Freshwater Algae Biostimulant in Mitigating Impacts of Saline Irrigation on Onions
Abstract
1. Introduction
2. Results
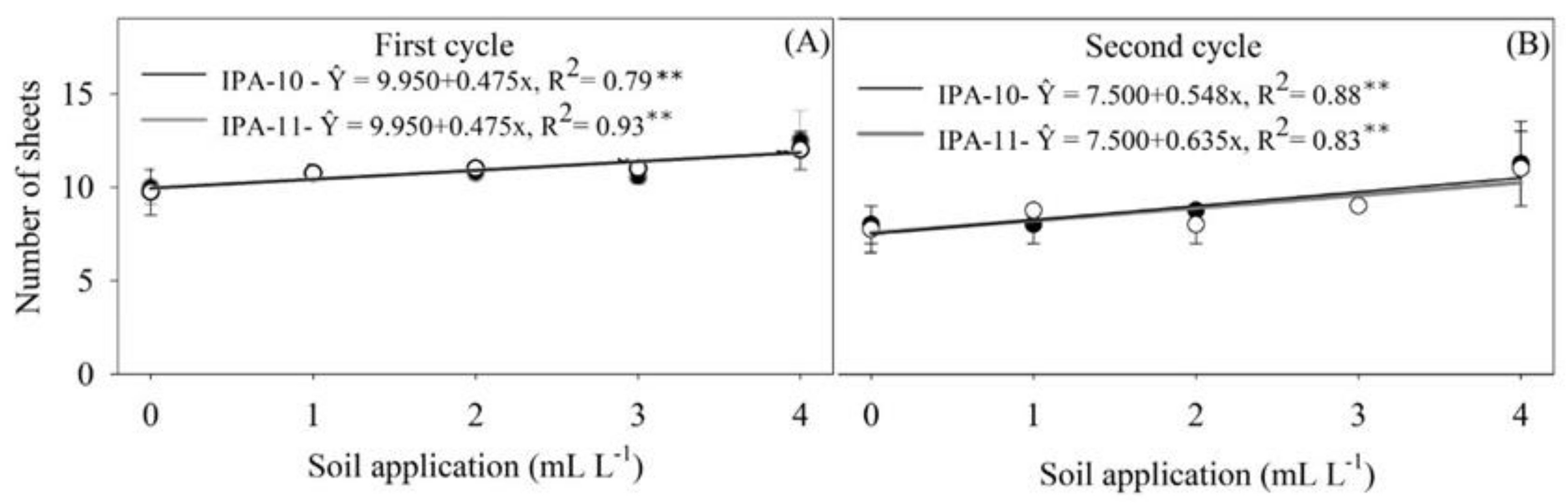
2.1. Growth Parameters
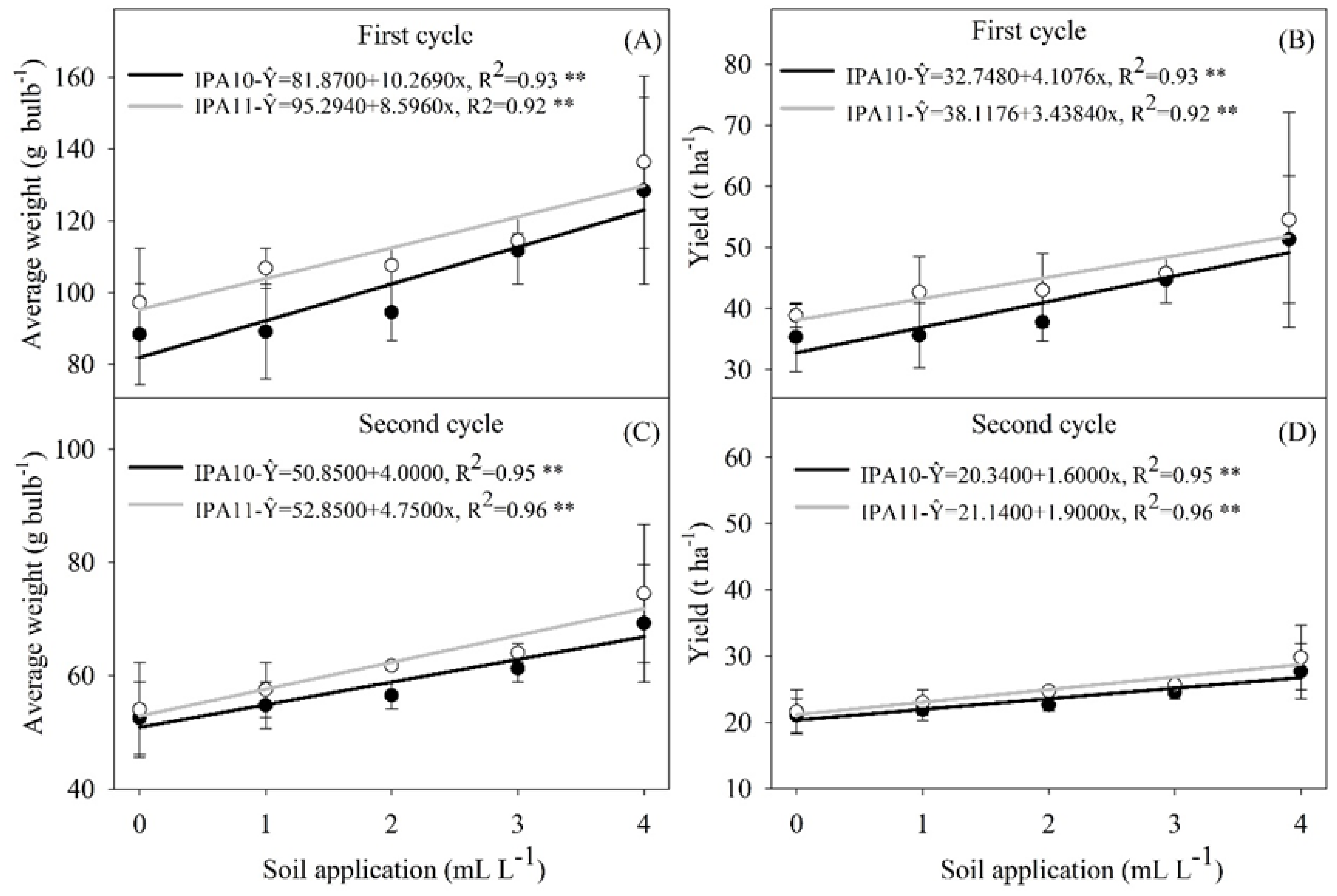
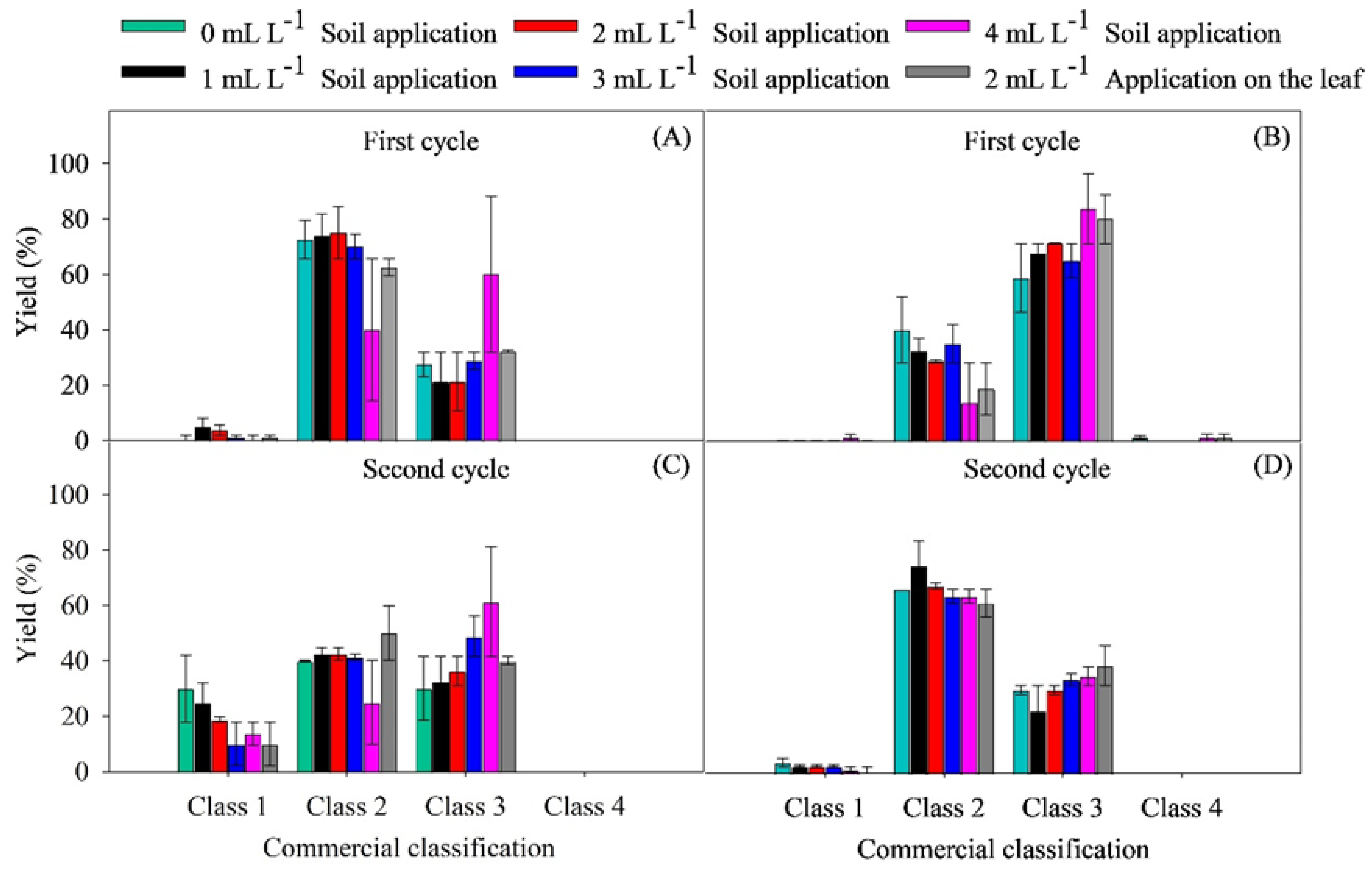
2.2. Yield Parameters
2.3. Physical–Chemical Characteristics (Bulb Firmness, Titratable Acidity, Soluble Solids, and pH)
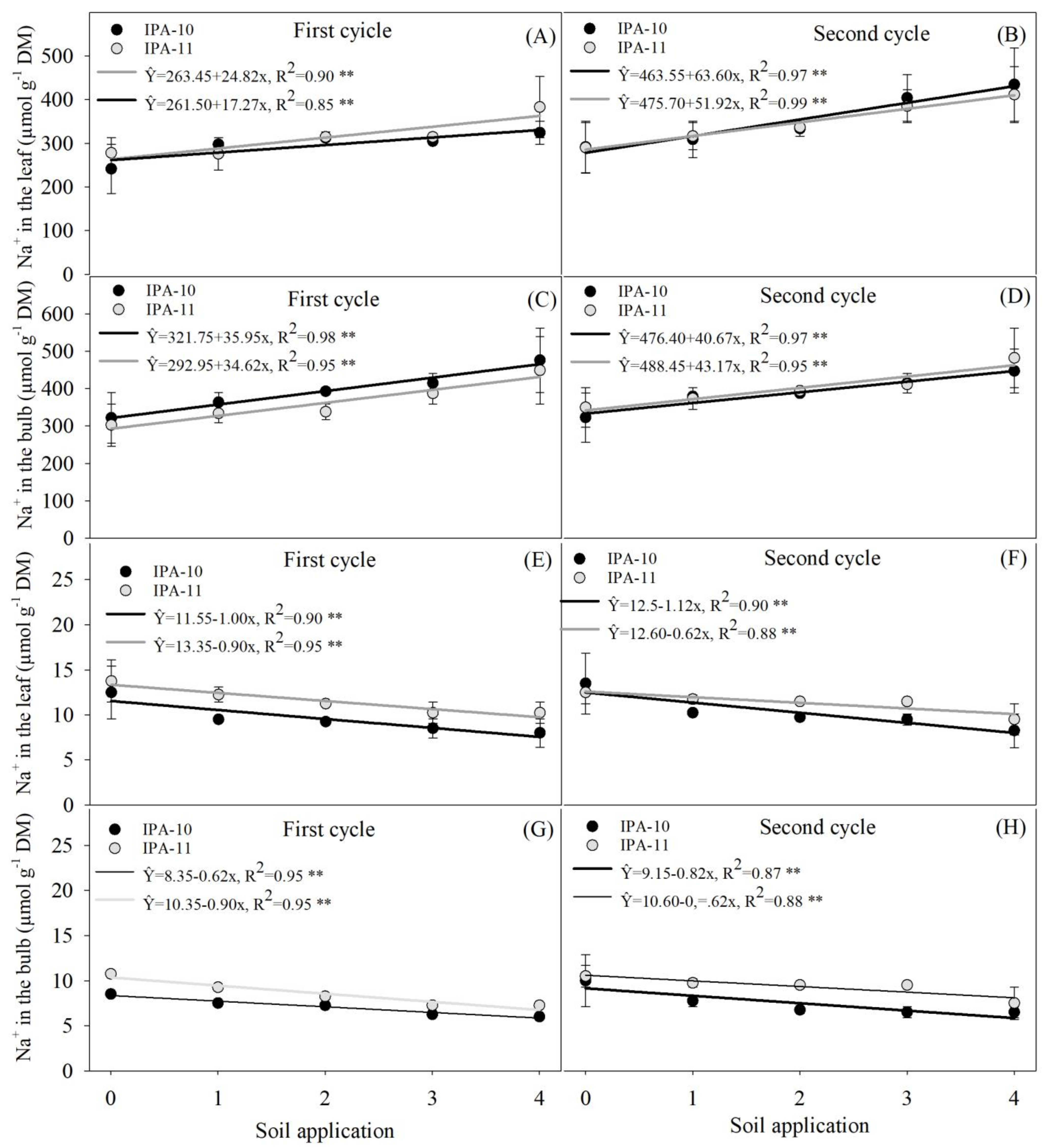
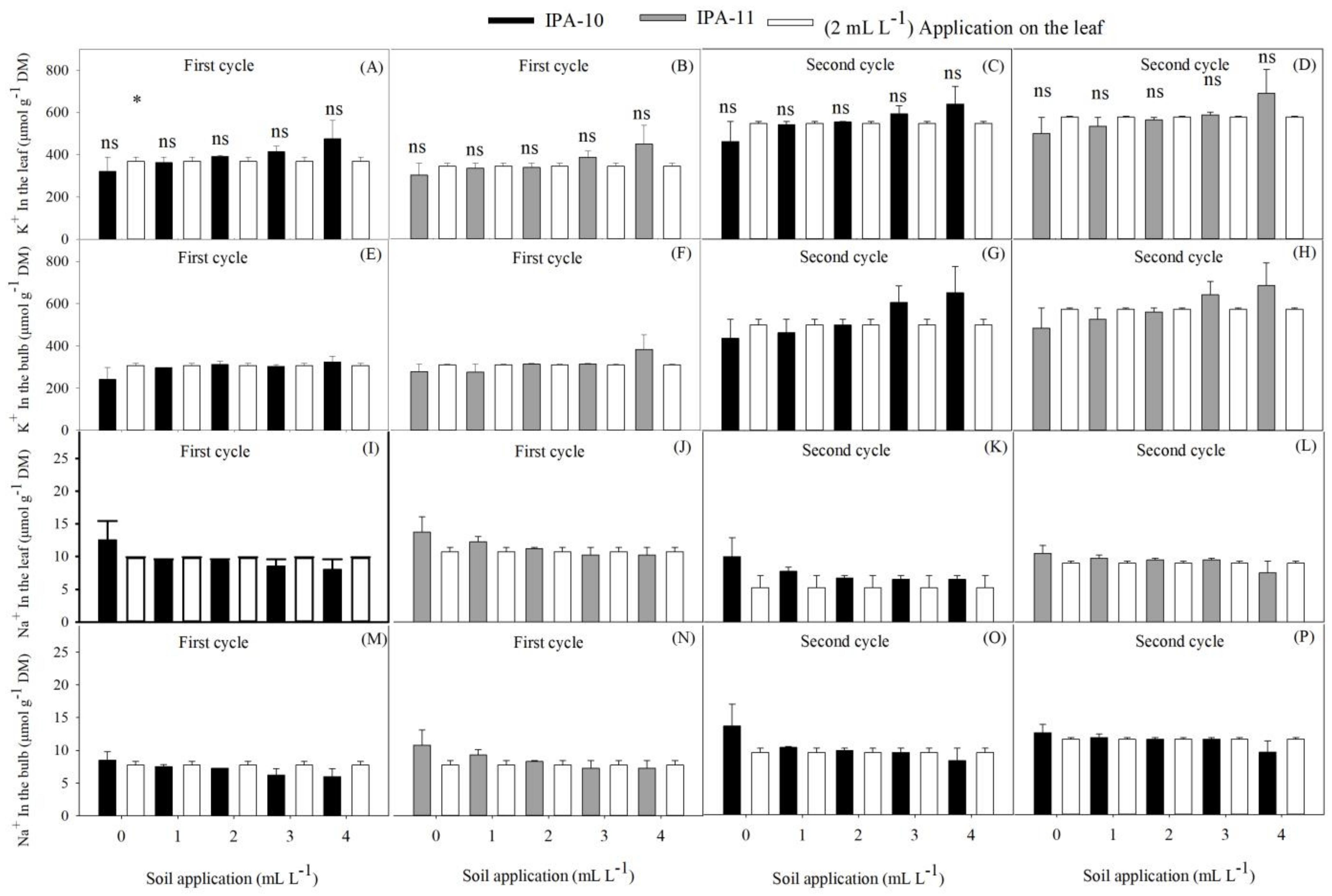
2.4. Evaluation of Potassium and Sodium Ions
2.5. Photosynthetic Pigments
2.6. Electrical Conductivity of the Soil Saturation Extract
3. Discussion
4. Materials and Methods
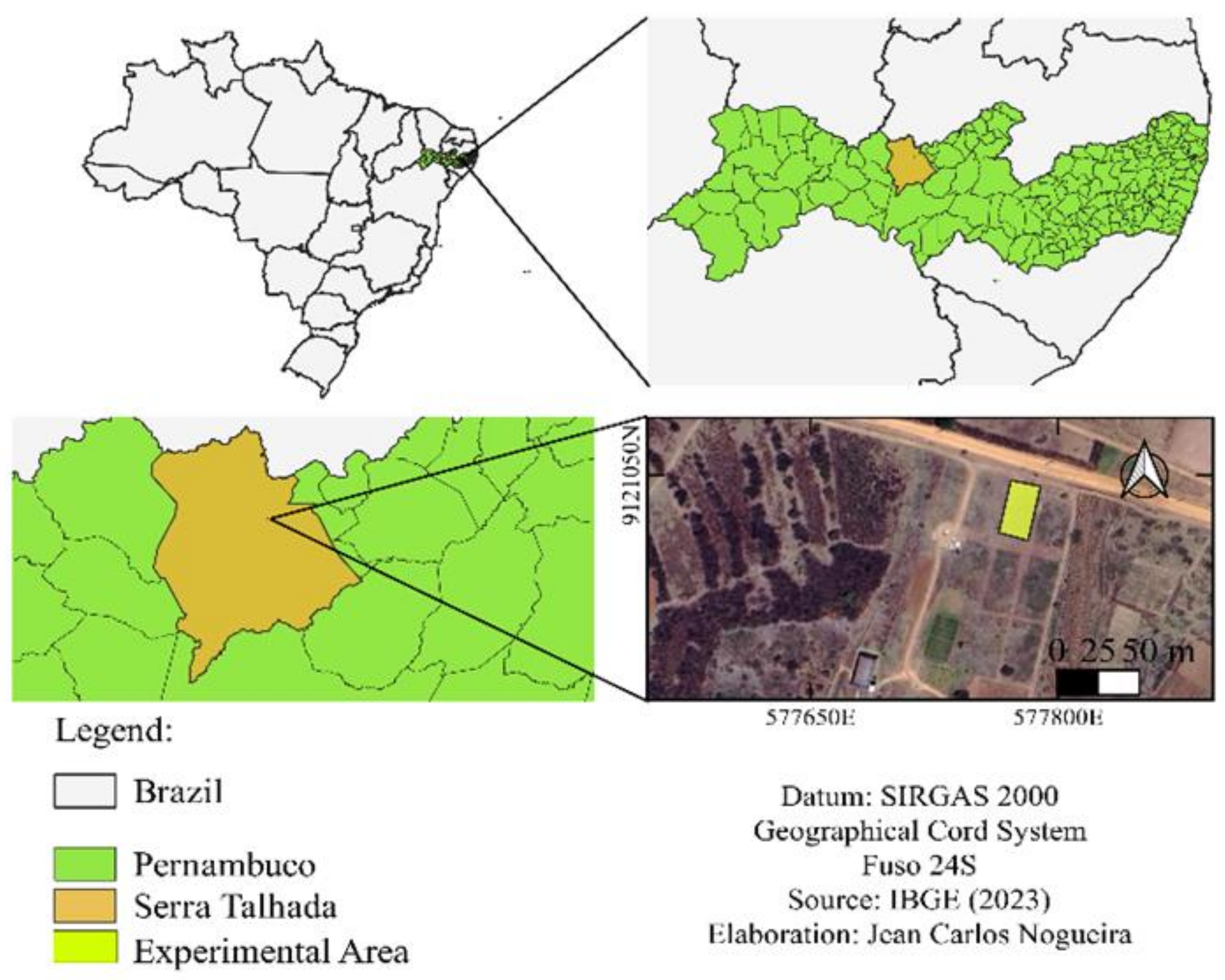
4.1. Study Area
4.2. Experimental Design
4.3. Management of the Experimental Area
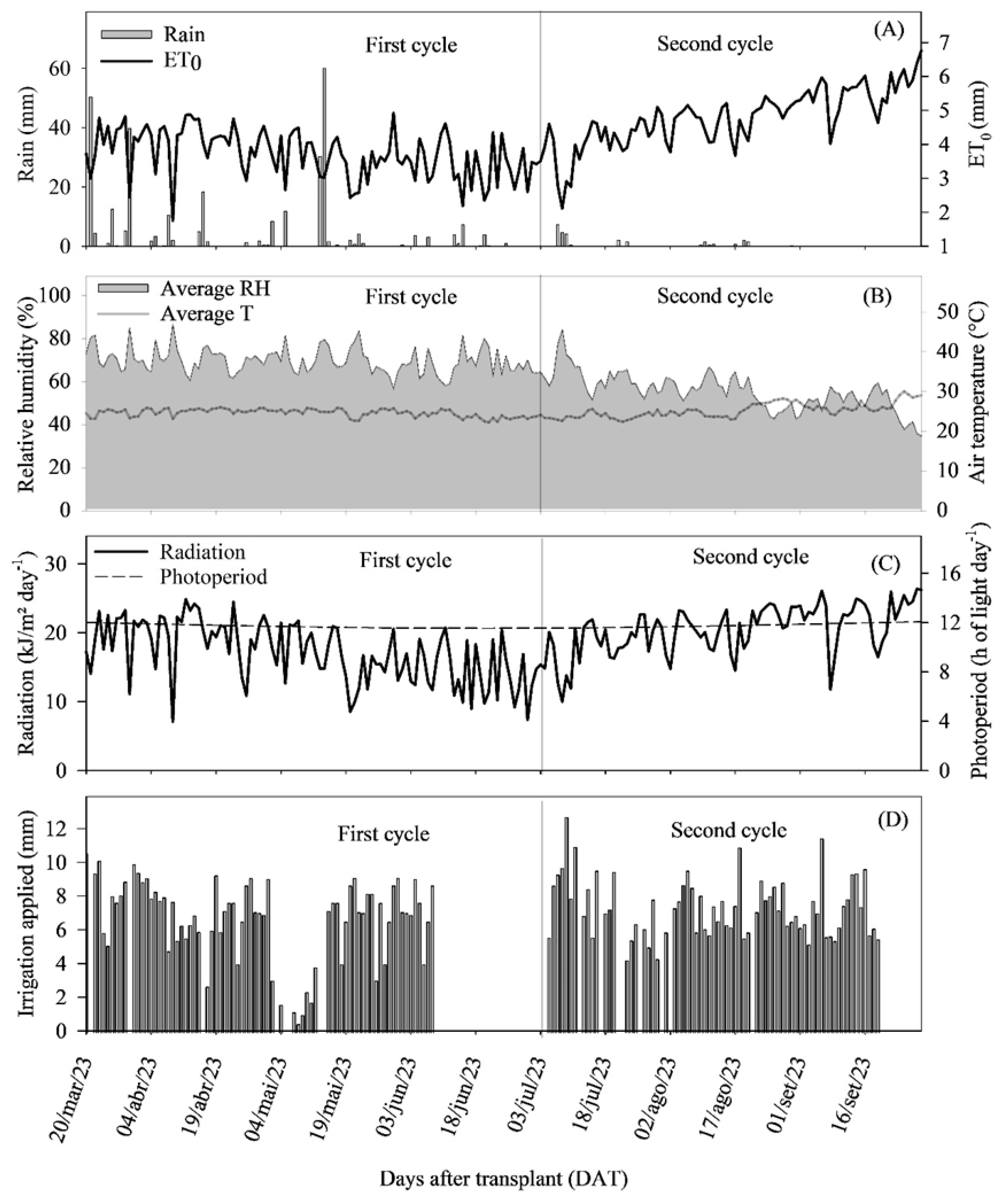
4.4. Climatic and Soil Conditions
4.5. Cultivation, Irrigation, and Fertilization
4.6. Data Collected
4.6.1. Growth Parameters
4.6.2. Yield Parameters
4.6.3. Physical–Chemical Characteristics of the Bulbs
4.6.4. Evaluation of Chloride, Potassium, and Sodium Ions in Plants
4.6.5. Photosynthetic Pigments in the Leaves
4.7. Data Treatment and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.G.M.; Freire, M.B.G.d.S.; dos Santos, R.L.; Freire, F.J.; dos Santos, P.R.; Miranda, M.F.A. Saline water irrigation in semiarid region: II-effects on growth and nutritional status of onions. Aust. J. Crop Sci. 2019, 13, 1177–1182. [Google Scholar] [CrossRef]
- Vojnović, Đ.; Maksimović, I.; Horecki, A.T.; Banjac, M.K.; Kovačević, S.; Daničić, T.; Podunavac-Kuzmanović, S.; Ilin, Ž. Onion (Allium cepa L.) Yield and Quality Depending on Biostimulants and Nitrogen Fertilization—A Chemometric Perspective. Processes 2023, 11, 684. [Google Scholar] [CrossRef]
- Morales-Meléndez, R.; Arroyo-Ramírez, A.; Camposeco-Montejo, N.; Méndez-López, A.; López-Pérez, M.C. Ascophyllum Nodosum and Calcium Nitrate as Biostimulants in the Development and Yield of Tomato Cultivation. Cienc. Lat. Multidiscip. J. 2024, 8, 1574–1589. [Google Scholar] [CrossRef]
- Dehnavi, A.R.; Zahedi, M.; Ludwiczak, A.; Perez, S.C.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Lavandula multifida response to salinity: Growth, nutrient uptake, and physiological changes. J. Plant Nutr. Soil Sci. 2017, 180, 96–104. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Pessoa, L.; Dos Freire, M.B.G.S.; Freire, F.J.; Menezes, D. Growth of onion irrigated with saline water in soils of the semi-arid region of Pernambuco. Braz. J. Agric. Sci. 2012, 7, 527–534. [Google Scholar] [CrossRef]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2022: Leveraging Automation in Agriculture to Transform Agri-Food Systems. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 26 September 2024).
- IBGE. Agricultural Production. Brazilian Institute of Geography and Statistics. 2023. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria (accessed on 26 September 2024).
- Lima, M.D.B.; Bull, L.T. Onion Production in Salinized Soil. Braz. J. Agric. Environ. Eng. 2008, 12, 231–235. [Google Scholar] [CrossRef]
- Carvalho, J.N.d.S.; Garrido, M.d.S.; da Silva, J.A.B.; Nagahama, H.d.J.; Simões, W.L.; Macedo, B.L.D.N.; Souza, E.M.d.S. Photosynthesis and accumulation of organic solutes in Dioscorea alata L. subjected to the application of manihot wastewater and biostimulant. Obs. Lat. Am. Econ. 2024, 22, e6995. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Rai, N.; Rai, S.P.; Sarma, B.K. Prospects for Abiotic Stress Tolerance in Crops Utilizing Phyto- and Bio-Stimulants. Front. Sustain. Food Syst. 2021, 5, 754853. [Google Scholar] [CrossRef]
- Righini, H.; Roberti, R.; Baraldi, E. Use of algae in strawberry management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Dias, L.R.C.; da Paz Filho, E.R.; Cavalcante, R.I.F.S.; Pereira, J.C.d.S.; Oliveira, L.d.J.M.G.d. The Sustainable Use of the Seaweed Ascophyllum nodosum (L.) Le Jol. in Agriculture: A Theoretical Framework. Pest Dis. Manag. Search Sustain. Control. Methods 2021, 1, 78–89. [Google Scholar] [CrossRef]
- Pedro, S.F.; Júnior, K.S.F.; Ribeiro, V.M.; Brigante, G.P. Effects of fertilizer based on seaweed extracts on the initial growth of coffee trees. Res. Soc. Dev. 2022, 11, e79111738844. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Piero, A.R.L.; Baglieri, A. Biostimulant Effect and Biochemical Response in Lettuce Seedlings Treated with A Scenedesmus quadricauda Extract. Plants 2020, 9, 123. [Google Scholar] [CrossRef]
- Mógor, A.F.; Amatussi, J.O.; Mógor, G.; Gemin, L.G. Biostimulant action of Lithothamnium sp. promoting growth, yield, and biochemical and chemical changes on onion. J. Appl. Phycol. 2021, 33, 1905–1913. [Google Scholar] [CrossRef]
- Rivera-Solís, L.L.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Flores-López, M.L.; Robledo-Olivo, A.; González-Morales, S. Biostimulant effect of nanochitosan-iodine on the growth and vigor of tomato plants. Ecosist. Recur. Agropecu. 2024, 11, e3623. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Verde, D.d.S.V.; Peixoto, C.P.; Costa, J.A. Physiological Quality of Papaya Seeds Treated with Bio-Stimulant and Germinated Under Different Light Conditions. Delos J. 2024, 17, e1400. [Google Scholar] [CrossRef]
- Gomes, M.M.d.A.; Costa, C.C.; Pereira, U.d.S.; de Sousa, M.E.; de Sousa, C.A.A.; Lopes, K.P.; Diniz, G.L.; da Silva, G.C. Foliar biostimulant application on the growth and development of Citrullus lanatus seedlings grown in salinized substrate. Cad. Pedagógico 2024, 21, e8350. [Google Scholar] [CrossRef]
- Batista, L.A.; Melo, P.R.; de Andrade, C.L.L.; Filho, F.R.C.; Ventura, M.V.A. Agronomic performance of soybean subjected to different ICL nutritional solutions. Braz. J. Agric. Spray. 2024, 1, 47–55. [Google Scholar] [CrossRef]
- Michalak, I.; Dmytryk, A.; Wieczorek, P.P.; Rój, E.; Łęska, B.; Górka, B.; Messyasz, B.; Lipok, J.; Mikulewicz, M.; Wilk, R.; et al. Supercritical Algal Extracts: A Source of Biologically Active Compounds from Nature. J. Chem. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Hans, S.; Feitosa, G. Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados, 2nd ed.; INCTSal: Fortaleza, Brazil, 2016; Volume 2, 504p. [Google Scholar]
- Nurbekova, Z.; Satkanov, M.; Beisekova, M.; Akbassova, A.; Ualiyeva, R.; Cui, J.; Chen, Y.; Wang, Z.; Zhangazin, S. Strategies for Achieving High and Sustainable Plant Productivity in Saline Soil Conditions. Horticulturae 2024, 10, 878. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Melo, P.; Abreu, C.; Bahcevandziev, K.; Araujo, G.; Pereira, L. Biostimulant Effect of Marine Macroalgae Bioextract on Pepper Grown in Greenhouse. Appl. Sci. 2020, 10, 4052. [Google Scholar] [CrossRef]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Chojnacka, K.; Michalak, I.; Dmytryk, A.; Gramza, M.; Słowiński, A.; Górecki, H. Algal Extracts as Plant Growth Biostimulants. In Algal Extracts as Plant Growth Biostimulants—Marine Algae Extracts: Processes, Products, and Applications; Willey: Hoboken, NJ, USA, 2015; Volume 2, pp. 189–212. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- Tian, S.-L.; Khan, A.; Zheng, W.-N.; Song, L.; Liu, J.-H.; Wang, X.-Q.; Li, L. Effects of Chlorella extracts on growth of Capsicum annuum L. seedlings. Sci. Rep. 2022, 12, 15455. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wszelaczyńska, E.; Pobereżny, J.; Ochmian, I. Response of Onion (Allium cepa L.) to the Method of Seaweed Biostimulant Application. Acta Sci. Pol. Hortorum Cultus 2017, 16, 113–122. Available online: https://czasopisma.up.lublin.pl/index.php/asphc/article/view/2301 (accessed on 8 September 2023).
- Oancea, F.; Velea, S.; Fatu, V.; Mincea, C.; Ilie, L. Micro-algae based plant biostimulant and its effect on water stressed tomato plants. Rom. J. Plant Prot. 2013, 6, 104–117. Available online: https://www.researchgate.net/publication/318489795 (accessed on 10 September 2023).
- Dias, G.A.; Rocha, R.H.C.; Araújo, J.L.; Lima, J.F.; Guedes, W.A. Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semin. Ciências Agrárias 2016, 37, 3893–3902. [Google Scholar] [CrossRef]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Hajnal-Jafari, T.; Seman, V.; Stamenov, D.; Đurić, S. Effect of Chlorella vulgaris on Growth and Photosynthetic Pigment Content in Swiss Chard (Beta vulgaris L. subsp. cicla). Pol. J. Microbiol. 2020, 69, 235–238. [Google Scholar] [CrossRef]
- Filho, A.M.d.S.; Gheyi, H.R.; de Melo, A.S.; Silva, P.C.C.; da Silva, T.I.; Bonou, S.I.; de Alencar, R.S.; Dias, G.F.; de Lacerda, C.F.; Ferraz, R.L.d.S. NPK fertilization modulates enzyme activity and mitigates the impacts of salinity on West Indian cherry. Braz. J. Biol. 2024, 84, e282616. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus Growth is Promoted by Ascophyllum nodosum (L.) Le Jol. Seaweed Extract: Microarray Analysis and Physiological Characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Dias, P.R.R.; Bardiviesso, D.M.; Barbosa, R.T.; Ferreira, I.B.P.A.; Boas, J.K.V. Germination and initial growth of corn with the application of bio-stimulant doses and saline water irrigation. Cultiv. Knowl. J. 2024, 17, 158–173. Available online: https://cultivandosaber.fag.edu.br/index.php/cultivando/article/view/1274 (accessed on 22 October 2024).
- Rodríguez, D.J.-D.; Ramírez-Pérez, C.; Ramírez-Rodríguez, H.; Villarreal-Quintanilla, J.Á.; Hernández-Pérez, A.; Díaz-Jimenez, M.L.V.; Peña-Ramos, F.M. Plant Biostimulants from the Semidesert on the Root and Aerial Growth of Tomato Seedlings. Ecosist. Recur. Agropecu. 2024, 11, e4145. [Google Scholar] [CrossRef]
- Jain, G.; Kushwah, S.S.; Singh, O.P.; Verma, K.S. Effect of different doses of nitrogen and sulphur on growth, yield and quality of onion (Allium cepa). Indian J. Agric. Sci. 2014, 84, 788–792. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant biostimulants from seaweeds or vegetal proteins enhance the salinity tolerance in greenhouse lettuce by modulating plant metabolism in a distinctive manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Baier, J.E.; de Resende, J.T.V.; Galvão, A.G.; Battistelli, G.M.; Machado, M.M.; Faria, M.V. Productivity and commercial yield of onion bulbs as a function of planting density. Sci. Agrotechnol. 2009, 33, 496–501. [Google Scholar] [CrossRef]
- Kurtz, C.; Ernani, P.R.; Coimbra, J.L.M.; Petry, E. Yield and Storage of Onion Altered by Nitrogen Dose and Split Application. Braz. J. Soil Sci. 2012, 36, 865–876. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Shim, C.-K.; Kim, Y.-K.; Ko, B.-G.; Park, J.-H.; Hwang, S.-G.; Kim, B.-H. Effect of biostimulator chlorella fusca on improving growth and qualities of chinese chives and spinach in organic farm. Plant Pathol. J. 2018, 34, 567–574. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Ahluwalia, A.S.; Bansal, R.; Babu, S.; Singh, R.; Shivay, Y.S.; Nain, L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertilizer for wheat. Environ. Sci. Pollut. Res. 2016, 23, 6608–6620. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.Q.; Lv, K.; Cheng, J.J.; Chen, X.L.; Ge, Y.; Yu, X.Y. Soil microalgae modulate grain arsenic accumulation by reducing dimethylarsinic acid and enhancing nutrient uptake in rice (Oryza sativa L.). Plant Soil 2018, 430, 99–111. [Google Scholar] [CrossRef]
- Veloso, L.L.d.S.A.; de Azevedo, C.A.V.; da Silva, A.A.R.; de Lima, G.S.; Gheyi, H.R.; da Nóbrega, R.A.; Pinheiro, F.W.A.; Lucena, R.C.M. Effects of saline water and exogenous application of hydrogen peroxide (H2O2) on Soursop (Annona muricata L.) at vegetative stage. Aust. J. Crop Sci. 2019, 13, 472–479. [Google Scholar] [CrossRef]
- Rios, C.V.; Souza, G.V.D.; de Brito, E.P.; Pagliosa, M.; DA Silva, C.P. Efeito do cálcio, boro e potássio no crescimento inicial de hortelã-verde (Mentha spicata L.) em solução nutritiva. Científic@ Multidiscip. J. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Alvarenga, C.F.d.S.; da Silva, E.M.; Nobre, R.G.; Gheyi, H.R.; de Lima, G.S.; Silva, L.d.A. Morphophysiology of acerola trees irrigated with saline waters under combinations of nitrogen and potassium doses. Agric. Sci. J. 2019, 42, 191–200. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Tofei, A.M.; Boscaiu, M.; Moreno-Ramón, H.; Ibáñez-Asensio, S.; Vicente, O. Effect of a Biostimulant Based on Polyphenols and Glycine Betaine on Tomato Plants’ Responses to Salt Stress. Agronomy 2022, 12, 2142. [Google Scholar] [CrossRef]
- Hoque, N.; Imran, S.; Hannan, A.; Paul, N.C.; Mahamud, A.; Chakrobortty, J.; Sarker, P.; Irin, I.J.; Brestic, M.; Rhaman, M.S. Organic Amendments for Mitigation of Salinity Stress in Plants: A Review. Life 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Ashour, H.A.; Esmail, S.E.A.; Kotb, M.S. Alleviative effects of chitosan or humic acid on Vitex trifolia ‘Purpurea’ grown under salinity stress. Ornam. Hortic. 2020, 27, 88–102. [Google Scholar] [CrossRef]
- Kano, C.; Cardoso, A.I.I.; Bôas, R.L.V. Influence of Potassium Doses on Macronutrient Levels in Lettuce Plants and Seeds. Hortic. Bras. 2010, 28, 287–291. [Google Scholar] [CrossRef]
- Viana, E.M.; Kiehl, J.d.C. Nitrogen and Potassium Doses on Wheat Growth. Bragantia 2010, 69, 975–982. [Google Scholar] [CrossRef]
- Alves, C.P.; da Silva, T.G.F.; Júnior, G.D.N.A.; de Souza, L.S.B.; Jardim, A.M.d.R.F.; de Araújo, G.G.L.; Queiroz, M.A.A.; e Silva, S.M.S.; Pinheiro, A.G.; Salvador, K.R.d.S. Morphophysiological indicators, phenophase and cutting time in an irrigated forage cactus–sorghum intercropping system under strategies of agricultural resilience and agriculture biosaline. Irrig. Drain. 2024, 74, 251–275. [Google Scholar] [CrossRef]
- MAPA. Standards for Identity, Quality, Packaging, and Presentation of Onions. Organization: Ministry of Agriculture, Livestock, and Supply. 1995. Available online: https://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=740098373 (accessed on 26 September 2024).
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; de la Rosa, A.P.B. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; 201p. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2023. Available online: https://www.R-project.org/ (accessed on 1 January 2023).
- Systat Software, Inc. SigmaPlot for Windows, Version 13.0; Systat Software, Inc.: San Jose, CA, USA, 2014. Available online: https://systatsoftware.com/products/sigmaplot/ (accessed on 1 January 2023).





| First Cycle | ||||||||
| Firmness (N) | SS (Brix°) | TA (%) | pH | |||||
| Doses (mL L−1) | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 |
| 0—Soil application | 60 ns | 65 ns | 10.3 ns | 9.6 ns | 0.66 ns | 0.65 ns | 5.38 ns | 5.50 ns |
| 1—Soil application | 61 ns | 61 ns | 10.3 ns | 9.9 ns | 0.65 ns | 0.65 ns | 5.28 ns | 5.39 ns |
| 2—Soil application | 50 ns | 63 ns | 10.0 ns | 9.9 ns | 0.66 ns | 0.65 ns | 5.42 ns | 5.32 ns |
| 3—Soil application | 62 ns | 63 ns | 10.3 ns | 9.7 ns | 0.66 ns | 0.65 ns | 5.33 ns | 5.35 ns |
| 4—Soil application | 63 ns | 69 ns | 10.1 ns | 9.6 ns | 0.67 ns | 0.66 ns | 5.32 ns | 5.36 ns |
| 2—Application on the leaf | 62 ns | 62 ns | 9.25 ns | 10.0 ns | 0.65 ns | 0.68 ns | 5.68 ns | 5.68 ns |
| p-value | 0.20 | 0.33 | 0.32 | 0.56 | 0.25 | 0.11 | 0.20 | 0.41 |
| Second Cycle | ||||||||
| Firmness (N) | SS (Brix°) | AT (%) | pH | |||||
| Doses (mL L−1) | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 |
| 0—Soil application | 52 ns | 57 ns | 9.0 ns | 9.2 ns | 0.56 ns | 0.55 ns | 5.34 ns | 5.42 ns |
| 1—Soil application | 52 ns | 57 ns | 8.5 ns | 9.2 ns | 0.55 ns | 0.53 ns | 5.24 ns | 5.31 ns |
| 2—Soil application | 50 ns | 52 ns | 8.9 ns | 9.0 ns | 0.56 ns | 0.54 ns | 5.38 ns | 5.24 ns |
| 3—Soil application | 42 ns | 55 ns | 8.8 ns | 9.1 ns | 0.54 ns | 0.54 ns | 5.29 ns | 5.27 ns |
| 4—Soil application | 54 ns | 69 ns | 9.9 ns | 9.1 ns | 0.53 ns | 0.54 ns | 5.26 ns | 5.28 ns |
| 2—Application on the leaf | 55 ns | 60 ns | 9.0 ns | 9.1 ns | 0.53 ns | 0.54 ns | 5.28 ns | 5.25 ns |
| p-value | 0.20 | 0.33 | 0.54 | 0.25 | 0.59 | 0.51 | 0.63 | 0.73 |
| First Cycle | ||||||||
| Chlorophyll a (mg g−1) | Chlorophyll b (mg g−1) | Total Chlorophyll (mg g−1) | Carotenoids (mg g−1) | |||||
| Doses (mlL−1) | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 |
| 0—Soil application | 8.74 ns | 9.10 ns | 3.99 ns | 5.02 ns | 12.73 ns | 14.12 ns | 2.27 ns | 1.13 ns |
| 1—Soil application | 8.74 ns | 8.34 ns | 3.67 ns | 4.08 ns | 12.41 ns | 12.42 ns | 1.98 ns | 1.77 ns |
| 2—Soil application | 11.55 ns | 12.07 ns | 5.01 ns | 5.52 ns | 16.56 ns | 17.60 ns | 2.80 ns | 2.42 ns |
| 3—Soil application | 11.72 ns | 13.85 ns | 4.80 ns | 5.56 ns | 16.53 ns | 19.41 ns | 2.57 ns | 3.60 ns |
| 4—Soil application | 12.87 ns | 11.39 ns | 5.45 ns | 5.06 ns | 18.33 ns | 16.45 ns | 2.80 ns | 2.58 ns |
| 2—Application on the leaf | 11.55 ns | 11.63 ns | 5.05 ns | 5.65 ns | 16.61 ns | 17.29 ns | 2.50 ns | 2.11 ns |
| p-value | 0.44 | 0.45 | 0.83 | 0.15 | 0.52 | 0.29 | 0.99 | 0.55 |
| Second Cycle | ||||||||
| Chlorophyll a (mg g−1) | Chlorophyll b (mg g−1) | Total Chlorophyll (mg g−1) | Carotenoids (mg g−1) | |||||
| Doses (mlL−1) | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 | IPA-10 | IPA-11 |
| 0—Soil application | 8.42 ns | 6.48 ns | 3.12 ns | 3.02 ns | 11.54 ns | 9.05 ns | 2.08 ns | 1.53 ns |
| 1—Soil application | 8.05 ns | 6.51 ns | 3.10 ns | 4.73 ns | 11.15 ns | 11.24 ns | 1.99 ns | 1.54 ns |
| 2—Soil application | 8.19 ns | 6.94 ns | 3.75 ns | 3.32 ns | 12.94 ns | 10.24 ns | 2.18 ns | 1.63 ns |
| 3—Soil application | 9.04 ns | 8.07 ns | 3.68 ns | 3.48 ns | 12.72 ns | 11.55 ns | 2.21 ns | 1.87 ns |
| 4—Soil application | 9.53 ns | 8.29 ns | 4.19 ns | 3.49 ns | 13.72 ns | 11.78 ns | 2.21 ns | 1.87 ns |
| 2—Application on the leaf | 8.94 ns | 6.06 ns | 3.61 ns | 3.15 ns | 12.55 ns | 9.21 ns | 2.20 ns | 1.62 ns |
| p-value | 0.30 | 0.51 | 0.01 | 0.07 | 0.70 | 0.46 | 0.39 | 0.77 |
| First Cycle | |||
| CEs (dS m−1) | |||
| Doses (mL L−1) | Beginning of Cycle | End of Cycle | |
| IPA-10 | IPA-11 | ||
| 0—Soil application | 1.7 a | 1.6 | |
| 1—Soil application | 1.5 ab | 1.47 | |
| 2—Soil application | 0.85 | 1.5 ab | 1.48 |
| 3—Soil application | 1.46 ab | 1.4 | |
| 4—Soil application | 1.2 b | 1.5 | |
| 2—Application on the leaf | 1.52 ab | 1.7 | |
| p-value | - | 0.85 | 0.06 |
| Second Cycle | |||
| ECs (dS m−1) | |||
| Doses (mL L−1) | Beginning of Cycle | End of Cycle | |
| IPA-10 | IPA-11 | ||
| 0—Soil application | 4.70 | 4.69 | |
| 1—Soil application | 4.44 | 4.53 | |
| 2—Soil application | 4.46 | 4.57 | |
| 3—Soil application | 1.5 | 4.55 | 4.46 |
| 4—Soil application | 4.56 | 4.44 | |
| 2—Application on the leaf | 4.49 | 4.48 | |
| p-value | 0.34 | 0.29 | |
| Physical–Hydric Characterization of the Soil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Depth | Ø | FC | Ds | Sand | Silt | Clay | EC-I | EC-II | ||
| cm | % | % Weight | g cm−3 | g kg−1 | dS m−1 | |||||
| 0–30 | 48.0 | 14.0 | 1.39 | 809.24 | 105.69 | 85.07 | 0.85 | 1.50 | ||
| Chemical Characterization of the Soil During the First Cycle | ||||||||||
| Depth (cm) | pH | O.M | V | H+Al | CTC | K+ | Ca2+ | Mg2+ | Na+ | |
| g kg−1 | % | cmolc dm−3 | ||||||||
| 6.7 | 8.8 | 75.1 | 1.8 | 7.3 | 0.54 | 3.7 | 1.21 | 0.04 | ||
| 0–30 | P | 2- S-SO4 | Fe2+ | Mn2+ | Cu2+ | Zn2+ | B | |||
| mg dm−3 | ||||||||||
| 57.8 | 4.56 | 85.0 | 46.4 | 1.4 | 1.4 | 0.32 | ||||
| Chemical Characterization of the Soil During the Second Cycle | ||||||||||
| Depth (cm) | pH | O.M | V | H+Al | CTC | K+ | Ca2+ | Mg2+ | Na+ | |
| g kg−1 | % | cmolc dm−3 | ||||||||
| 7.4 | 6.6 | 99.7 | 0.02 | 9.28 | 0.88 | 5.03 | 3.13 | 0.23 | ||
| 0–30 | P | 2- S-SO4 | Fe2+ | Mn2+ | Cu2+ | Zn2+ | B | |||
| mg dm−3 | ||||||||||
| 93.0 | 3.0 | 143.6 | 68.0 | 1.78 | 2.6 | 0.64 | ||||
| Properties of Irrigation Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC | pH | Hardness-CaCO3 | B | Cu | Mn | Fe | Zn | |||
| dS m−1 | mg L−1 | |||||||||
| 1.68 | 6.63 | 610.5 | 0.129 | 0.019 | 0.04 | <LQ | ||||
| Ca2+ | Mg2+ | K+ | Na+ | CO32− | NaHCO3 | Cl− | SO42− | RAS | ||
| mmolc L−1 | ||||||||||
| 5.8 | 6.3 | 0.30 | 2.04 | 0.00 | 3.90 | 11.6 | 0.09 | 0.83 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, J.C.; dos Santos Gomes Calaça, J.; Barbosa de Souza Gomes, C.V.; Callou Menezes, L.E.; Inácio Silva, J.R.; Jardim, A.M.d.R.F.; Pessoa, L.G.M.; Barbosa da Silva, J.H.; Freire da Silva, R.; Dias, T.J.; et al. Freshwater Algae Biostimulant in Mitigating Impacts of Saline Irrigation on Onions. Plants 2025, 14, 1559. https://doi.org/10.3390/plants14101559
Nogueira JC, dos Santos Gomes Calaça J, Barbosa de Souza Gomes CV, Callou Menezes LE, Inácio Silva JR, Jardim AMdRF, Pessoa LGM, Barbosa da Silva JH, Freire da Silva R, Dias TJ, et al. Freshwater Algae Biostimulant in Mitigating Impacts of Saline Irrigation on Onions. Plants. 2025; 14(10):1559. https://doi.org/10.3390/plants14101559
Chicago/Turabian StyleNogueira, Jean Carlos, Jefferson dos Santos Gomes Calaça, Carla Veronica Barbosa de Souza Gomes, Luiz Emanuel Callou Menezes, José Raliuson Inácio Silva, Alexandre Maniçoba da Rosa Ferraz Jardim, Luiz Guilherme Medeiros Pessoa, João Henrique Barbosa da Silva, Ramon Freire da Silva, Thiago Jardelino Dias, and et al. 2025. "Freshwater Algae Biostimulant in Mitigating Impacts of Saline Irrigation on Onions" Plants 14, no. 10: 1559. https://doi.org/10.3390/plants14101559
APA StyleNogueira, J. C., dos Santos Gomes Calaça, J., Barbosa de Souza Gomes, C. V., Callou Menezes, L. E., Inácio Silva, J. R., Jardim, A. M. d. R. F., Pessoa, L. G. M., Barbosa da Silva, J. H., Freire da Silva, R., Dias, T. J., & Barros Júnior, G. (2025). Freshwater Algae Biostimulant in Mitigating Impacts of Saline Irrigation on Onions. Plants, 14(10), 1559. https://doi.org/10.3390/plants14101559







