Synergistic Effects of Flower Color and Mechanical Barriers on Pollinator Selection Within the Papilionoideae of Fabaceae
Abstract
1. Introduction
2. Results
2.1. Correlations Among Floral Traits and Biomechanics
2.2. Observation of Visiting Insects
2.3. Strength of Pollinators
2.4. Relationship Between Flower Operative Strength and Bee Strength
2.5. Flower Visiting Preferences of Insects
2.6. Relationship Between Flower Color and Mechanistic Screening Mechanisms
3. Discussion
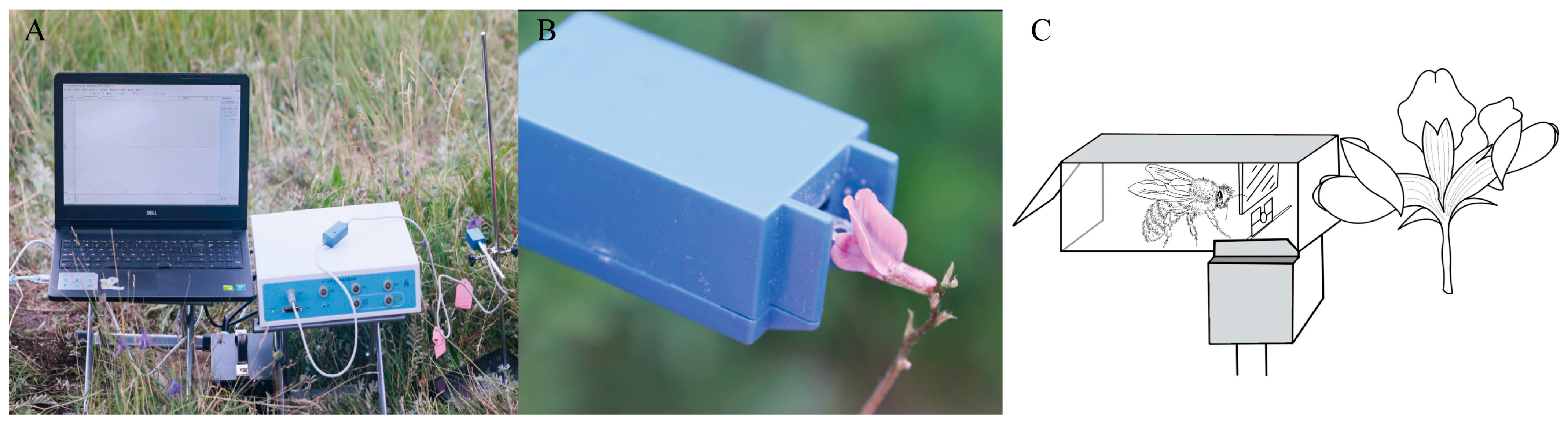
4. Materials and Methods
4.1. Study Species and Locations
4.2. Floral Morphometry and Color Measurement
4.3. Measurement of the Flowers Operative Strength
4.4. Observations of Flower-Visiting Insects and Analysis of Visiting Preferences
4.5. Measurement of the Mass and Strength of Pollinators
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ollerton, J. Pollinator diversity: Distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Artamendi, M.; Martin, P.A.; Bartomeus, I.; Magrach, A. Loss of pollinator diversity consistently reduces reproductive success for wild and cultivated plants. Nat. Ecol. Evol. 2025, 9, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M. The Evolution and Ecology of Floral Color in the Biological Partnership of Flowering Plants and Pollinators; Monash University: Melbourne, Australia, 2013. [Google Scholar]
- Córdoba, S.A.; Cocucci, A.A. Flower power: Its association with bee power and floral functional morphology in papilionate legumes. Ann. Bot. 2011, 108, 919–931. [Google Scholar] [CrossRef] [PubMed]
- van der Kooi, C.J.; Dyer, A.G.; Kevan, P.G.; Lunau, K. Functional significance of the optical properties of flowers for visual signalling. Ann. Bot. 2019, 123, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Trunschke, J.; Lunau, K.; Pyke, G.H.; Ren, Z.-X.; Wang, H. Flower color evolution and the evidence of pollinator-Mediated selection. Front. Plant Sci. 2021, 12, 617851. [Google Scholar] [CrossRef]
- Shen, Y.; Rao, Y.; Ma, M.; Li, Y.; He, Y.; Wang, Z.; Liang, M.; Ning, G. Coordination among flower pigments, scents and pollinators in ornamental plants. Hortic. Adv. 2024, 2, 6. [Google Scholar] [CrossRef]
- Sinha, S.K.; Dolai, A.; Roy, A.B.; Manna, S.; Das, A. The Flower color Influences Spontaneous Nectaring in Butterflies: A Case Study with Twenty Subtropical Butterflies. Neotrop. Entomol. 2023, 52, 1027–1040. [Google Scholar] [CrossRef]
- Shrestha, M.; Dyer, A.G.; Burd, M. Evaluating the spectral discrimination capabilities of different pollinators and their effect on the evolution of flower colors. Commun. Integr. Biol. 2013, 6, 301–310. [Google Scholar] [CrossRef]
- Lunau, K.; Papiorek, S.; Eltz, T.; Sazima, M. Avoidance of achromatic colors by bees provides a private niche for hummingbirds. J. Exp. Biol. 2011, 214, 1607–1612. [Google Scholar] [CrossRef]
- Ehmet, N.; Shao, W.J.; Gao, R.C.; Yang, G.; Xu, Y.-F.; Sun, K.; Hou, Q.-Z. The geographic covariation of functional traits of flowers and pollinators in Delphinium caeruleum. Flora 2025, 326, 152704. [Google Scholar] [CrossRef]
- Cardona, J.; Lara, C.; Ornelas, J.F. Pollinator divergence and pollination isolation between hybrids with different floral color and morphology in two sympatric Penstemon species. Sci. Rep. 2020, 10, 8126. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.Z.; Shao, W.J.; Ehmet, N.; Yang, G.; Zhong, Y.-Q.; Min, W.-R.; Xu, Y.-F.; Gao, R.-C. The biomechanical screening game between visitor power and staminode operative strength of Delphinium caeruleum (Ranunculaceae). Plants 2022, 11, 2319. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Keasar, T. Morphological complexity as a floral signal: From perception by insect pollinators to co-evolutionary implications. Int. J. Mol. Sci. 2018, 19, 1681. [Google Scholar] [CrossRef]
- Brantjes, N.B.M. Floral mechanics in Phlomis (Lamiaceae). Ann. Bot. 1981, 47, 279–282. [Google Scholar] [CrossRef]
- Yang, C.F.; Gituru, R.W.; Guo, Y.H. Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): How does the same pollinator type avoid interspecific pollen transfer? Biol. J. Linn. Soc. 2007, 90, 37–48. [Google Scholar] [CrossRef]
- Lázaro, A.; Vignolo, C.; Santamaría, L. Long corollas as nectar barriers in Lonicera implexa: Interactions between corolla tube length and nectar volume. Evol. Ecol. 2015, 29, 419–435. [Google Scholar] [CrossRef]
- Gurevich, Y.; Hadany, L. Floral complexity can help maintain plant diversity by inducing pollinator specialization. J. Ecol. 2021, 109, 2897–2908. [Google Scholar] [CrossRef]
- Gegear, R.J.; Burns, R.; Swoboda-Bhattarai, K.A. “Hummingbird” floral traits interact synergistically to discourage visitation by bumble bee foragers. Ecology 2017, 98, 489–499. [Google Scholar] [CrossRef]
- Wang, H.; Ran, N.; Jiang, H.Q.; Wang, Q.-Q.; Ye, M.; Bowler, P.A.; Jin, X.-F.; Ye, Z.-M. Complex floral traits shape pollinator attraction to flowering plants in urban greenspaces. Urban For. Urban Green. 2024, 91, 128165. [Google Scholar] [CrossRef]
- Wester, P.; Cairampoma, L.; Haag, S.; Schramme, J.; Neumeyer, C.; Claßen-Bockhoff, R. Bee exclusion in bird-pollinated Salvia flowers: The role of flower color versus flower construction. Int. J. Plant Sci. 2020, 181, 770–786. [Google Scholar] [CrossRef]
- de Souza, L.G.M.P.; Falcão, M.J.A.; Basso-Alves, J.P.; de Freitas Mansano, V. Floral developmental insights into two species of Erythrina (Fabaceae: Papilionoideae: Phaseoleae) pollinated by hummingbirds and passerines. J. Plant Res. 2024, 138, 253–272. [Google Scholar] [CrossRef]
- Córdoba, S.A.; Benitez-Vieyra, S.; Cocucci, A.A. Functional modularity in a forcible flower mechanism: Relationships among morphology, biomechanical features and fitness. Evol. Ecol. 2015, 29, 719–732. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S. Legume-based agroecosystem for sustainable intensification: An overview. In Advances in Legumes for Sustainable Intensification; Academic Press: Cambridge, MA, USA, 2022; pp. 3–8. [Google Scholar]
- Ågren, J. Pollinators, herbivores, and the evolution of floral traits. Science 2019, 364, 122–123. [Google Scholar] [CrossRef]
- Wei, N.; Kaczorowski, R.L.; Arceo-Gómez, G.; O’neill, E.M.; Hayes, R.A.; Ashman, T.-L. Pollinators contribute to the maintenance of flowering plant diversity. Nature 2021, 597, 688–692. [Google Scholar] [CrossRef]
- Naghiloo, S.; Nikzat-Siahkolaee, S.; Esmaillou, Z. Size-matching as an important driver of plant-pollinator interactions. Plant Biol. 2021, 23, 583–591. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, T.; Yang, G.; Shao, W.; Min, W.; Zhong, Y. A Decrease in the Staminode-Mediated Visitor Screening Mechanism in Response to Nectar Robbers Positively Affects Reproduction in Delphinium caeruleum Jacq. ex Camb.(Ranunculaceae). Biology 2022, 11, 1203. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Kessler, A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol. 2010, 188, 393–402. [Google Scholar] [CrossRef]
- Teixido, A.L.; Aizen, M.A. Reproductive assurance weakens pollinator-mediated selection on flower size in an annual mixed-mating species. Ann. Bot. 2019, 123, 1067–1077. [Google Scholar] [CrossRef]
- Lemaitre, A.B.; Pinto, C.F.; Niemeyer, H.M. Generalized pollination system: Are floral traits adapted to different pollinators? Arthropod-Plant Interact. 2014, 8, 261–272. [Google Scholar] [CrossRef]
- Delgado, T.; Leal, L.C.; El Ottra, J.H.L.; Brito, V.L.G.; Nogueira, A. Flower size affects bee species visitation pattern on flowers with poricidal anthers across pollination studies. Flora 2024, 299, 152198. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Schaefer, H.M. Floral color change as a potential signal to pollinators. Curr. Opin. Plant Biol. 2016, 32, 96–100. [Google Scholar] [CrossRef]
- Hirota, S.K.; Nitta, K.; Kim, Y.; Kato, A.; Kawakubo, N.; Yasumoto, A.A.; Yahara, T. Relative role of flower color and scent on pollinator attraction: Experimental tests using F1 and F2 hybrids of daylily and nightlily. PLoS ONE 2012, 7, e39010. [Google Scholar] [CrossRef]
- Koski, M.H. The role of sensory drive in floral evolution. New Phytol. 2020, 227, 1012–1024. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Visual-, olfactory-, and nectar-taste-based flower aposematism. Plants 2024, 13, 391. [Google Scholar] [CrossRef]
- Caruso, C.M.; Eisen, K.E.; Martin, R.A.; Sletvold, N. A meta-analysis of the agents of selection on floral traits. Evolution 2019, 73, 4–14. [Google Scholar] [CrossRef]
- Sletvold, N.; Tye, M.; Ågren, J. Resource-and pollinator-mediated selection on floral traits. Funct. Ecol. 2017, 31, 135–141. [Google Scholar] [CrossRef]
- Rosas-Guerrero, V.; Aguilar, R.; Martén-Rodríguez, S.; Ashworth, L.; Lopezaraiza-Mikel, M.; Bastida, J.M.; Quesada, M. A quantitative review of pollination syndromes: Do floral traits predict effective pollinators? Ecol. Lett. 2014, 17, 388–400. [Google Scholar] [CrossRef]
- Hemingway, C.T.; Leonard, A.S.; MacNeill, F.T.; Pimplikar, S.; Muth, F. Pollinator cognition and the function of complex rewards. Trends Ecol. Evol. 2024, 39, 1047–1058. [Google Scholar] [CrossRef]
- Lagomarsino, L.P.; Condamine, F.L.; Antonelli, A.; Mulch, A.; Davis, C.C. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). New Phytol. 2016, 210, 1430–1442. [Google Scholar] [CrossRef]
- Beentje, H. The Kew Plant Glossary: An Illustrated Dictionary of Plant Identification Terms; Bibliovault OAI Repository: London, UK, 2010. [Google Scholar]
- Attwal, K.; Singh, A. Exploring SPSS statistics for data mining and statistical modeling. Int. J. Stat. Appl. Math. 2024, 9, 9–16. [Google Scholar] [CrossRef]
- da Costa Pina, W.; Bonfim, M.S.; Silva, S.O.; Almeida, I.R.R. Abelhas (Hymenoptera: Apoidea) visitantes das flores de urucum (Bixa orellana Linnaeus 1753) em Teixeira de Freitas, Bahia, Brasil. Sci. Plena 2015, 11, 058001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]

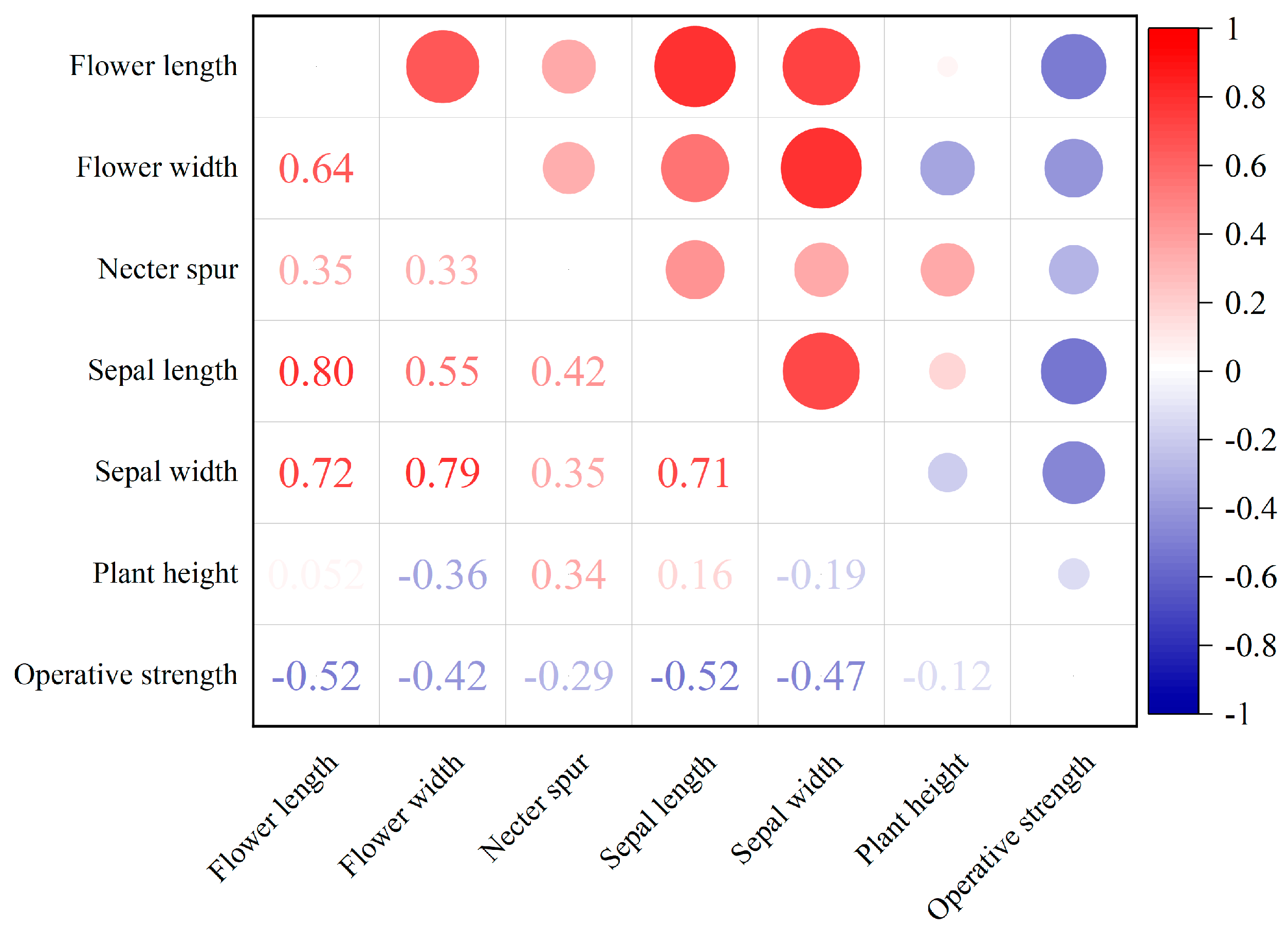


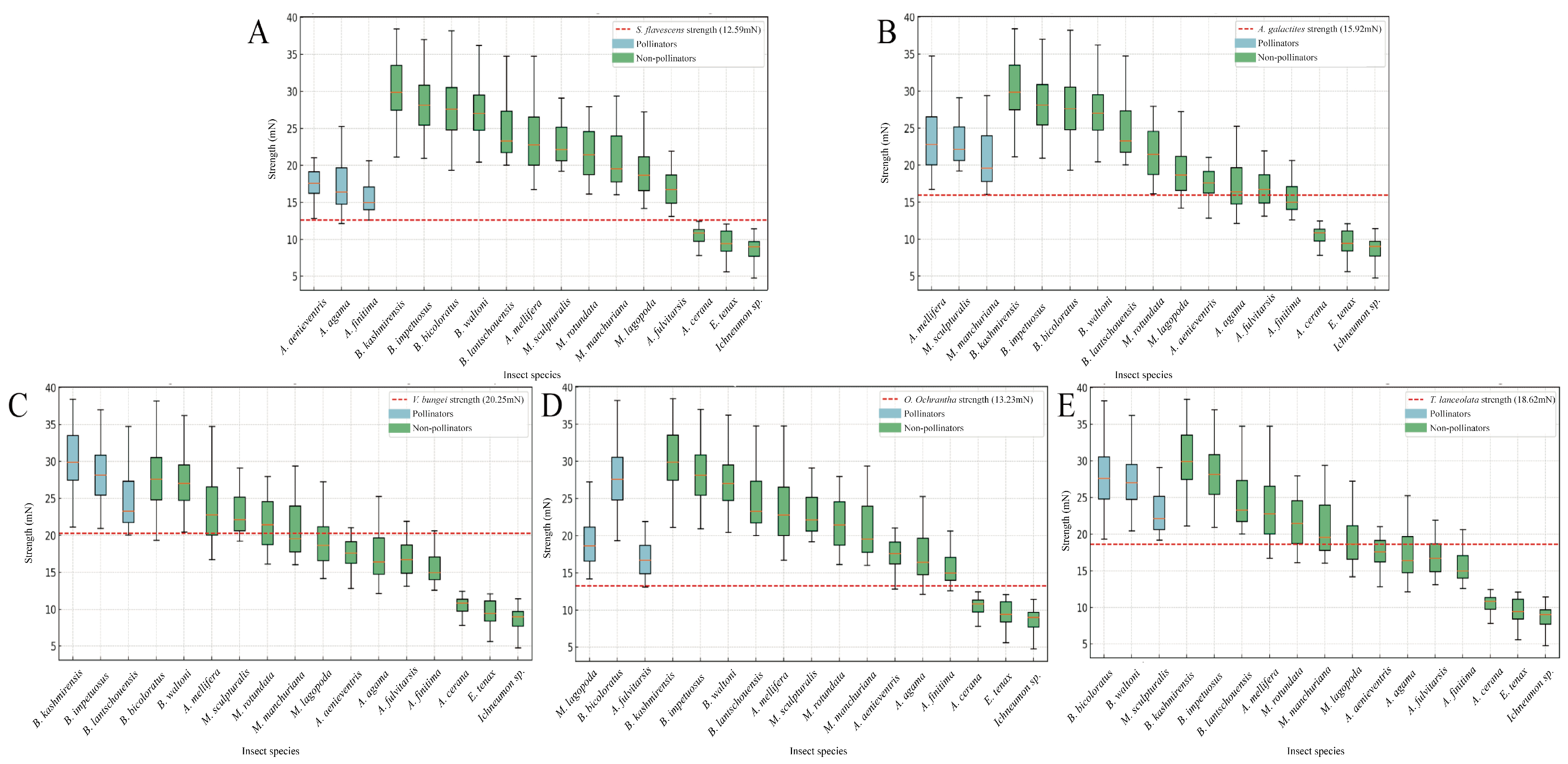

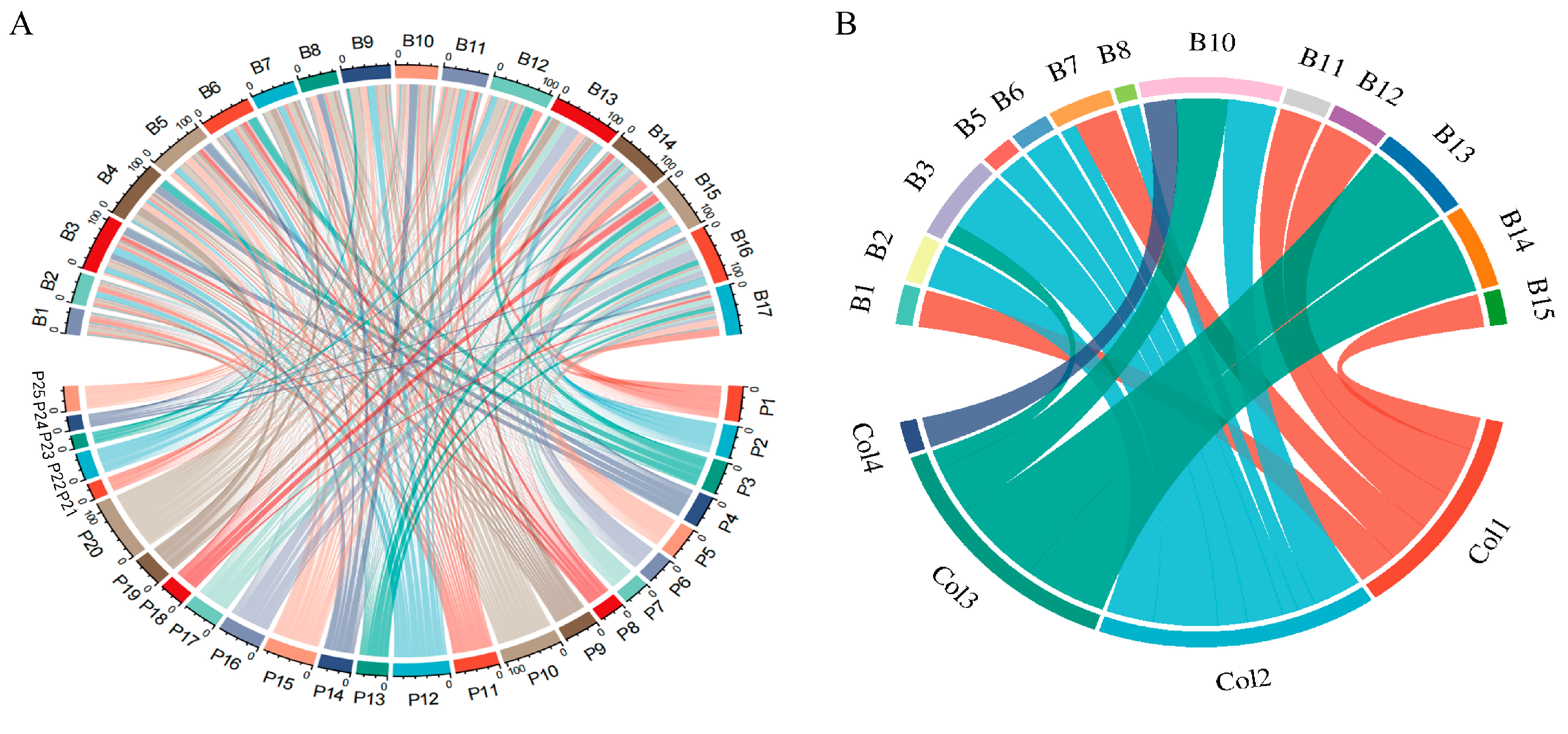
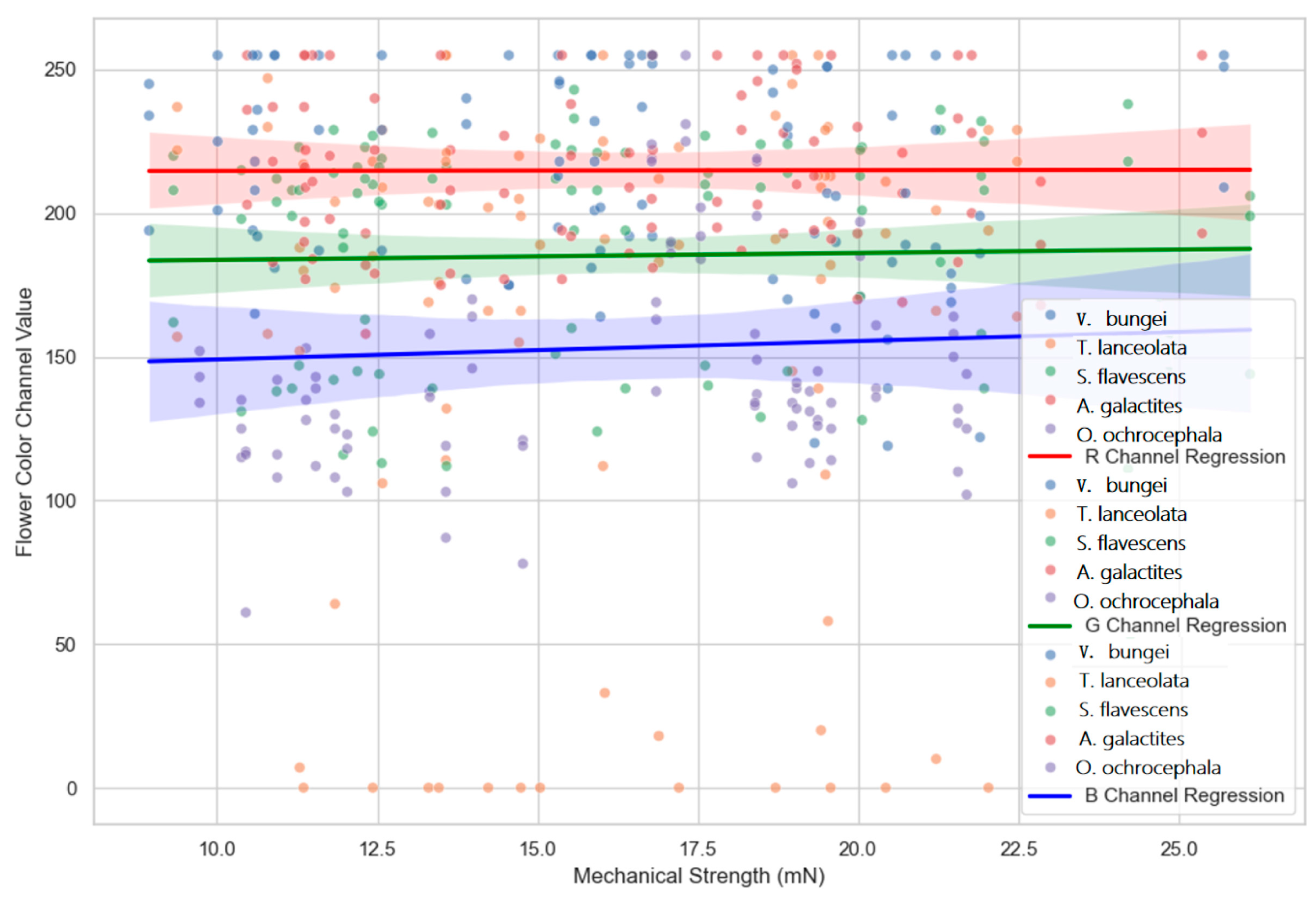


| Species | Flower Length/mm | Flower Width/mm | Nectar Spur/mm | Sepal Length/mm | Sepal Width/mm | Plant Height/mm | Operative Strength/mN |
|---|---|---|---|---|---|---|---|
| Vicia bungei | 16.46 ± 1.07 a | 15.62 ± 1.37 a | 7.41 ± 0.36 a | 13.17 ± 1.40 a | 2.87 ± 0.36 a | 158.2 ± 29.6 d | 20.25 ± 2.88 a |
| Thermopsis lanceolata | 13.98 ± 1.15 b | 9.10 ± 1.04 b | 7.17 ± 0.60 a | 10.95 ± 1.08 b | 1.47 ± 1.59 b | 264.11 ± 35.66 c | 18.62 ± 1.87 b |
| Sophora flavescens | 11.72 ± 1.59 d | 5.57 ± 0.74 e | 5.87 ± 0.79 c | 5.62 ± 0.88 e | 0.82 ± 0.76 c | 409.89 ± 34.34 a | 12.59 ± 2.23 d |
| Astragalus galactites | 12.51 ± 1.47 c | 8.01 ± 0.95 c | 6.79 ± 0.87 b | 9.48 ± 0.96 c | 1.06 ± 0.26 c | 373.66 ± 66.27 b | 15.92 ± 3.43 c |
| Oxytropis ochrantha var. longisepala | 12.24 ± 1.20 c | 6.46 ± 0.88 d | 6.59 ± 1.07 b | 7.00 ± 0.67 d | 0.95 ± 0.24 c | 167.21 ± 18.97 d | 13.23 ± 3.10 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Gao, R.; Bai, J.; Rong, J.; Wei, X.; Wang, H.; Zhu, X.; Sun, K.; Hou, Q. Synergistic Effects of Flower Color and Mechanical Barriers on Pollinator Selection Within the Papilionoideae of Fabaceae. Plants 2025, 14, 1568. https://doi.org/10.3390/plants14111568
Zhao X, Gao R, Bai J, Rong J, Wei X, Wang H, Zhu X, Sun K, Hou Q. Synergistic Effects of Flower Color and Mechanical Barriers on Pollinator Selection Within the Papilionoideae of Fabaceae. Plants. 2025; 14(11):1568. https://doi.org/10.3390/plants14111568
Chicago/Turabian StyleZhao, Xiang, Ruochun Gao, Jie Bai, Jing Rong, Xuexia Wei, Hairong Wang, Xiaojuan Zhu, Kun Sun, and Qinzheng Hou. 2025. "Synergistic Effects of Flower Color and Mechanical Barriers on Pollinator Selection Within the Papilionoideae of Fabaceae" Plants 14, no. 11: 1568. https://doi.org/10.3390/plants14111568
APA StyleZhao, X., Gao, R., Bai, J., Rong, J., Wei, X., Wang, H., Zhu, X., Sun, K., & Hou, Q. (2025). Synergistic Effects of Flower Color and Mechanical Barriers on Pollinator Selection Within the Papilionoideae of Fabaceae. Plants, 14(11), 1568. https://doi.org/10.3390/plants14111568





