Invasion Status, Mechanisms, and Future Distribution Prediction of Solidago canadensis in the Trade Port Region: A Case Study of Ningbo Port, China
Abstract
1. Introduction
2. Results
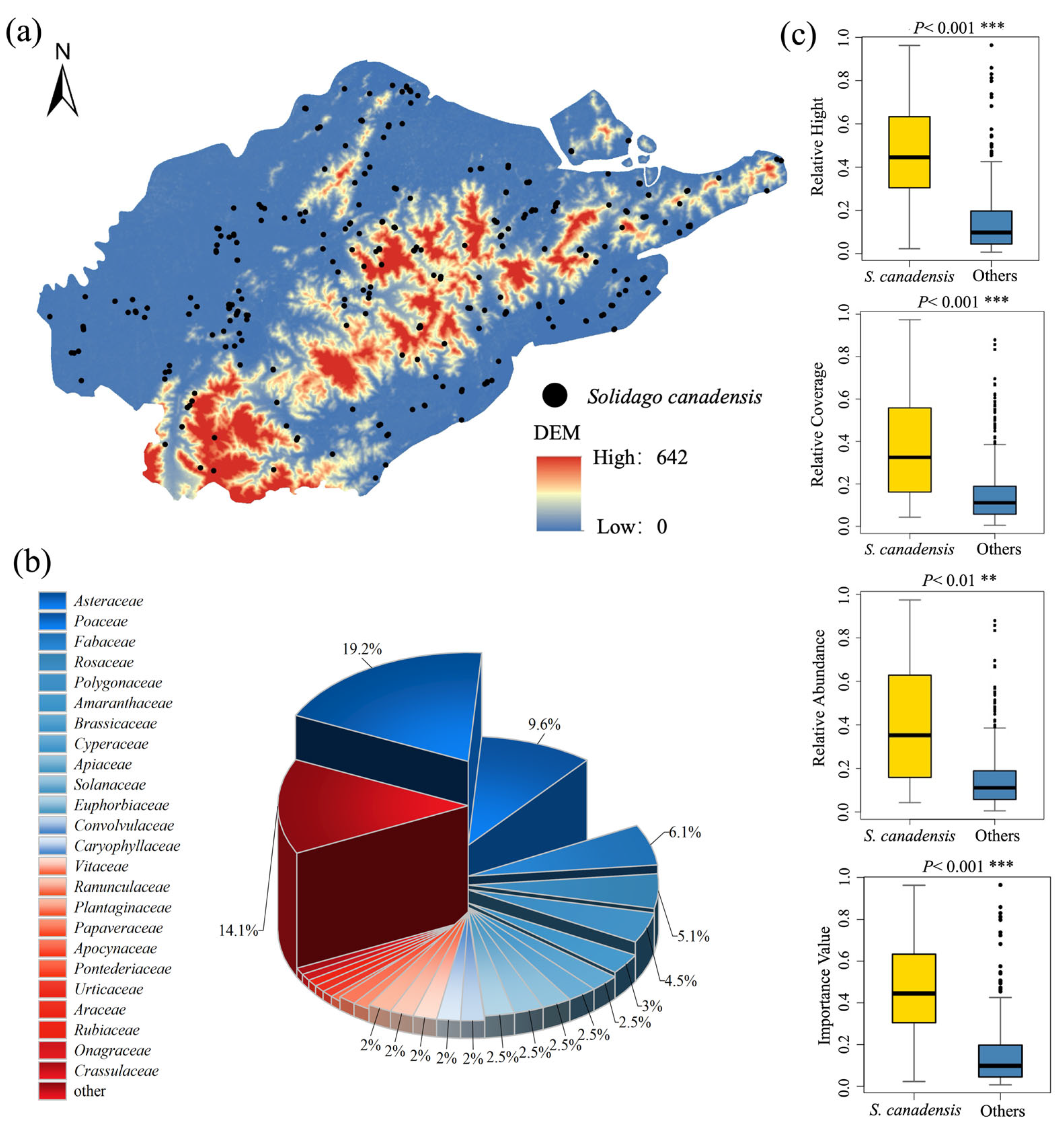
2.1. Invasion Status and Risk Level
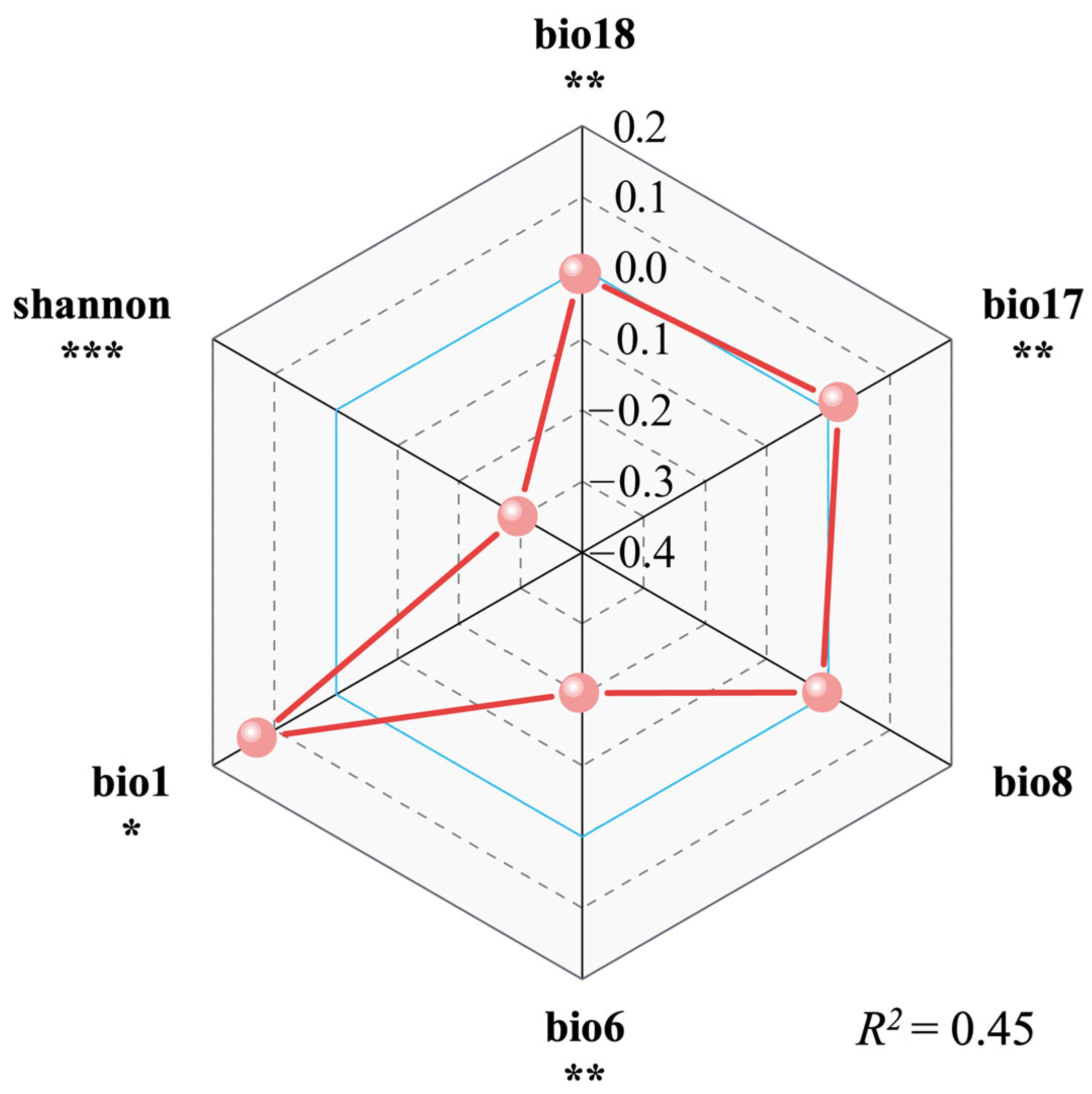
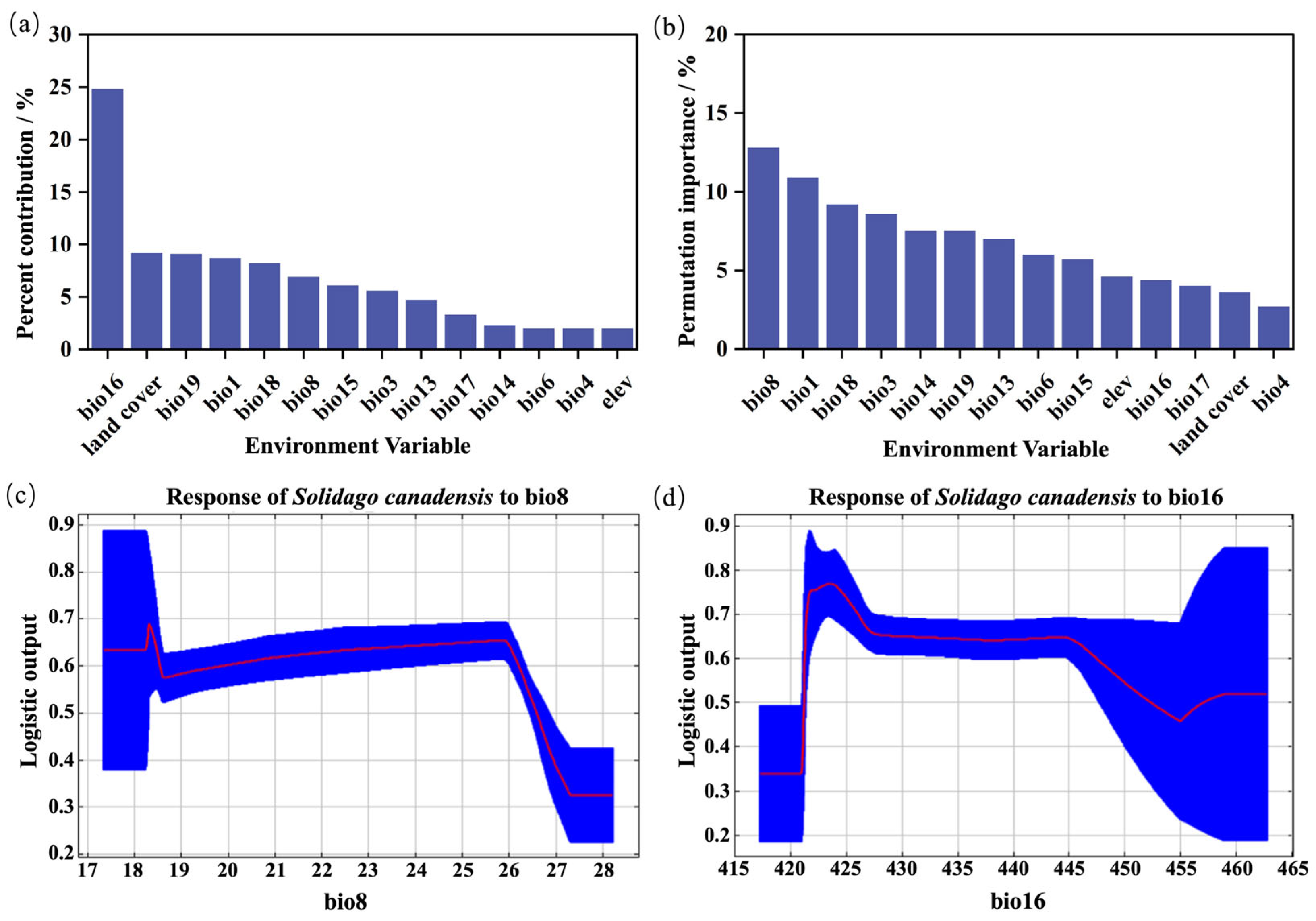
2.2. The Relationship Between Invasion Intensity, Plant Diversity, and Environmental Factors
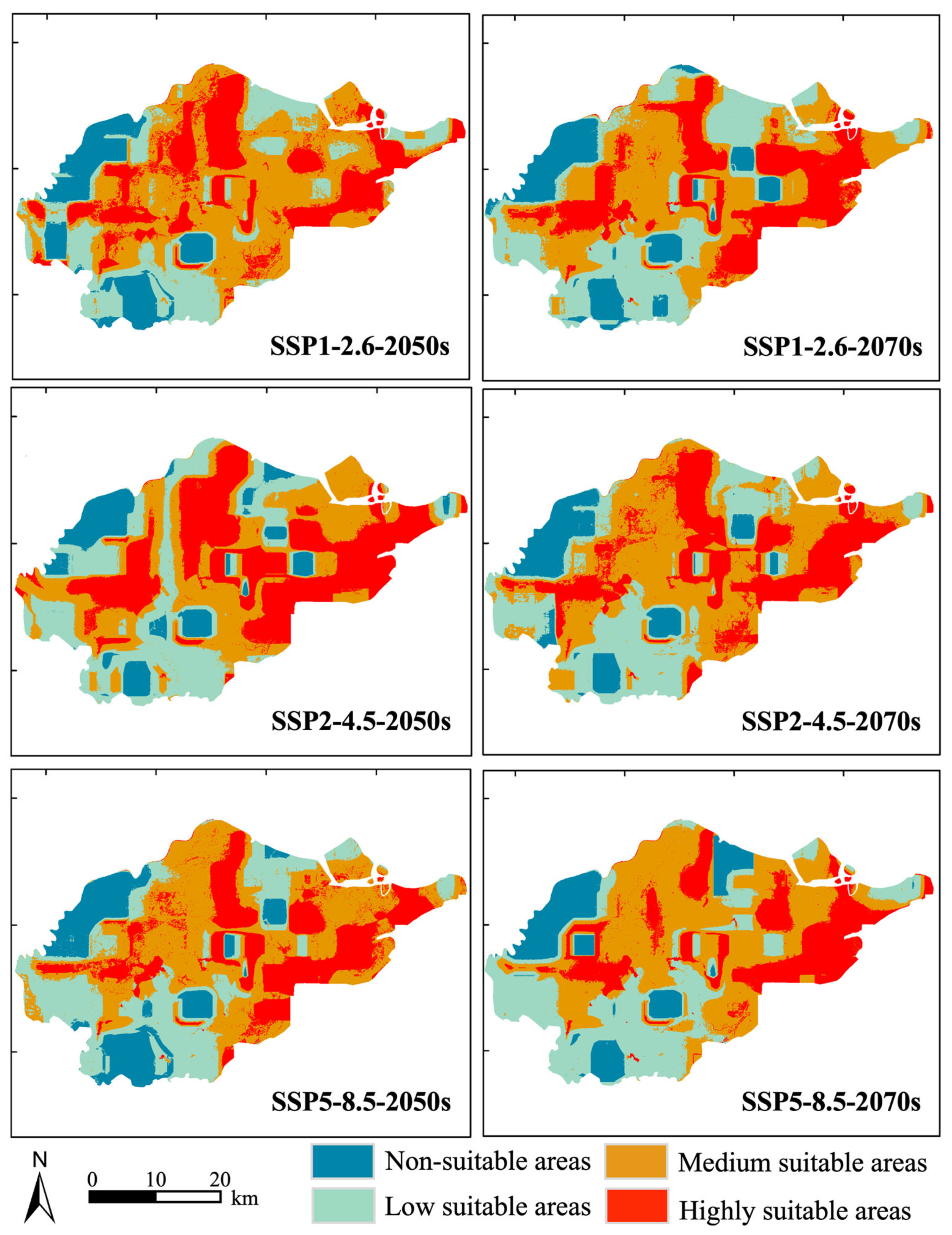
2.3. Potential Distribution of S. canadensis Under Climate Scenarios
3. Discussion
3.1. The Invasion of S. canadensis Was Severe in the Port Region
3.2. The Major Underlying Drivers of S. canadensis Invasion
3.3. The Potential Area of S. canadensis Under Different Climate Scenarios
4. Materials and Methods
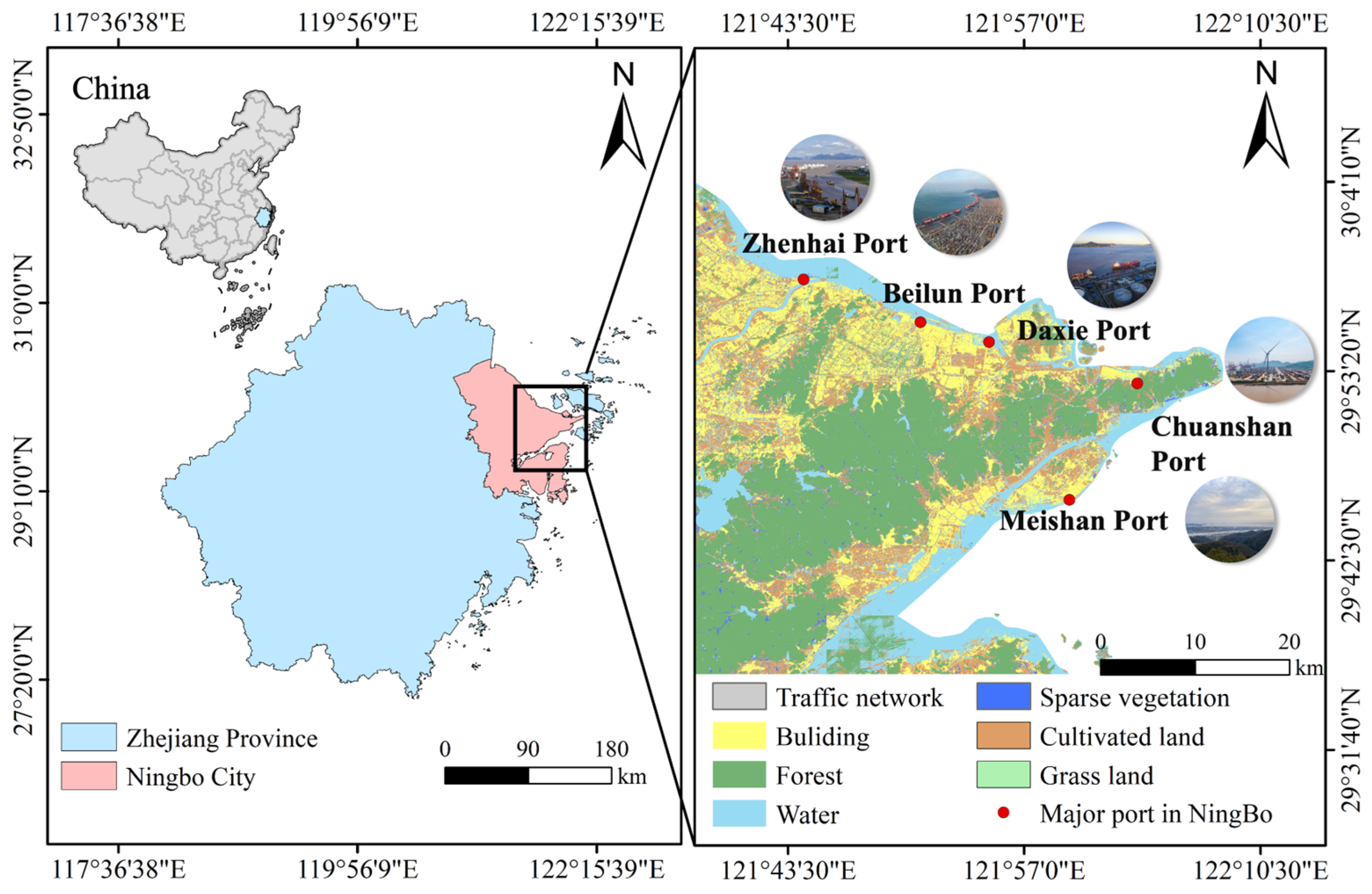
4.1. Study Area
4.2. Methods
4.2.1. Field Community Surveys
4.2.2. Environmental Data
4.2.3. Risk Assessment of Biological Invasions
4.2.4. Invasion Mechanism Analysis
4.2.5. Prediction of Suitable Habitat Area for S. canadensis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global Threats from Invasive Alien Species in the Twenty-First Century and National Response Capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Unwelcome Exchange: International Trade as a Direct and Indirect Driver of Biological Invasions Worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Hao, Q.; Ma, J.-S. Invasive Alien Plants in China: An Update. Plant Divers 2022, 45, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, H.; Li, M.; Qiang, S.; Guo, J.; Han, Z.; Huang, Z.; Sun, H.; He, S.; Wu, H.; et al. The Distribution and Economic Losses of Alien Species Invasion to China. Biol. Invasions 2006, 8, 1495–1500. [Google Scholar] [CrossRef]
- Kang, L.; Wu, W.; Yu, H.; Su, F. Global Container Port Network Linkages and Topology in 2021. Sensors 2022, 22, 5889. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Li, D.; Baiser, B.; Kühn, I.; Aronson, M.F.J.; Carboni, M.; Celesti-Grapow, L.; de Matos, A.C.L.; Lososová, Z.; Montaño-Centellas, F.A.; et al. Cities Shape the Diversity and Spread of Nonnative Species. Annu. Rev. Ecol. Evol. Syst. 2024, 55, 157–180. [Google Scholar] [CrossRef]
- Jehlík, V.; Dostálek, J.; Frantík, T. Alien Plants in Central European River Ports. Neobiota 2019, 45, 93–115. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; Van Kleunen, M.; Weber, E.; Winter, M.; et al. Global Trade Will Accelerate Plant Invasions in Emerging Economies under Climate Change. Glob. Change Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [PubMed]
- Sardain, A.; Sardain, E.; Leung, B. Global Forecasts of Shipping Traffic and Biological Invasions to 2050. Nat. Sustain. 2019, 2, 274–282. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Mooney, H.A. Invasive Alien Species in an Era of Globalization. Front. Ecol. Environ. 2007, 5, 199–208. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef] [PubMed]
- Dawson, W.; Rohr, R.P.; Van Kleunen, M.; Fischer, M. Alien Plant Species with a Wider Global Distribution Are Better Able to Capitalize on Increased Resource Availability. New Phytol. 2012, 194, 859–867. [Google Scholar] [CrossRef]
- Huenneke, L.F.; Hamburg, S.P.; Koide, R.; Mooney, H.A.; Vitousek, P.M. Effects of Soil Resources on Plant Invasion and Community Structure in Californian Serpentine Grassland. Ecology 1990, 71, 478–491. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wei, M.; Wang, S.; Wu, B.; Du, D. Plant Height and Leaf Size: Which One Is More Important in Affecting the Successful Invasion of Solidago Canadensis and Conyza Canadensis in Urban Ecosystems? Urban For. Urban Green. 2021, 59, 127033. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of Invasive Alien Plants on Native Plant Communities and Natura 2000 Habitats: State of the Art, Gap Analysis and Perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Lei, Y.-B.; Wang, R.-F.; Callaway, R.M.; Valiente-Banuet, A.; Inderjit; Li, Y.-P.; Zheng, Y.-L. Evolutionary Tradeoffs for Nitrogen Allocation to Photosynthesis versus Cell Walls in an Invasive Plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef]
- Huang, K.; Kong, D.-L.; Lu, X.-R.; Feng, W.-W.; Liu, M.-C.; Feng, Y.-L. Lesser Leaf Herbivore Damage and Structural Defense and Greater Nutrient Concentrations for Invasive Alien Plants: Evidence from 47 Pairs of Invasive and Non-Invasive Plants. Sci. Total Environ. 2020, 723, 137829. [Google Scholar] [CrossRef]
- Lin, T.; Doorduin, L.; Temme, A.; Pons, T.L.; Lamers, G.E.M.; Anten, N.P.R.; Vrieling, K. Enemies Lost: Parallel Evolution in Structural Defense and Tolerance to Herbivory of Invasive Jacobaea Vulgaris. Biol. Invasions 2015, 17, 2339–2355. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Springer Nature: Berlin, Germany, 2020; pp. 23–30. [Google Scholar]
- Funk, J.L.; Vitousek, P.M. Resource-Use Efficiency and Plant Invasion in Low-Resource Systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- Raheem, A.; Yohanna, P.; Li, G.; Noh, N.J.; Iqbal, B.; Tang, J.; Du, D.; Alahmadi, T.A.; Ansari, M.J.; Zhan, A.; et al. Unraveling the Ecological Threads: How Invasive Alien Plants Influence Soil Carbon Dynamics. J. Environ. Manag. 2024, 356, 120556. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, H.; Wu, B.; Jiang, K.; Wang, S.; Wei, M.; Du, D. The Functional Diversity of Native Ecosystems Increases during the Major Invasion by the Invasive Alien Species, Conyza canadensis. Ecol. Eng. 2021, 159, 106093. [Google Scholar] [CrossRef]
- Hejda, M.; Sádlo, J.; Kutlvašr, J.; Petřík, P.; Vítková, M.; Vojík, M.; Pyšek, P.; Pergl, J. Impact of Invasive and Native Dominants on Species Richness and Diversity of Plant Communities. Preslia 2021, 93, 181–201. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of Two Invasive Plant Invaders in Europe (Solidago canadensis and Solidago gigantea) as Possible Sources of Botanical Insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Zhou, J.; Wu, B. Solidago canadensis Invasion Affects Soil N-Fixing Bacterial Communities in Heterogeneous Landscapes in Urban Ecosystems in East China. Sci. Total Environ. 2018, 631–632, 702–713. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Dai, S.; Meng, L.; He, M.; Chen, S.; Zhao, C.; Dan, X.; Cai, Z.; Zhang, J.; et al. Effects of Solidago canadensis L. on Mineralization-Immobilization Turnover Enhance Its Nitrogen Competitiveness and Invasiveness. Sci. Total Environ. 2023, 882, 163641. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Liu, J.; Zhou, J.; Wu, B. Moderate and Heavy Solidago canadensis L. Invasion Are Associated with Decreased Taxonomic Diversity but Increased Functional Diversity of Plant Communities in East China. Ecol. Eng. 2018, 112, 55–64. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do Allelopathic Compounds in Invasive Solidago canadensis s.l. Restrain the Native European Flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Ningbo Municipal Bureau of Statistics. Ningbo Statistical Yearbook; China Statistics Press: Beijing, China, 2022; pp. 258–271.
- Wilson, J.R.U.; Dormontt, E.E.; Prentis, P.J.; Lowe, A.J.; Richardson, D.M. Something in the Way You Move: Dispersal Pathways Affect Invasion Success. Trends Ecol. Evol. 2009, 24, 136–144. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, Transport and Trouble: Managing Invasive Species Pathways in an Era of Globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Lan, R.; Qiao, B. Study on the Invasion Mechanism of Alien Species through Ship Activities. IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 052104. [Google Scholar] [CrossRef]
- Dafforn, K. Future-Proofing Our Ports against Biological Invasion. Curr. Opin. Environ. Sustain. 2025, 73, 101518. [Google Scholar] [CrossRef]
- Adomako, M.O.; Ning, L.; Tang, M.; Du, D.-L.; Van Kleunen, M.; Yu, F.-H. Diversity- and Density-Mediated Allelopathic Effects of Resident Plant Communities on Invasion by an Exotic Plant. Plant Soil 2019, 440, 581–592. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, G.; Bu, D.; Sun, G.; Qiang, S. Long−Distance Wind Dispersal Drives Population Range Expansion of Solidago Canadensis. Plants 2022, 11, 2734. [Google Scholar] [CrossRef]
- Vojík, M.; Boublík, K. Fear of the Dark: Decline in Plant Diversity and Invasion of Alien Species Due to Increased Tree Canopy Density and Eutrophication in Lowland Woodlands. Plant Ecol. 2018, 219, 749–758. [Google Scholar] [CrossRef]
- Biswas, S.R.; He, D.; Li, J.; Gong, L.; Biswas, P.L.; Zhuo, Z.; Xu, M.; Yang, X.; Yan, E. Putting Space into Trait Ecology: Trait, Environment and Biodiversity Relationships at Multiple Spatial Scales. J. Ecol. 2024, 112, 613–628. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of Increased Competitive Ability in Invasive Nonindigenous Plants: A Hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Mei, D.; Jian-Zhong, L.; Wen-Ju, Z.; Jia-Kuan, C.; Bo, L. Canada Goldenrod (Solidago canadensis): An Invasive Alien Weed Rapidly Spreading in China. J. Syst. Evol. 2006, 44, 72–85. [Google Scholar]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A Meta-analysis of Biotic Resistance to Exotic Plant Invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Byun, C.; De Blois, S.; Brisson, J. Plant Functional Group Identity and Diversity Determine Biotic Resistance to Invasion by an Exotic Grass. J. Ecol. 2013, 101, 128–139. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate Change and Biological Invasions: Evidence, Expectations, and Response Options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, S.; Wang, N.; Fan, P.; You, C.; Wang, R.; Zheng, P.; Wang, H. Dynamics of the Distribution of Invasive Alien Plants (Asteraceae) in China under Climate Change. Sci. Total Environ. 2023, 903, 166260. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
- Zhou, X.-H.; He, W.-M. Climate Warming Facilitates Seed Germination in Native but Not Invasive Solidago canadensis Populations. Front. Ecol. Evol. 2020, 8, 595214. [Google Scholar] [CrossRef]
- Li, H.; Ali, A.; Luo, X.; Liao, K.; Sun, N.; Xu, M.; Sha, L.; He, D.; Du, Y.; Sun, W.; et al. China’s Subtropical Deciduous Plants Are More Sensitive to Climate Change than Evergreen Plants by Flowering Phenology. Glob. Change Biol. 2024, 30, e17168. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Dawson, W.; Moser, D.; Van Kleunen, M.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; Blackburn, T.M.; et al. Global Hotspots and Correlates of Alien Species Richness across Taxonomic Groups. Nat. Ecol. Evol. 2017, 1, 0186. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Wu, Y.; Jiang, L. Are Hong Kong and Taiwan Stepping-Stones for Invasive Species to the Mainland of China? Ecol. Evol. 2018, 8, 1966–1973. [Google Scholar] [CrossRef]
- Li, X.; Fu, J.; Jiang, D.; Lin, G.; Cao, C. Land Use Optimization in Ningbo City with a Coupled GA and PLUS Model. J. Clean. Prod. 2022, 375, 134004. [Google Scholar] [CrossRef]
- Li, S.; Pan, Q.; Yang, X.; Gao, Y.; Xu, M. Differential Influences of Island Biogeographic Effects on the Diversity among Three Plant Life-Forms. Glob. Ecol. Conserv. 2025, 58, e03499. [Google Scholar] [CrossRef]
- Tang, Y.T.; Chan, F.K.S.; Griffiths, J. City Profile: Ningbo. Cities 2015, 42, 97–108. [Google Scholar] [CrossRef]
- Wu, Z. Chinese Vegetation, 1st ed.; Science Press: Beijing, China, 1980; pp. 823–836. [Google Scholar]
- Roy, H.E.; Rabitsch, W.; Scalera, R.; Stewart, A.; Gallardo, B.; Genovesi, P.; Essl, F.; Adriaens, T.; Bacher, S.; Booy, O.; et al. Developing a Framework of Minimum Standards for the Risk Assessment of Alien Species. J. Appl. Ecol. 2018, 55, 526–538. [Google Scholar] [CrossRef]
- Ou, J.; Lu, C.; O’Toole, D.K. A Risk Assessment System for Alien Plant Bio-Invasion in Xiamen, China. J. Environ. Sci. 2008, 20, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Kreft, H.; Jetz, W. Global Patterns and Determinants of Vascular Plant Diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef]
- Tello, R.; Crewson, P.E. Hypothesis Testing II: Means. Radiology 2003, 227, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Anibaba, Q.A.; Dyderski, M.K.; Jagodziński, A.M. Predicted Range Shifts of Invasive Giant Hogweed (Heracleum mantegazzianum) in Europe. Sci. Total Environ. 2022, 825, 154053. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the Potentially Suitable Areas of Litsea cubeba in China Based on Future Climate Change Using the Optimized MaxEnt Model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
| Community Type | Companion Species | Number of Plots | Coverage (Mean ± SD %) * | Height (Mean ± SD cm) * | |
|---|---|---|---|---|---|
| 1 | Solidago canadensis | Setaria viridis, Lactuca indica, Vicia sepium, Artemisia indica | 166 | 34.7 ± 19 | 64.5 ± 50.4 |
| 2 | Veronica persica | Geranium carolinianum, Vicia sepium, Stellaria media | 81 | 20.2 ± 16.9 | 7.1 ± 5.1 |
| 3 | Galium spurium | Veronica persica, Solidago canadensis, Artemisia indica | 60 | 12.50 ± 8.9 | 9.9 ± 17.3 |
| 4 | Alternanthera philoxeroides | Solidago canadensis, Erigeron canadensis | 45 | 32.50 ± 20.6 | 25.1 ± 13.3 |
| 5 | Artemisia indica | Veronica persica, Erigeron sumatrensis, Trifolium repens | 45 | 12.70 ± 5.9 | 6.2 ± 14.1 |
| 6 | Setaria viridis | Symphyotrichum subulatum, Solidago canadensis, Anthriscus sylvestris | 41 | 15.0 ± 5.9 | 72.4 ± 30.3 |
| 7 | Oxalis corniculata | Solidago canadensis, Stellaria media | 36 | 8.20 ± 3.8 | 9.4 ± 6.2 |
| 8 | Symphyotrichum subulatum | Artemisia argyi, Erigeron canadensis | 29 | 15.10 ± 8.6 | 67.1 ± 41.3 |
| 9 | Spartina alterniflora | Phragmites australis | 26 | 50.80 ± 28.2 | 75.7 ± 42.2 |
| 10 | Digitaria sanguinalis | Solidago canadensis, Symphyotrichum subulatum, Humulus scandens | 24 | 20.80 ± 13.2 | 41.2 ± 19.7 |
| 11 | Geranium carolinianum | Stellaria media, Cerastium glomeratum | 22 | 16.90 ± 7.8 | 8.4 ± 8.6 |
| 12 | Erigeron canadensis | Oxalis corniculata, Pseudognaphalium affine | 20 | 28.80 ± 12.5 | 55.6 ± 50.5 |
| Total number of plots | 595 |
| Type | Factors | Description |
|---|---|---|
| Climate data | bio1 | Average temperature for the year |
| bio2 | Mean daily temperature variation | |
| bio3 | The consistency of temperature (bio2/bio7) (×100) | |
| bio4 | Seasonal temperature variation | |
| bio5 | Highest temperature in the warmest month | |
| bio6 | Minimum temperature of the coldest month | |
| bio7 | Annual temperature variation (bio5-bio6) | |
| bio8 | Average temperature of the wettest season | |
| bio9 | Temperature average of the driest season | |
| bio10 | Temperature average of the warmest season | |
| bio11 | Temperature average of the coldest season | |
| bio12 | Total precipitation for the year | |
| bio13 | Total precipitation in the wettest month | |
| bio14 | Total precipitation in the driest month | |
| bio15 | Seasonal variation in precipitation (coefficient of variation) | |
| bio16 | Total precipitation in the wettest season | |
| bio17 | Precipitation during the driest season | |
| bio18 | Total precipitation in the warmest season | |
| bio19 | Total precipitation in the coldest season |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Shen, S.; Liao, K.; Li, S.; Pan, Q.; Ma, J.; Li, W.; Yang, X. Invasion Status, Mechanisms, and Future Distribution Prediction of Solidago canadensis in the Trade Port Region: A Case Study of Ningbo Port, China. Plants 2025, 14, 1546. https://doi.org/10.3390/plants14101546
Luo X, Shen S, Liao K, Li S, Pan Q, Ma J, Li W, Yang X. Invasion Status, Mechanisms, and Future Distribution Prediction of Solidago canadensis in the Trade Port Region: A Case Study of Ningbo Port, China. Plants. 2025; 14(10):1546. https://doi.org/10.3390/plants14101546
Chicago/Turabian StyleLuo, Xu, Sixiao Shen, Ke Liao, Saiqiang Li, Qinqin Pan, Jiahao Ma, Weiqiang Li, and Xiaodong Yang. 2025. "Invasion Status, Mechanisms, and Future Distribution Prediction of Solidago canadensis in the Trade Port Region: A Case Study of Ningbo Port, China" Plants 14, no. 10: 1546. https://doi.org/10.3390/plants14101546
APA StyleLuo, X., Shen, S., Liao, K., Li, S., Pan, Q., Ma, J., Li, W., & Yang, X. (2025). Invasion Status, Mechanisms, and Future Distribution Prediction of Solidago canadensis in the Trade Port Region: A Case Study of Ningbo Port, China. Plants, 14(10), 1546. https://doi.org/10.3390/plants14101546







