Multi-Omics and Functional Insights into Triterpenoid Biosynthesis Pathways in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Callus Induction
2.3. Metabolite Profiling Using UPLC-MS/MS
2.4. RNA-Seq Analysis
2.5. Phylogenetic Analysis
2.6. Vector Construction
2.7. Characterization Analysis of NsOSC2 in Yeast
2.8. Stable Transformation of N. scrophulariiflora
2.9. RT-qPCR Analysis
2.10. Statistical Analysis
3. Results
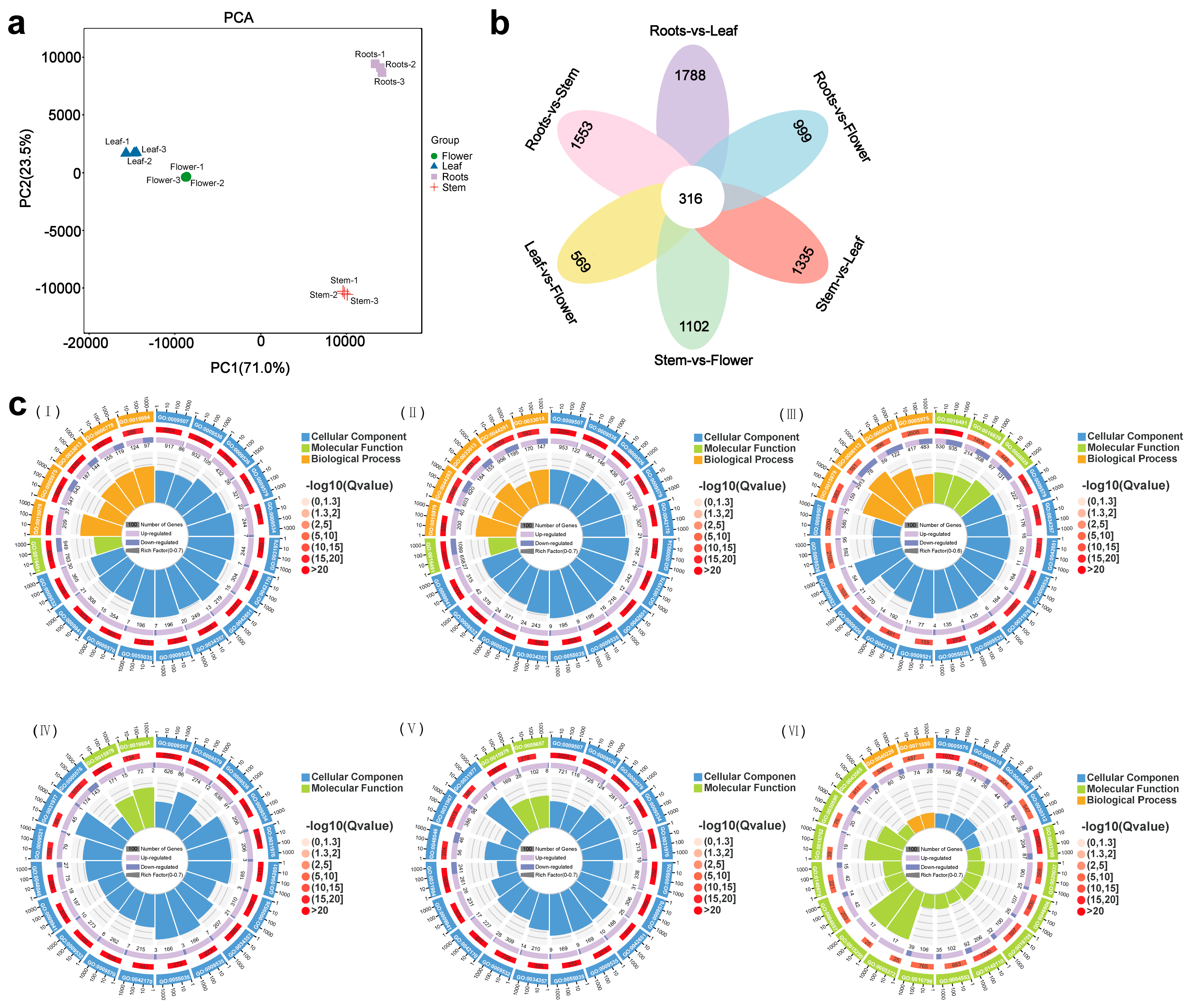
3.1. The Metabolome Profiling in N. scrophulariiflora
3.2. Identification of Differentially Expressed Genes (DEGs)
3.3. Phylogenetic and Expression Analysis Related to Triterpenoid Biosynthesis
3.4. Verification of Genes Related to Triterpenoid Biosynthesis Using RT-qPCR
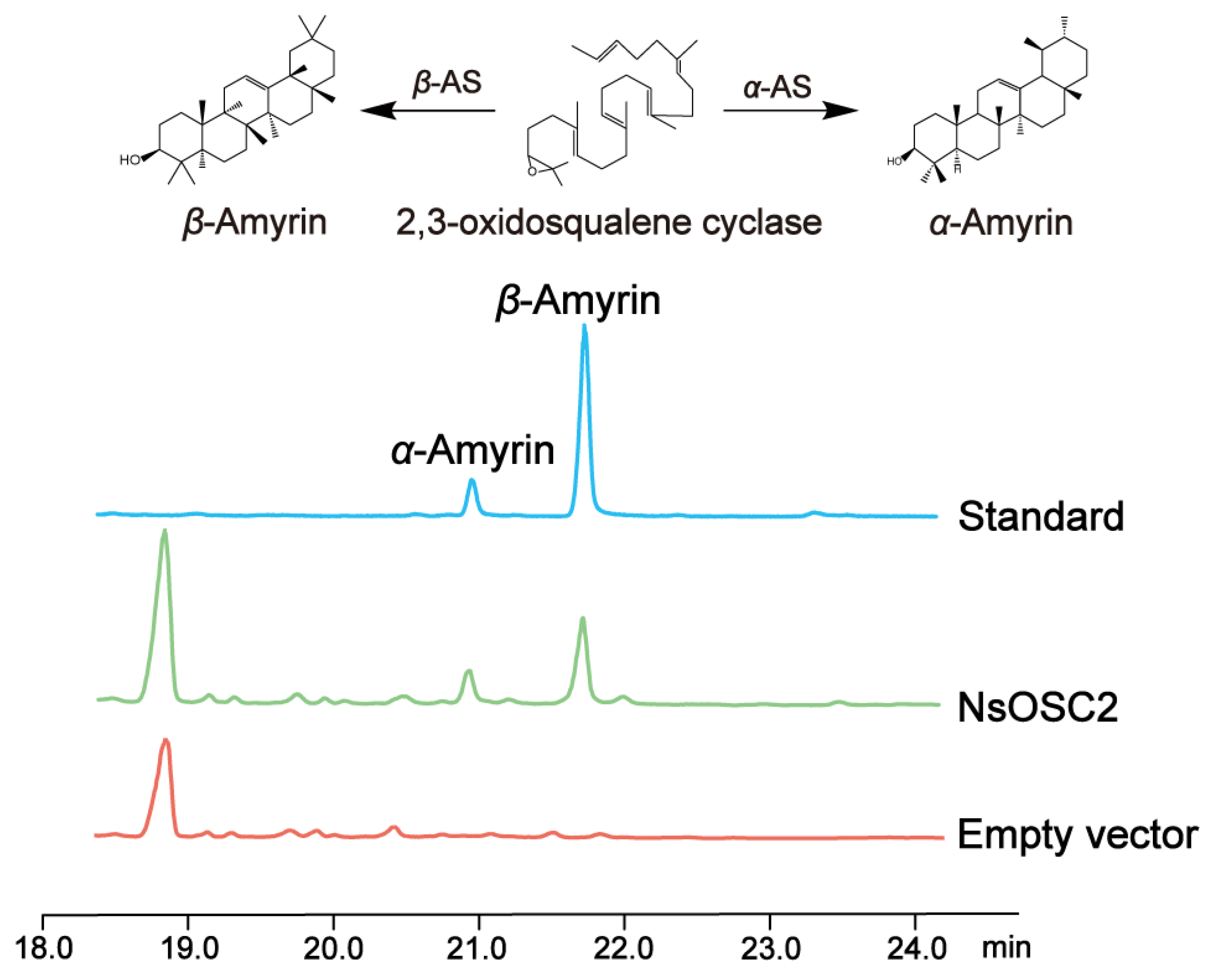
3.5. Heterologous Expression of NsOSCs in Yeast
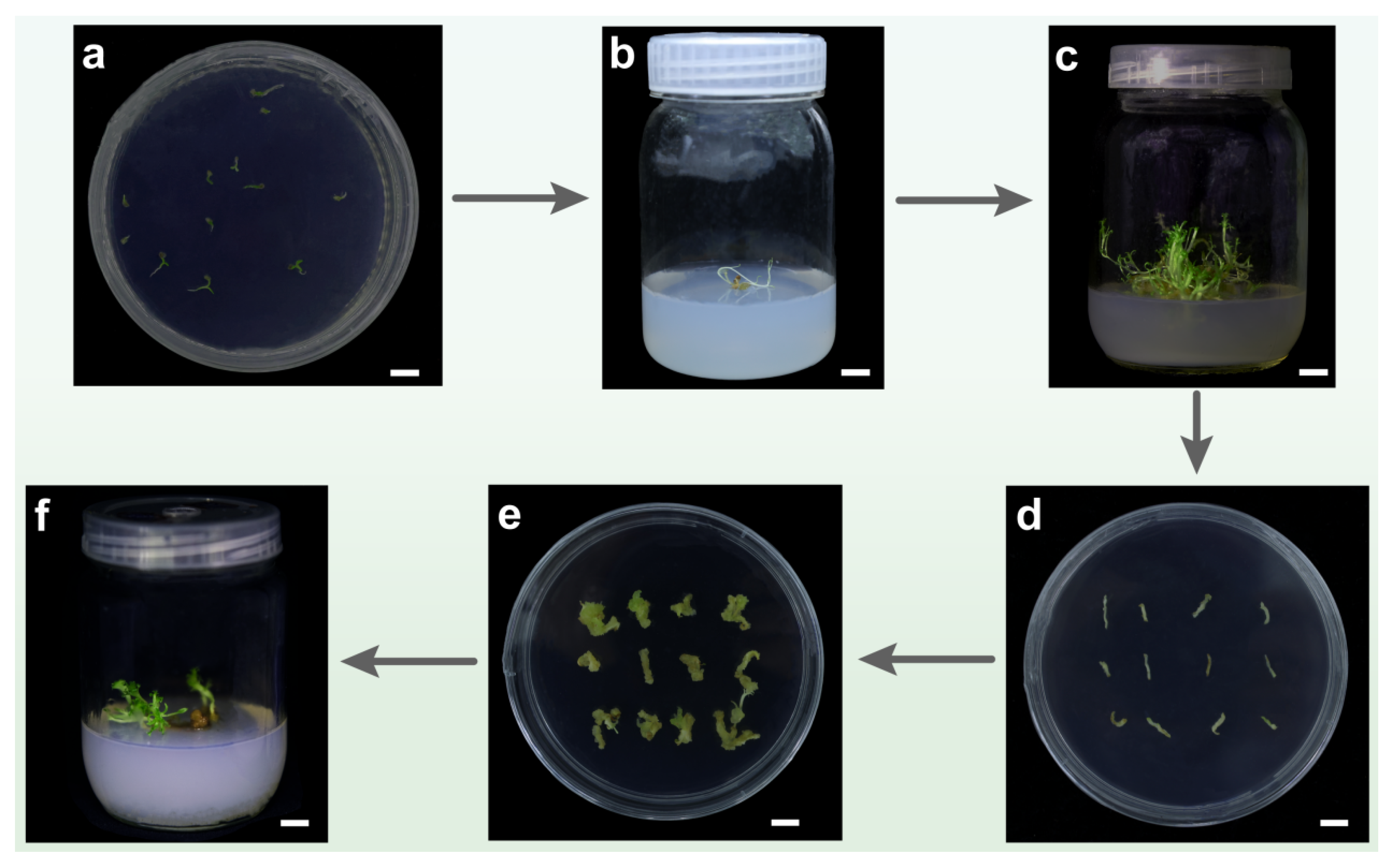
3.6. Establishment of Regeneration System
3.7. Establishment of Genetic Transformation System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnesen, J.A.; Belmonte Del Ama, A.; Jayachandran, S.; Dahlin, J.; Rago, D.; Andersen, A.J.C.; Borodina, I. Engineering of Yarrowia lipolytica for the production of plant triterpenoids: Asiatic, madecassic, and arjunolic acids. Metab. Eng. Commun. 2022, 14, e00197. [Google Scholar] [CrossRef] [PubMed]
- Czarnotta, E.; Dianat, M.; Korf, M.; Granica, F.; Merz, J.; Maury, J.; Baallal Jacobsen, S.A.; Förster, J.; Ebert, B.E.; Blank, L.M. Fermentation and purification strategies for the production of betulinic acid and its lupane-type precursors in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Cammareri, M.; Biazzi, E.; Pecchia, P.; Fevereiro, M.P.; Balestrazzi, A.; Tava, A.; Conicella, C. Enhanced triterpene saponin biosynthesis and root nodulation in transgenic barrel medic (Medicago truncatula Gaertn.) expressing a novel beta-amyrin synthase (AsOXA1) gene. Plant Biotechnol. J. 2009, 7, 172–182. [Google Scholar] [CrossRef]
- Chen, Q.X. Research progress on the traditional Chinese medicinal herb Plantago asiatica. Cardiovasc. Dis. Electron. J. Integr. Tradit. Chin. West. Med. 2019, 7, 151–152. [Google Scholar]
- Dai, L.; Liu, C.; Zhu, Y.; Zhang, J.; Men, Y.; Zeng, Y.; Sun, Y. Functional characterization of cucurbitadienol synthase and triterpene glycosyltransferase involved in biosynthesis of mogrosides from Siraitia grosvenorii. Plant Cell Physiol. 2015, 56, 1172–1182. [Google Scholar] [CrossRef]
- Feng, L.; Yao, Y.; Kang, M.; Yang, W.; Han, Y.; Liu, W.; Li, X.; Li, N.; Hu, Y.; Liu, J.; et al. Integrated genomic, transcriptomic, and metabolomic analyses of Ilex hylonoma provide insights into the triterpenoid saponin biosynthesis. Plant J. 2024, 120, 1176–1189. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Cao, J.; Xiang, C.; Yuan, C.; Li, X.; Wang, J.; Zhou, P.; Li, L.; Liu, J.; et al. MetaDb: A database for metabolites and their regulation in plants with an emphasis on medicinal plants. Mol. Hortic. 2024, 4, 17. [Google Scholar] [CrossRef]
- Hayashi, H.; Huang, P.; Kirakosyan, A.; Inoue, K.; Hiraoka, N.; Ikeshiro, Y.; Kushiro, T.; Shibuya, M.; Ebizuka, Y. Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosyntheses in licorice. Biol. Pharm. Bull. 2001, 24, 912–916. [Google Scholar] [CrossRef]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.J.; Bao, J.; Huang, L.; Nielsen, J.; Krivoruchko, A. Metabolic engineering of Saccharomyces cerevisiae for production of germacrene A, a precursor of beta-elemene. J. Ind. Microbiol. Biotechnol. 2017, 44, 1065–1072. [Google Scholar] [CrossRef]
- Kang, K.B.; Jun, J.B.; Kim, J.W.; Kim, H.W.; Sung, S.H. Ceanothane- and lupane-type triterpene esters from the roots of Hovenia dulcis and their antiproliferative activity on HSC-T6 cells. Phytochemistry 2017, 142, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kant, K.; Walia, M.; Agnihotri, V.K.; Pathania, V.; Singh, B. Evaluation of antioxidant activity of Picrorhiza kurroa (Leaves) extracts. Indian J. Pharm. Sci. 2013, 75, 324–329. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ueda, S.; Kanazawa, J.; Naoe, H.; Yamada, T.; Tanaka, R. Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds. Molecules 2014, 19, 4802–4813. [Google Scholar] [CrossRef]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. β-Amyrin synthase. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Gao, Q.Q.; Xiang, C.F.; Yuan, C.X.; Li, X.N.; Shu, Y.Y.; Zhang, G.H.; Liang, Y.L.; Yang, S.C.; et al. Site-directed mutagenesis identified the key active site residues of 2,3-oxidosqualene cyclase HcOSC6 responsible for cucurbitacins biosynthesis in Hemsleya chinensis. Front. Plant Sci. 2023, 14, 1138893. [Google Scholar] [CrossRef]
- Liu, H. Research progress on pharmacological effects of ursolic acid. Guangzhou Chem. Ind. 2023, 51, 28–30. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Liu, J.; Yin, X.; Kou, C.; Thimmappa, R.; Hua, X.; Xue, Z. Classification, biosynthesis, and biological functions of triterpene esters in plants. Plant Commun. 2024, 5, 100845. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, G.; Yang, L.P.; Tang, D.Y.; Xiao, Z. Resource status and protection countermeasures of endangered Tibetan medicine Neopicrorhiza scrophulariiflora. J. Yunnan Coll. Tradit. Chin. Med. 2008, 31, 3–6. [Google Scholar]
- Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent advances in antiviral activities of triterpenoids. Pharmaceuticals 2022, 15, 1169. [Google Scholar] [CrossRef]
- Meng, F.; Chu, T.; Feng, P.; Li, N.; Song, C.; Li, C.; Leng, L.; Song, X.; Chen, W. Genome assembly of Polygala tenuifolia provides insights into its karyotype evolution and triterpenoid saponin biosynthesis. Hortic. Res. 2023, 10, uhad139. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.; Iñigo, S.; Kreft, L.; Pollier, J.; De Bo, C.; Botzki, A.; Coppens, F.; Bak, S.; Goossens, A. The TriForC database: A comprehensive up-to-date resource of plant triterpene biosynthesis. Nucleic Acids Res. 2018, 46, D586–D594. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P.T. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential partitioning of triterpenes and triterpene esters in apple peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Zhong, R.; Li, X.; Pant, S.D.; Shen, X.; BinMowyna, M.N.; Luo, L.; Lei, H. Ganoderma lucidum triterpenoids investigating their role in medicinal applications and genomic protection. J. Pharm. Pharmacol. 2024, 76, 1535–1551. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Parajuli, B.; Bhatta, K.P.; Timsina, B. Neopicrorhiza scrophulariiflora (Pennell) Hong: A comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. J. Ethnopharmacol. 2020, 247, 112250. [Google Scholar] [CrossRef]
- Schühly, W.; Heilmann, J.; Çalis, I.; Sticher, O. New triterpenoids with antibacterial activity from Zizyphus joazeiro. Planta Medica 1999, 65, 740–743. [Google Scholar] [CrossRef]
- Seki, H.; Ohyama, K.; Sawai, S.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Akashi, T.; Aoki, T.; Saito, K.; Muranaka, T. Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc. Natl. Acad. Sci. USA 2008, 105, 14204–14209. [Google Scholar] [CrossRef]
- Shang, J.-H.; Sun, W.-J.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. New hydroperoxylated and 20,24-epoxylated dammarane triterpenes from the rot roots of Panax notoginseng. J. Ginseng. Res. 2020, 44, 405–412. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Xiang, T.; Ohyama, K.; Seki, H.; Saito, K.; Muranaka, T.; Hayashi, H.; Katsube, Y.; Kushiro, T.; Shibuya, M.; et al. Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol. 2006, 47, 565–571. [Google Scholar] [CrossRef]
- Tansakul, P.; Shibuya, M.; Kushiro, T.; Ebizuka, Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett. 2006, 580, 5143–5149. [Google Scholar] [CrossRef]
- Thani, P.R. A comprehensive review on Picrorhiza kurroa Royle ex Benth. J. Pharmacogn. Phytochem. 2021, 10, 307–313. [Google Scholar] [CrossRef]
- Wang, G. Study on the chemical constituents and pharmacological effects of Plantago asiatica. Heilongjiang Med. J. 2014, 27, 864–865. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.H.; Xiang, C.F.; Zhou, P.H.; Li, L.S.; Li, X.; Yang, S.C.; Zhang, G.H.; Zhao, Y. Establishment and application of highly efficient regeneration, genetic transformation and genome editing system for cucurbitacins biosynthesis in Hemsleya chinensis. BMC Plant Biol. 2024, 24, 1052. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, P.H.; Li, C.H.; Liang, Y.L.; Liu, G.Z.; Yang, S.C.; Xiao, Y.; Zhao, Y. Progress on medicinal plant regeneration and the road ahead. Med. Plant Biol. 2024, 3, e030. [Google Scholar] [CrossRef]
- Wendt, K.U.; Poralla, K.; Schulz, G.E. Structure and function of a squalene cyclase. Science 1997, 277, 1811–1815. [Google Scholar] [CrossRef]
- Wu, Y.; Zou, H.D.; Cheng, H.; Zhao, C.Y.; Sun, L.F.; Su, S.Z.; Li, S.P.; Yuan, Y.P. Cloning and characterization of a β-amyrin synthase gene from the medicinal tree Aralia elata (Araliaceae). Genet. Mol. Res. 2012, 11, 2301–2314. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Ri, H.C.; An, Z.; Wang, X.; Zhou, J.-N.; Zheng, D.; Wu, H.; Wang, P.; Yang, J.; et al. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata. Nat. Commun. 2022, 13, 2224. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Zeng, F.; Zhou, X.; Shu, Y. Research progress on anti-inflammatory and anti-oxidation of oleanolic acid. Biot. Resour. 2023, 45, 504–509. [Google Scholar] [CrossRef]
- Xia, L.H.; Jin, G.Q.; Sun, L.; Yang, J. Research progress on the chemical constituents and pharmacological effects of Plantago asiatica. Chin. Pharm. 2013, 16, 294–296. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.K.; Fu, J.; Wang, H.Q.; Li, B.M.; Chen, R.Y.; Kang, J. Advances in studies on structure and pharmacological activities of natural tirucallane-type triterpenoids. Zhongguo Zhong Yao Za Zhi 2020, 45, 3617–3630. [Google Scholar]
- Yang, S.H.; Chen, C.; Guo, C.G.; Kang, P.D.; Xue, R.G.; Xu, Z.Z. Biological characteristics, endangered reasons and protection countermeasures of Neopicrorhiza scrophulariiflora in Yunnan. Southwest Agric. J. 2009, 22, 1482–1485. [Google Scholar]
- Yang, Y.J.; Zhou, Q.G.; Zeng, H.; Chu, H.B.; Liang, Z.C.; Li, Q.Y. Research progress on the chemical constituents and novel biological activities of Plantago asiatica. Chin. Tradit. Pat. Med. 2011, 33, 1771–1776. [Google Scholar] [CrossRef]
- Zhang, S.; Meng, F.; Pan, X.; Qiu, X.; Li, C.; Lu, S. Chromosome-level genome assembly of Prunella vulgaris, L. provides insights into pentacyclic triterpenoid biosynthesis. Plant J. 2024, 118, 731–752. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Peng, Y.Q.; Xiang, G.S.; Song, W.L.; Feng, L.; Jiang, X.Y.; Li, X.J.; He, S.M.; Yang, S.C.; Zhao, Y.; et al. Functional characterization of genes related to triterpene and flavonoid biosynthesis in Cyclocarya paliurus. Planta 2024, 259, 50. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Qu, W.; Liang, J.Y. Research progresses on chemical constituents and pharmacological activities of Plantago spp. Strait Pharm. J. 2013, 25, 1–8. [Google Scholar]
- Zhao, Y.; Liu, G.; Yang, F.; Liang, Y.; Gao, Q.; Xiang, C.; Li, X.; Yang, R.; Zhang, G.; Jiang, H.; et al. Multilayered regulation of secondary metabolism in medicinal plants. Mol. Hortic. 2023, 3, 11. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, T.; Gao, L.; Su, P.; Zhang, Y.; Zhao, Y.; Chen, S.; Tu, L.; Song, Y.; Wang, X.; et al. Friedelane-type triterpene cyclase in celastrol biosynthesis from Tripterygium wilfordii and its application for triterpenes biosynthesis in yeast. New Phytol. 2019, 223, 722–735. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Wang, J.; Li, C.; Li, L.; Duan, L.; Wang, W.; Liu, X.; Arshad, K.T.; Liang, Y.; Zhao, Y. Multi-Omics and Functional Insights into Triterpenoid Biosynthesis Pathways in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong. Plants 2025, 14, 1562. https://doi.org/10.3390/plants14101562
Zhou P, Wang J, Li C, Li L, Duan L, Wang W, Liu X, Arshad KT, Liang Y, Zhao Y. Multi-Omics and Functional Insights into Triterpenoid Biosynthesis Pathways in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong. Plants. 2025; 14(10):1562. https://doi.org/10.3390/plants14101562
Chicago/Turabian StyleZhou, Pinhan, Juan Wang, Chaohui Li, Lesong Li, Luyuan Duan, Weihao Wang, Xirui Liu, Khadija Tehseen Arshad, Yanli Liang, and Yan Zhao. 2025. "Multi-Omics and Functional Insights into Triterpenoid Biosynthesis Pathways in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong" Plants 14, no. 10: 1562. https://doi.org/10.3390/plants14101562
APA StyleZhou, P., Wang, J., Li, C., Li, L., Duan, L., Wang, W., Liu, X., Arshad, K. T., Liang, Y., & Zhao, Y. (2025). Multi-Omics and Functional Insights into Triterpenoid Biosynthesis Pathways in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong. Plants, 14(10), 1562. https://doi.org/10.3390/plants14101562





