp. Bot. 2002, 53, 1503–1514. [Google Scholar] [PubMed]
- Puglisi, I.; Nicolosi, E.; Vanella, D.; Piero, A.; Stagno, F.; Saitta, D.; Roccuzzo, G.; Consoli, S.; Baglieri, A. Physiological and Biochemical Responses of Orange Trees to Different Deficit Irrigation Regimes. Plants 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.J.; Zhang, J. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Einhorn, T.C.; Caspari, H.W.; Green, S. Total Soil Water Content accounts for Augmented ABA Leaf Concentration and Stomatal Regulation of Split-rooted Apple Trees during Heterogeneous Soil Drying. J. Exp. Bot. 2012, 63, 5365–5376. [Google Scholar] [CrossRef]
- Liu, F.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Physiological Responses of Potato (Solanum tuberosum L.) to Partial Root-zone Drying: ABA Signalling, Leaf Gas Exchange, and Water Use Efficiency. J. Exp. Bot. 2006, 57, 3727–3735. [Google Scholar] [CrossRef]
- Ahmad, P. Water Stress and Crop Plants: A Sustainable Approach Chapter 3: Stomatal Responses to Drought Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Xiong, D.; Flexas, J. From One Side to Two Sides: The Effects of Stomatal Distribution on Photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Hu, W.; Loka, D.A.; Yang, Y.; Wu, Z.; Wang, J.; Liu, L.; Wang, S.; Zhou, Z. Partial Root-zone Drying Irrigation Improves Intrinsic Water-use Efficiency and Maintains High Photosynthesis by Uncoupling Stomatal and Mesophyll Conductance in Cotton Leaves. Plant Cell Environ. 2024, 47, 3147–3165. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Z.; Ma, Y.; Liu, J.; Liu, F. Effects of Biochar Amendment and Reduced Irrigation on Growth, Physiology, Water-use Efficiency and Nutrients Uptake of Tobacco (Nicotiana tabacum L.) on Two Different Soil Types. Sci. Total Environ. 2021, 770, 144769. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Z.; Manevski, K.; Liu, J.; Ma, Y.; Andersen, M.N.; Liu, F. Partial Root-zone Drying Irrigation Increases Water-use Efficiency of Tobacco Plants Amended with Biochar. Ind. Crops Prod. 2021, 166, 113487. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Z.; Hou, J.; Wan, H.; Zhang, Q.; Ma, Y.; Liu, F. Partial Root-zone Drying Irrigation Improves Growth and Physiology of Tobacco Amended with Biochar by Modulating Phytohormonal Profile and Antioxidant System. Plant Soil 2022, 474, 561–579. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.; Jensen, C.R.; Liu, F. Alternate Partial Root-zone Irrigation Reduces Bundle-sheath Cell Leakage to CO2 and Enhances Photosynthetic Capacity in Maize Leaves. J. Exp. Bot. 2012, 63, 1145–1153. [Google Scholar] [CrossRef]
- Xu, H.; Qin, F.; Xu, Q.; Tan, J.; Liu, G. Applications of Xerophytophysiology in Plant Production—The Potato Crop improved by Partial Root-zone Drying of Early Season but Not Whole Season. Sci. Hortic. 2011, 129, 528–534. [Google Scholar] [CrossRef]
- Abboud, S.; Dbara, S.; Abidi, W.; Braham, M. Differential Agro-physiological Responses Induced by Partial Root-zone Drying Irrigation in Olive Cultivars Grown in Semi-Arid Conditions. Environ. Exp. Bot. 2019, 167, 103863. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Liu, S.; Huang, X.; Wang, W. Does Partial Root-zone Drying have Advantages over Regulated Deficit Irrigation in Pear Orchard under Desert Climates? Sci. Hortic. 2020, 262, 109099. [Google Scholar] [CrossRef]
- Beauclaire, Q.; Heinesch, B.; Longdoz, B. Non-stomatal Processes are Responsible for the Decrease in Gross Primary Production of a Potato Crop during Edaphic Drought. Agric. For. Meteorol. 2023, 343, 109782. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Zhang, J.; Liu, H.; Guo, H.; Peng, L.; Yin, B.; Zhang, Y.; Yang, C. Erodium oxyrhinchum Sustains Severe Drought Stress by Maintaining Stable Photosynthetic Electron Transport under Progressive Drought Conditions. Environ. Exp. Bot. 2023, 211, 105374. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.X.; Pang, X.P.; Yu, C.; Wang, Q.; Zhou, Y.P.; Lin, L.G.; Guo, Z.G. Effect of Partial Root-zone Drying Irrigation (PRD) on the Gas Exchange and Antioxidant Enzymatic Activities in Alfalfa. J. Soil Sci. Plant Nutr. 2019, 19, 127–136. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Zhang, J.; Li, F.; Hu, X. Yield and Physiological Responses of Cotton to Partial Root-zone Irrigation in the Oasis Field of Northwest China. Agric. Water Manag. 2006, 84, 41–52. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Yan, B.; Zhang, J. Alternate Furrow Irrigation: A Practical Way to Improve Grape Quality and Water Use Efficiency in Arid Northwest China. J. Integr. Agric. 2013, 12, 509–519. [Google Scholar] [CrossRef]
- Fu, Z.; Ciais, P.; Prentice, I.C.; Gentine, P.; Makowski, D.; Bastos, A.; Luo, X.; Green, J.K.; Stoy, P.C.; Yang, H.; et al. Atmospheric Dryness Reduces Photosynthesis Along a Large Range of Soil Water Deficits. Nat. Commun. 2022, 13, 989. [Google Scholar] [CrossRef]
- Peng, J.; Tang, J.; Xie, S.; Wang, Y.; Liao, J.; Chen, C.; Sun, C.; Mao, J.; Zhou, Q.; Niu, S. Evidence for the Acclimation of Ecosystem Photosynthesis to Soil Moisture. Nat. Commun. 2024, 15, 9795. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of Climate Warming on Photosynthesis in Boreal Tree Species Depend on Soil Moisture. Nature 2018, 562, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, X.; Manevski, K.; Li, S.; Wei, Z.; Andersen, M.N.; Liu, F. Physiological and Growth Responses of Potato (Solanum Tuberosum L.) to Air Temperature and Relative Humidity under Soil Water Deficits. Plants 2022, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Requirements; Irrigation and Drainage Paper No. 56; Food and Agricultural Organization of the United Nations: Rome, Italy, 1988. [Google Scholar]
- Romero, P.; Dodd, I.C.; Martinez-Cutillas, A. Contrasting Physiological Effects of Partial Root Zone Drying in Field-grown Grapevine (Vitis vinifera L. Cv. Monastrell) according to Total Soil Water Availability. J. Exp. Bot. 2012, 63, 4071–4083. [Google Scholar]
- Liu, X.; Liu, W.; Su, Z.; Lu, J.; Zhang, P.; Cai, M.; Li, W.; Liu, F.; Andersen, M.N.; Manevski, K. Biochar Addition and Reduced Irrigation Modulates Leaf Morpho-physiology and Biological Nitrogen Fixation in Faba Bean-ryegrass Intercropping. Sci. Total Environ. 2024, 925, 171731. [Google Scholar] [CrossRef]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of Elevated CO2, Temperature and Drought on Dry Matter Partitioning and Photosynthesis Before and After Cutting of Nodulated Alfalfa. Plant Sci. 2006, 170, 1059–1067. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Pérez, P.; Hernández, L.; Irigoyen, J.J.; Zita, G.; Martínez-Carrasco, R.; Sánchez-Díaz, M. The Response of Nodulated Alfalfa to Water Supply, Temperature and Elevated CO2: Photosynthetic Downregulation. Physiol. Plant. 2010, 123, 348–358. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Irigoyen, J.J.; Sánchez-Díaz, M. Effect of Elevated Temperature and Water Availability on CO2 Exchange and Nitrogen Fixation of Nodulated Alfalfa Plants. Environ. Exp. Bot. 2007, 59, 99–108. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Molero, G.; Erice, G.; Avice, J.C.; Nogues, S. Plant Physiology and Proteomics Reveals the Leaf Response to Drought in Alfalfa (Medicago sativa L.). J. Exp. Bot. 2010, 62, 111–123. [Google Scholar] [CrossRef]
- Volaire, F.; Norton, M.R.; Lelièvre, F. Summer Drought Survival Strategies and Sustainability of Perennial Temperate Forage Grasses in Mediterranean Areas. Crop Sci. 2009, 49, 2386–2392. [Google Scholar] [CrossRef]
- Volaire, F. A Unified Framework of Plant Adaptive Strategies to Drought: Crossing Scales and Disciplines. Glob. Change Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M. Mechanisms Underlying Plant Resilience to Water Deficits: Prospects for Water-saving Agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [PubMed]
- Pembleton, K.G.; Rawnsley, R.P.; Donaghy, D.J.; Volenec, J.J. Water Deficit alters Canopy Structure but Not Photosynthesis During the Regrowth of Alfalfa. Crop Sci. 2009, 49, 722–731. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, M.; Li, C.; Li, B.; Zhuo, S.; Yang, Y.; Chen, Y.; Zhong, A.; Liu, H.; Lai, W.; et al. Response Mechanism of Water Status and Photosynthetic Characteristics of Cotoneaster multiflorus under Drought Stress and Rehydrated Conditions. Front. Plant Sci. 2025, 15, 1457955. [Google Scholar] [CrossRef]
- Li, N.N.; Li, J.H.; Shi, X.J.; Shi, F.; Tian, Y.; Wang, J.; Hao, X.Z.; Luo, H.H.; Wang, Z.B. Increasing Cotton Lint Yield and Water Use Efficiency for Subsurface Drip Irrigation without Mulching. Front. Plant Sci. 2024, 15, 1433719. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Tcherkez, G.; Molero, G.; Gilard, F.; Avice, J.C.; Nogues, S. Concerted Changes in N and C Primary Metabolism in Alfalfa (Medicago sativa) under Water Restriction. J. Exp. Bot. 2013, 64, 885–897. [Google Scholar] [CrossRef]
- Nicolodi, C.; Massacci, A.; Marco, G. Water Status Effects on Net Photosynthesis in Field-grown Alfalfa. Crop Sci. 1988, 28, 918–944. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Cui, P.; Su, D. Effects of Partial Root-zone Drying on Alfalfa Growth, Yield and Quality under Subsurface Drip Irrigation. Agric. Water Manag. 2021, 245, 106608. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Gu, Q.; Shi, Y.; Chen, J.; Wu, H.; He, J.; Li, X.; Han, L.; Su, D. Partial Root-zone Drying Subsurface Drip Irrigation Increased the Alfalfa Quality Yield but Decreased the Alfalfa Quality Content. Front. Plant Sci. 2024, 15, 1297468. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, D.; Muneer, M.A.; Fang, G.; Su, D. The Effects of Irrigation Regimes on Soil Moisture Dynamics, Yield and Quality of Lucerne under Subsurface Drip Irrigation. Appl. Ecol. Environ. Res. 2020, 18, 4179–4194. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Gao, K.; Han, L.; Li, X.; He, J.; Su, D. Deficit Irrigation Provides a Trade-Off Between Water Use and Alfalfa Quality. Agronomy 2025, 15, 932. [Google Scholar] [CrossRef]
- Li, Y.; Su, D. Alfalfa Water Use and Yield under Different Sprinkler Irrigation Regimes in North Arid Regions of China. Sustainability 2017, 9, 1380. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Pang, X.P.; Xu, H.P.; Wang, J.; Zhang, W.N.; Guo, Z.G. Effect of Partial Root-zone Drying Irrigation (PRDI) on the Biomass, Water Productivity and Carbon, Nitrogen and Phosphorus Allocations in different Organs of Alfalfa. Agric. Water Manag. 2021, 243, 106525. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Jia, T.T.; Pang, X.P.; Guo, Z.G. Effects of Alternate Furrow Irrigation on the Biomass and Quality of Alfalfa (Medicago sativa). Agric. Water Manag. 2015, 161, 147–154. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, X.P.; Wang, Q.; Yang, D.; Qiao, F.Y.; Zhi, D.G.; Guo, Z.G. PRDI can Maintain Aboveground Biomass and Increase Economic Benefits in Alfalfa through Regulating N:P Ratios in Roots and Leaves. Field Crops Res. 2020, 253, 107821. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.G. Effect of Partial Root-zone Drying Irrigation (PRDI) on Soil C:N:P Stoichiometry in N-fixing Crops: Insights from a Three-year Field Study with Alfalfa. Agric. Water Manag. 2025, 307, 109258. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Cui, Z.; Fang, Y.; He, H.; Liu, B.; Wu, G. Soil Water Storage Deficit of Alfalfa (Medicago sativa) Grasslands along Ages in Arid Area (China). Field Crops Res. 2018, 221, 1–6. [Google Scholar] [CrossRef]
- Berry, J.A.; Downton, W.J.S. Environmental Regulation of Photosynthesis. In Photosynthesis: Development, Carbon Metabolism and Productivity; Academic Press: New York, NY, USA, 1982; Volume Ⅱ, pp. 263–343. [Google Scholar]
- Ramanjulu, S.; Sreenivasulu, N.; Sudhakar, C.S. Effect of Water Stress on Photosynthesis in Two Mulberry Genotypes with Different Drought Tolerance. Photosynthetica 1998, 35, 279–283. [Google Scholar] [CrossRef]
- Fernández, J.E.; Alcon, F.; Diaz-Espejo, A.; Hernandez-Santana, V.; Cuevas, M.V. Water Use Indicators and Economic Analysis for On-farm Irrigation Decision: A Case Study of a Super High Density Olive Tree Orchard. Agric. Water Manag. 2020, 237, 106074. [Google Scholar] [CrossRef]

| Treatments | Degree of Water Deficit | Seedling Irrigation /mm | Irrigation Frequency /No. | Effective Rainfall /mm | Irrigation Quota/mm | Irrigation Time/(M-D) | Irrigation Volume/mm |
|---|---|---|---|---|---|---|---|
| PRD0 | Severe water deficit | 50 a | 5 b | 13.4 | 0 | 17 June, 24 June, 1 July, 8 July, 15 July | 50 |
| PRD1 | Moderate water deficit (45% ET0) | 50 a | 5 b | 13.4 | 10 | 100 | |
| PRD2 | Full irrigaiton (89% ET0) | 50 a | 5 b | 13.4 | 20 | 150 | |
| PRD3 | Over-irrigation (134% ET0) | 50 a | 5 b | 13.4 | 30 | 200 |
| Soil Depth/cm | Photosynthetic Parameters | Regression Coefficient | Coefficient of Determination | ||
|---|---|---|---|---|---|
| y0 | A | R0 | R2 | ||
| 10 | Pn/(μmol m−2 s−1) | 36.6897 | −715.0556 | −0.4910 | 0.9864 |
| Tr/(mmol m−2 s−1) | 23.2425 | −316.3945 | −0.4158 | 0.8571 | |
| Gs/(mmol m−2 s−1) | 0.6295 | −3.7961 | −0.2889 | 0.8462 | |
| 20 | Pn/(μmol m−2 s−1) | 38.5017 | −2479.1211 | −0.4939 | 0.8820 |
| Tr/(mmol m−2 s−1) | 24.6012 | −1189.0500 | −0.4453 | 0.6913 | |
| Gs/(mmol m−2 s−1) | 0.6756 | −10.2782 | −0.3129 | 0.6951 | |
| Treatments | Hay Yield/(kg hm−2) | WP/(kg hm−2 mm−1) | IWP/(kg hm−2 mm−1) |
|---|---|---|---|
| PRD0 | 2752.27 ± 278.09 Dd | 22.81 ± 2.29 Bb | 55.05 ± 5.56 Aa |
| PRD1 | 3546.00 ± 221.22 Cc | 20.88 ± 1.49 Bb | 35.46 ± 2.21 Bb |
| PRD2 | 4356.80 ± 109.02 Bb | 22.91 ± 0.64 Bb | 29.05 ± 0.73 Bc |
| PRD3 | 5825.47 ± 275.44 Aa | 30.50 ± 1.16 Aa | 29.13 ± 1.38 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Q.; Ju, M.; Gao, K.; Han, L.; Li, X.; He, J.; Su, D. Alfalfa Photosynthesis Under Partial Root-Zone Drying: Diurnal Patterns and Its Non-Stomatal Limitations. Plants 2025, 14, 1573. https://doi.org/10.3390/plants14111573
Wang Y, Zhang Q, Ju M, Gao K, Han L, Li X, He J, Su D. Alfalfa Photosynthesis Under Partial Root-Zone Drying: Diurnal Patterns and Its Non-Stomatal Limitations. Plants. 2025; 14(11):1573. https://doi.org/10.3390/plants14111573
Chicago/Turabian StyleWang, Yadong, Qiuchi Zhang, Mingxiu Ju, Kai Gao, Liliang Han, Xingfu Li, Jing He, and Derong Su. 2025. "Alfalfa Photosynthesis Under Partial Root-Zone Drying: Diurnal Patterns and Its Non-Stomatal Limitations" Plants 14, no. 11: 1573. https://doi.org/10.3390/plants14111573
APA StyleWang, Y., Zhang, Q., Ju, M., Gao, K., Han, L., Li, X., He, J., & Su, D. (2025). Alfalfa Photosynthesis Under Partial Root-Zone Drying: Diurnal Patterns and Its Non-Stomatal Limitations. Plants, 14(11), 1573. https://doi.org/10.3390/plants14111573





