Effects of Early-Stage Treeline Shifts on Soil Microbial Biomass and Catabolic Diversity in Reserved and Grazed Subalpine Meadows
Abstract
1. Introduction
2. Results
2.1. Plant Residues
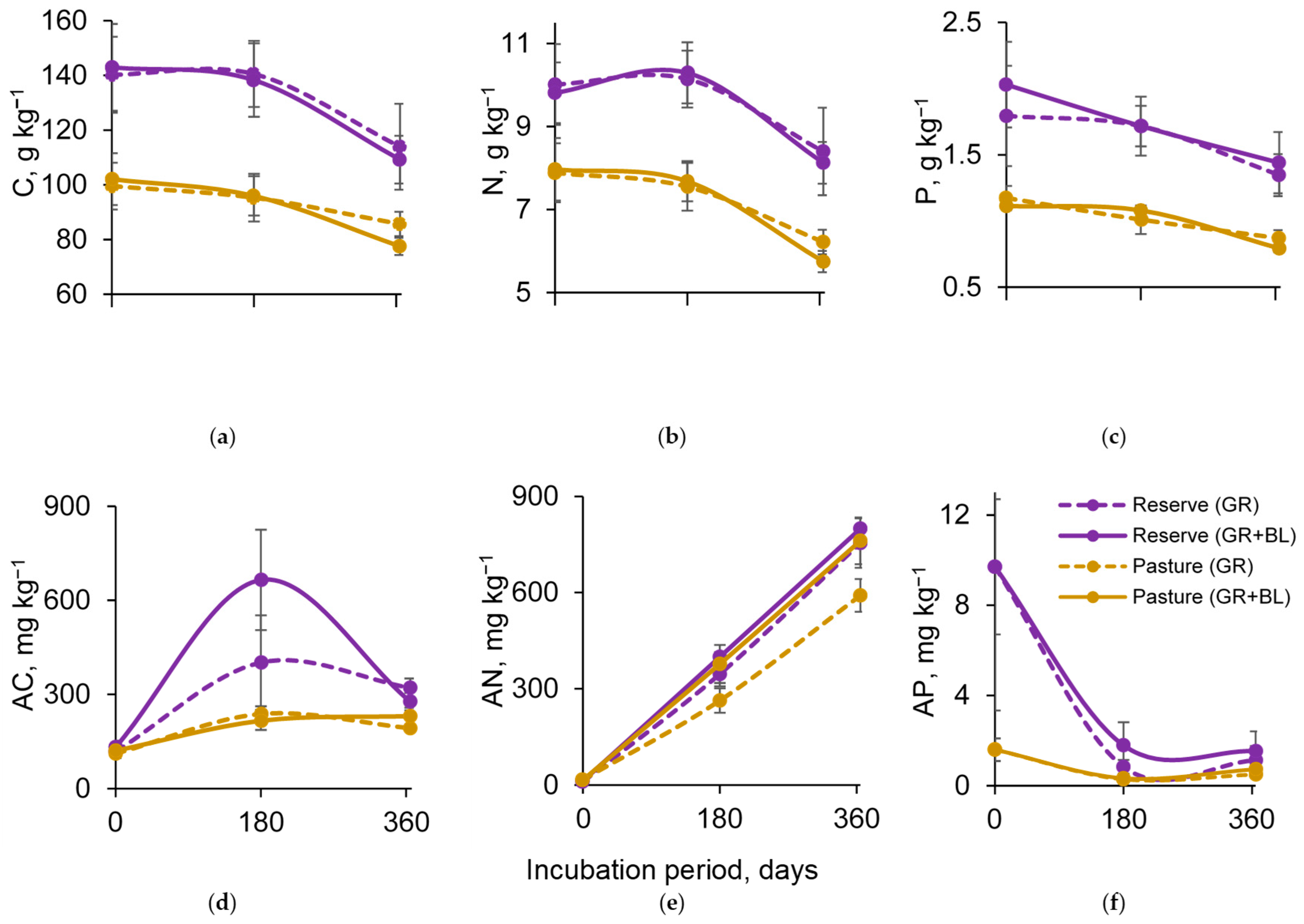
2.2. Soil C, N, and P Content
2.3. C, N, and P Content in Soil Microbial Biomass
2.4. Catabolic Activity and Diversity of the Soil Microbial Community
2.5. Quality of Plant Residues and Dynamics of Soil Microbial Properties
3. Discussion
3.1. Early Changes in Soil Microbial Biomass and Activity Induced by Plant Residue Quality
3.2. Enhancing Microbial Catabolic Diversity with Plant Residue Recalcitrance
3.3. Study Limitations and Future Research
4. Materials and Methods
4.1. Study Sites
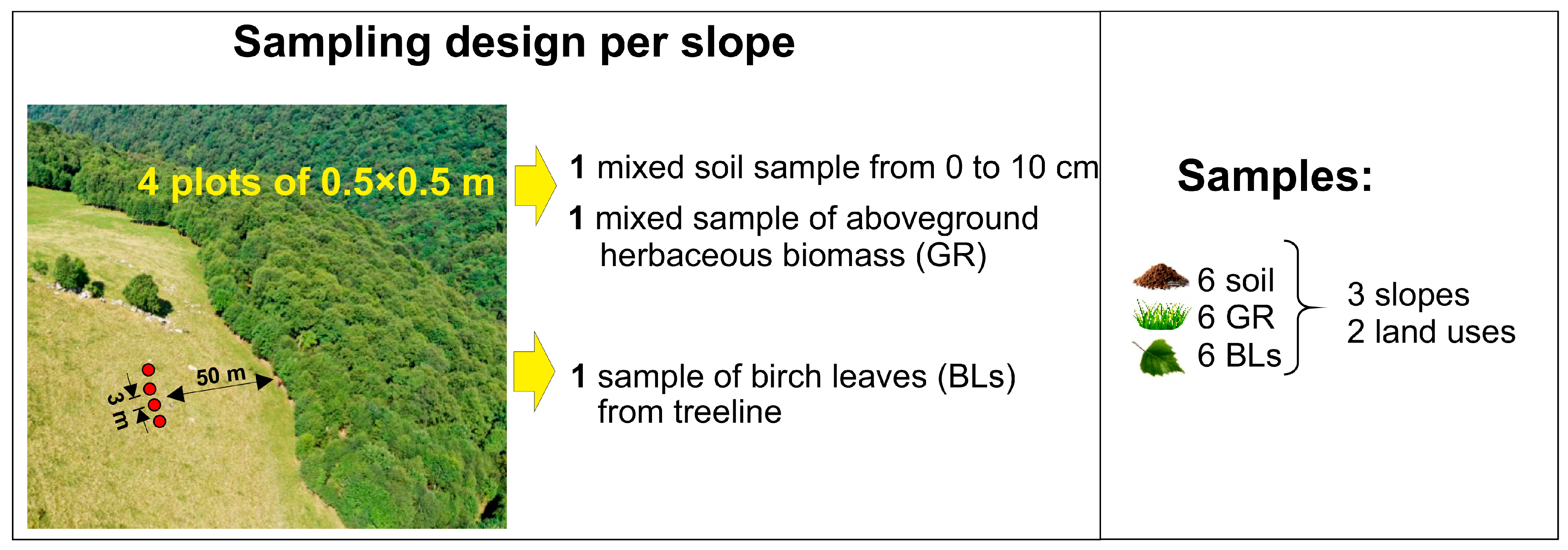
4.2. Sampling and Treatments
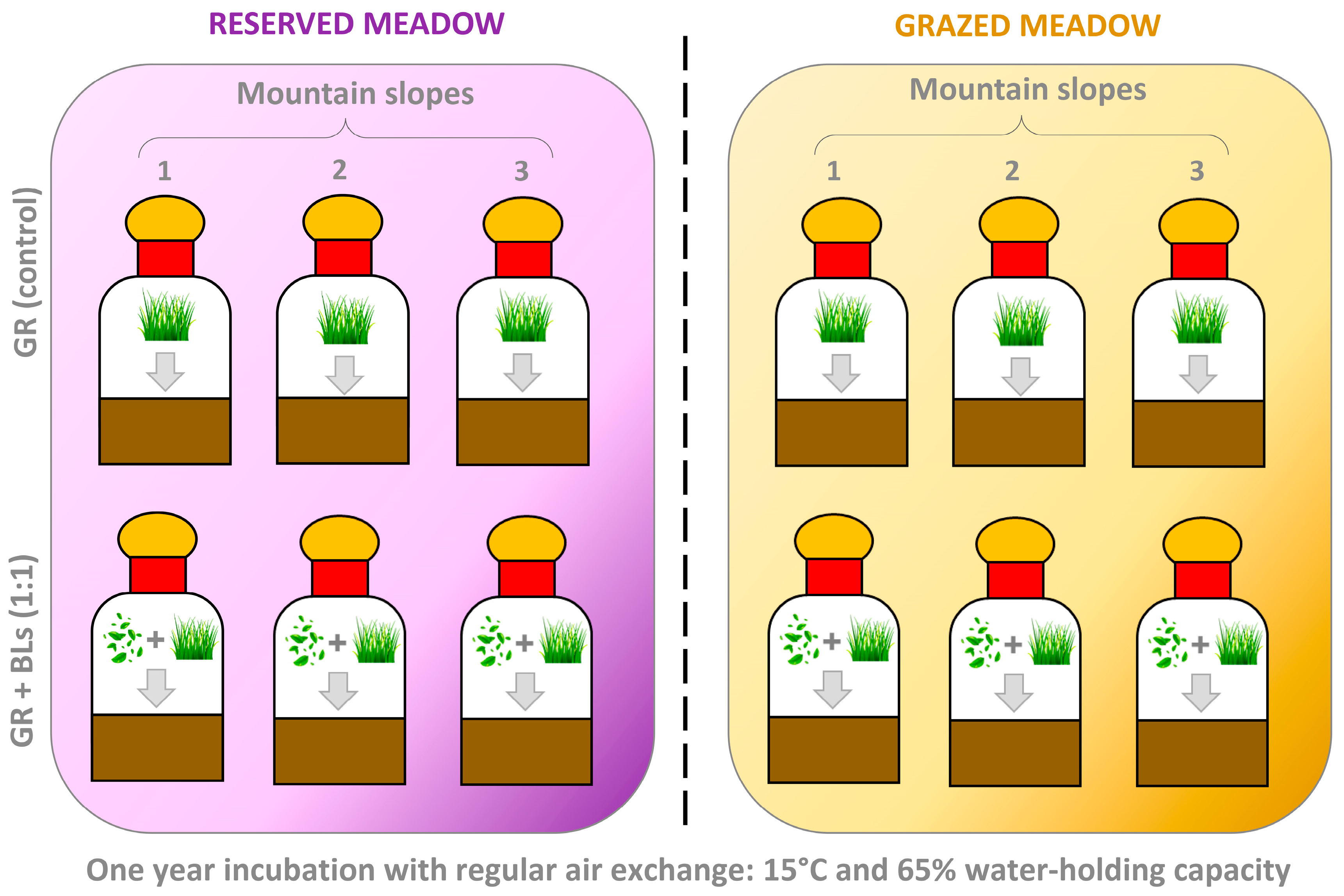
4.3. The Incubation Experiment
4.4. Soil Samples’ Analysis
4.5. Plant Residuals’ Analysis
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.; Chandra Garkoti, S.; Borgaonkar, H.P. Composition, Age-Structure and Dendroecology of High Altitude Treeline Forest in the Western Himalaya, India. For. Ecol. Manag. 2023, 549, 121494. [Google Scholar] [CrossRef]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are Treelines Advancing? A Global Meta-Analysis of Treeline Response to Climate Warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef]
- Lu, X.; Liang, E.; Wang, Y.; Babst, F.; Camarero, J.J. Mountain Treelines Climb Slowly despite Rapid Climate Warming. Glob. Ecol. Biogeogr. 2021, 30, 305–315. [Google Scholar] [CrossRef]
- Gaire, N.P.; Fan, Z.-X.; Chhetri, P.K.; Shah, S.K.; Bhuju, D.R.; Wang, J.; Sharma, B.; Shi, P.; Dhakal, Y.R. Treeline Dynamics in Nepal Himalaya in a Response to Complexity of Factors. In Ecology of Himalayan Treeline Ecotone; Singh, S.P., Reshi, Z.A., Joshi, R., Eds.; Springer Nature: Singapore, 2023; pp. 519–563. ISBN 978-981-19-4476-5. [Google Scholar]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhary, R.P.; Müller, M.; Scholten, T.; Schwab, N.; Weidinger, J. The Treeline Ecotone in Rolwaling Himal, Nepal: Pattern-Process Relationships and Treeline Shift Potential. In Ecology of Himalayan Treeline Ecotone; Singh, S.P., Reshi, Z.A., Joshi, R., Eds.; Springer Nature: Singapore, 2023; pp. 95–145. ISBN 978-981-19-4476-5. [Google Scholar]
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Tree Line Shifts in the Swiss Alps: Climate Change or Land Abandonment? J. Veg. Sci. 2007, 18, 571–582. [Google Scholar] [CrossRef]
- Brofas, G.; Karetsos, G.; Dimopoulos, P.; Tsagari, C. The Natural Environment of Cupressus Sempervirens in Greece as a Basis for Its Use in the Mediterranean Region. Land Degrad. Dev. 2006, 17, 645–659. [Google Scholar] [CrossRef]
- Sushko, S.; Ivashchenko, K.; Komarova, A.; Yudina, A.; Makhantseva, V.; Elsukova, E.; Blagodatsky, S. Shifting Mountain Tree Line Increases Soil Organic Carbon Stability Regardless of Land Use. Plants 2024, 13, 1193. [Google Scholar] [CrossRef]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above- and Belowground Linkages Shape Responses of Mountain Vegetation to Climate Change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial eficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Pingthaisong, W.; Blagodatsky, S.; Vityakon, P.; Cadisch, G. Mixing Plant Residues of Different Quality Reduces Priming Effect and Contributes to Soil Carbon Retention. Soil Biol. Biochem. 2024, 188, 109242. [Google Scholar] [CrossRef]
- Canals, R.M.; Múgica, L.; Durán, M.; Emeterio, L.S. Soil Bacterial Functional Diversity Mirrors the Loss of Plant Diversity by the Expansion of a Native Tall-Grass in High Mountain Grasslands. Plant Soil 2019, 445, 243–257. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Hines, J.; Isbell, F.; van der Plas, F.; Hobbie, S.E.; Kazanski, C.E.; Lehmann, A.; Liu, M.; Lochner, A.; Rillig, M.C.; et al. Plant Diversity Maintains Multiple Soil Functions in Future Environments. eLife 2018, 7, e41228. [Google Scholar] [CrossRef]
- Zhang, Y.; Ai, J.; Sun, Q.; Li, Z.; Hou, L.; Song, L.; Tang, G.; Li, L.; Shao, G. Soil Organic Carbon and Total Nitrogen Stocks as Affected by Vegetation Types and Altitude across the Mountainous Regions in the Yunnan Province, South-Western China. CATENA 2021, 196, 104872. [Google Scholar] [CrossRef]
- Ivashchenko, K.; Sushko, S.; Selezneva, A.; Ananyeva, N.; Zhuravleva, A.; Kudeyarov, V.; Makarov, M.; Blagodatsky, S. Soil Microbial Activity along an Altitudinal Gradient: Vegetation as a Main Driver beyond Topographic and Edaphic Factors. Appl. Soil Ecol. 2021, 168, 104197. [Google Scholar] [CrossRef]
- Komarova, A.; Ivashchenko, K.; Sushko, S.; Zhuravleva, A.; Vasenev, V.; Blagodatsky, S. Temperature Sensitivity of Topsoil Organic Matter Decomposition Does Not Depend on Vegetation Types in Mountains. Plants 2022, 11, 2765. [Google Scholar] [CrossRef]
- Wang, H.; Boutton, T.W.; Xu, W.; Hu, G.; Jiang, P.; Bai, E. Quality of Fresh Organic Matter Affects Priming of Soil Organic Matter and Substrate Utilization Patterns of Microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial Decomposition of Soil Organic Matter Is Mediated by Quality and Quantity of Crop Residues: Mechanisms and Thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Agumas, B.; Blagodatsky, S.; Balume, I.; Musyoki, M.K.; Marhan, S.; Rasche, F. Microbial Carbon Use Efficiency during Plant Residue Decomposition: Integrating Multi-Enzyme Stoichiometry and C Balance Approach. Appl. Soil Ecol. 2021, 159, 103820. [Google Scholar] [CrossRef]
- Pingthaisong, W.; Vityakon, P. Nonadditive Effects on Decomposition of a Mixture of Rice Straw and Groundnut Stover Applied to a Sandy Soil. Agronomy 2021, 11, 1030. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter Decomposition: What Controls It and How Can We Alter It to Sequester More Carbon in Forest Soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadisch, G.; Giller, K.E. Organic Inputs for Soil Fertility Management in Tropical Agroecosystems: Application of an Organic Resource Database. Agric. Ecosyst. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Selezneva, A.E.; Ivashchenko, K.V.; Sushko, S.V.; Zhuravleva, A.I.; Ananyeva, N.D.; Blagodatsky, S.A. Microbial Respiration and Functional Diversity of Soil Microbial Community under Treeline Shifts in the Northwestern Caucasus. RUDN J. Agron. Anim. Ind. 2021, 16, 226–237. [Google Scholar] [CrossRef]
- Almagro, M.; Ruiz-Navarro, A.; Díaz-Pereira, E.; Albaladejo, J.; Martínez-Mena, M. Plant Residue Chemical Quality Modulates the Soil Microbial Response Related to Decomposition and Soil Organic Carbon and Nitrogen Stabilization in a Rainfed Mediterranean Agroecosystem. Soil Biol. Biochem. 2021, 156, 108198. [Google Scholar] [CrossRef]
- Bonanomi, G.; Capodilupo, M.; Incerti, G.; Mazzoleni, S. Nitrogen Transfer in Litter Mixture Enhances Decomposition Rate, Temperature Sensitivity, and C Quality Changes. Plant Soil 2014, 381, 307–321. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-59630-9. [Google Scholar]
- Moorhead, D.L.; Sinsabaugh, R.L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Higuchi, T. Lignin Biochemistry: Biosynthesis and Biodegradation. Wood Sci. Technol. 1990, 24, 23–63. [Google Scholar] [CrossRef]
- Coûteaux, M.-M.; Bottner, P.; Berg, B. Litter Decomposition, Climate and Liter Quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Arunkumar, N.; Banu, J.G.; Gopalakrishnan, N.; Prakash, A.H. Wax Degrading Bacteria: Scope and Applications in Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 649–664. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Carreiro, M.M.; Alvarez, S. Enzyme and Microbial Dynamics during Litter Decomposition. In Enzymes in the Environment: Activity, Ecology, and Applications; Burns, R.G., Dick, R.P., Eds.; CRC Press, Inc.: Oxford, UK, 2002; pp. 249–266. [Google Scholar]
- Snajdr, J.; Cajthaml, T.; Valášková, V.; Merhautová, V.; Petránková, M.; Spetz, P.; Leppänen, K.; Baldrian, P. Transformation of Quercus Petraea Litter: Successive Changes in Litter Chemistry Are Reflected in Differential Enzyme Activity and Changes in the Microbial Community Composition: Transformation of Quercus Petraea Litter. FEMS Microbiol. Ecol. 2011, 75, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A New Conceptual Model for the Fate of Lignin in Decomposing Plant Litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef]
- He, M.; Zhao, R.; Tian, Q.; Huang, L.; Wang, X.; Liu, F. Predominant Effects of Litter Chemistry on Lignin Degradation in the Early Stage of Leaf Litter Decomposition. Plant Soil 2019, 442, 453–469. [Google Scholar] [CrossRef]
- Bourget, M.Y.; Fanin, N.; Fromin, N.; Hättenschwiler, S.; Roumet, C.; Shihan, A.; Huys, R.; Sauvadet, M.; Freschet, G.T. Plant Litter Chemistry Drives Long-lasting Changes in the Catabolic Capacities of Soil Microbial Communities. Funct. Ecol. 2023, 37, 2014–2028. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Russian Geological Research Institute. Available online: https://karpinskyinstitute.ru/en/ (accessed on 28 April 2025).
- Sushko, S.; Ovsepyan, L.; Gavrichkova, O.; Yevdokimov, I.; Komarova, A.; Zhuravleva, A.; Blagodatsky, S.; Kadulin, M.; Ivashchenko, K. Contribution of Microbial Activity and Vegetation Cover to the Spatial Distribution of Soil Respiration in Mountains. Front. Microbiol. 2023, 14, 1165045. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- ISO 14240-2:1997; Soil Quality—Determination of Soil Microbial Biomass—Part 2: Fumigation-Extraction Method. ISO: Geneva, Switzerland, 1997. Available online: https://www.iso.org/standard/23951.html (accessed on 29 March 2025).
- Bilyera, N.; Blagodatskaya, E.; Yevdokimov, I.; Kuzyakov, Y. Towards a Conversion Factor for Soil Microbial Phosphorus. Eur. J. Soil Biol. 2018, 87, 1–8. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The Fumigation-Extraction Method to Estimate Soil Microbial Biomass: Calibration of the kEN Value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A Rapid Microtiter Plate Method To Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Secondi, L.; Marabottini, R.; Papp, R.; Stazi, S.R.; Mania, E.; Marinari, S. Assessment of Soil Microbial Functional Diversity: Land Use and Soil Properties Affect CLPP-MicroResp and Enzymes Responses. Pedobiologia 2018, 66, 36–42. [Google Scholar] [CrossRef]
- Ryan, M.G.; Melillo, J.M.; Ricca, A. A comparison of methods for determining proximate carbon fractions of forest litter available. Can. J. For. Res. 1990, 20, 166–171. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 28 April 2025).




| Fractions | MBC | MBN | MBP | H | RSCH | RSAA | RSCX | RSPH |
|---|---|---|---|---|---|---|---|---|
| 6 months | ||||||||
| WS | 0.57 ** | 0.05 | 0.15 | 0.44 ** | 0.17 | 0.15 | 0.31 * | 0.35 ** |
| NE | 0.01 | 0.26 * | 0.03 | 0.30 * | 0.53 ** | 0.00 | 0.61 ** | 0.24 |
| AS | 0.00 | 0.26 * | 0.00 | 0.51 ** | 0.56 ** | 0.00 | 0.59 ** | 0.27 * |
| AIS | 0.12 | 0.43 ** | 0.02 | 0.19 | 0.28 * | 0.05 | 0.28 * | 0.06 |
| 12 months | ||||||||
| WS | 0.57 ** | 0.16 | 0.00 | 0.01 | 0.01 | 0.17 | 0.07 | 0.07 |
| NE | 0.00 | 0.30 * | 0.21 | 0.11 | 0.05 | 0.29 * | 0.05 | 0.05 |
| AS | 0.02 | 0.26 * | 0.15 | 0.02 | 0.00 | 0.46 ** | 0.09 | 0.00 |
| AIS | 0.03 | 0.14 | 0.16 | 0.05 | 0.00 | 0.30 * | 0.03 | 0.02 |
| Color scale for R2 | ||||||||
| 0.00 | 0.15 | 0.25 | 0.30 | 0.35 | 0.45 | 0.50 | 0.60 | |
| Slope | PC (%) | NS | Dominant Species (≥10% of PC) |
|---|---|---|---|
| Reserved | |||
| 1 | 81 ± 20 | 14 ± 5 | Calamagrostis arundinacea (Schrad.) DC., Betonica macrantha K. Koch, Trollius ranunculinus (Sm.) Stearn, Centaurea phrygia L., Carex pallescens L., Cirsium simplex C.A. Mey |
| 2 | 96 ± 3 | 9 ± 2 | Calamagrostis arundinacea (Schrad.) DC., Betonica macrantha K. Koch |
| 3 | 91 ± 10 | 6 ± 2 | Calamagrostis arundinacea (Schrad.) DC., Betonica macrantha K. Koch |
| Grazed | |||
| 1 | 46 ± 8 | 14 ± 4 | Agrostis vinealis Schreb., Betonica macrantha K. Koch |
| 2 | 66 ± 11 | 16 ± 1 | Nardus stricta L., Potentilla erecta (L.) Raeusch. |
| 3 | 97 ± 5 | 21 ± 2 | Agrostis vinealis Schreb., Carum caucasicum Boiss, Betonica macrantha K. Koch, Avenella flexuosa (L.) Drejer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivashchenko, K.; Romanova, A.; Sushko, S.; Zhuravleva, A.; Kvitkina, A.; Khodzhaeva, A.; Ananyeva, N. Effects of Early-Stage Treeline Shifts on Soil Microbial Biomass and Catabolic Diversity in Reserved and Grazed Subalpine Meadows. Plants 2025, 14, 1541. https://doi.org/10.3390/plants14101541
Ivashchenko K, Romanova A, Sushko S, Zhuravleva A, Kvitkina A, Khodzhaeva A, Ananyeva N. Effects of Early-Stage Treeline Shifts on Soil Microbial Biomass and Catabolic Diversity in Reserved and Grazed Subalpine Meadows. Plants. 2025; 14(10):1541. https://doi.org/10.3390/plants14101541
Chicago/Turabian StyleIvashchenko, Kristina, Anastasiya Romanova, Sofia Sushko, Anna Zhuravleva, Anna Kvitkina, Anna Khodzhaeva, and Nadezhda Ananyeva. 2025. "Effects of Early-Stage Treeline Shifts on Soil Microbial Biomass and Catabolic Diversity in Reserved and Grazed Subalpine Meadows" Plants 14, no. 10: 1541. https://doi.org/10.3390/plants14101541
APA StyleIvashchenko, K., Romanova, A., Sushko, S., Zhuravleva, A., Kvitkina, A., Khodzhaeva, A., & Ananyeva, N. (2025). Effects of Early-Stage Treeline Shifts on Soil Microbial Biomass and Catabolic Diversity in Reserved and Grazed Subalpine Meadows. Plants, 14(10), 1541. https://doi.org/10.3390/plants14101541






