The Divergence History of Two Japanese Torreya Taxa (Taxaceae): Implications for Species Diversification in the Japanese Archipelago
Abstract
1. Introduction
2. Results
2.1. Genetic Diversity and Neutrality Tests
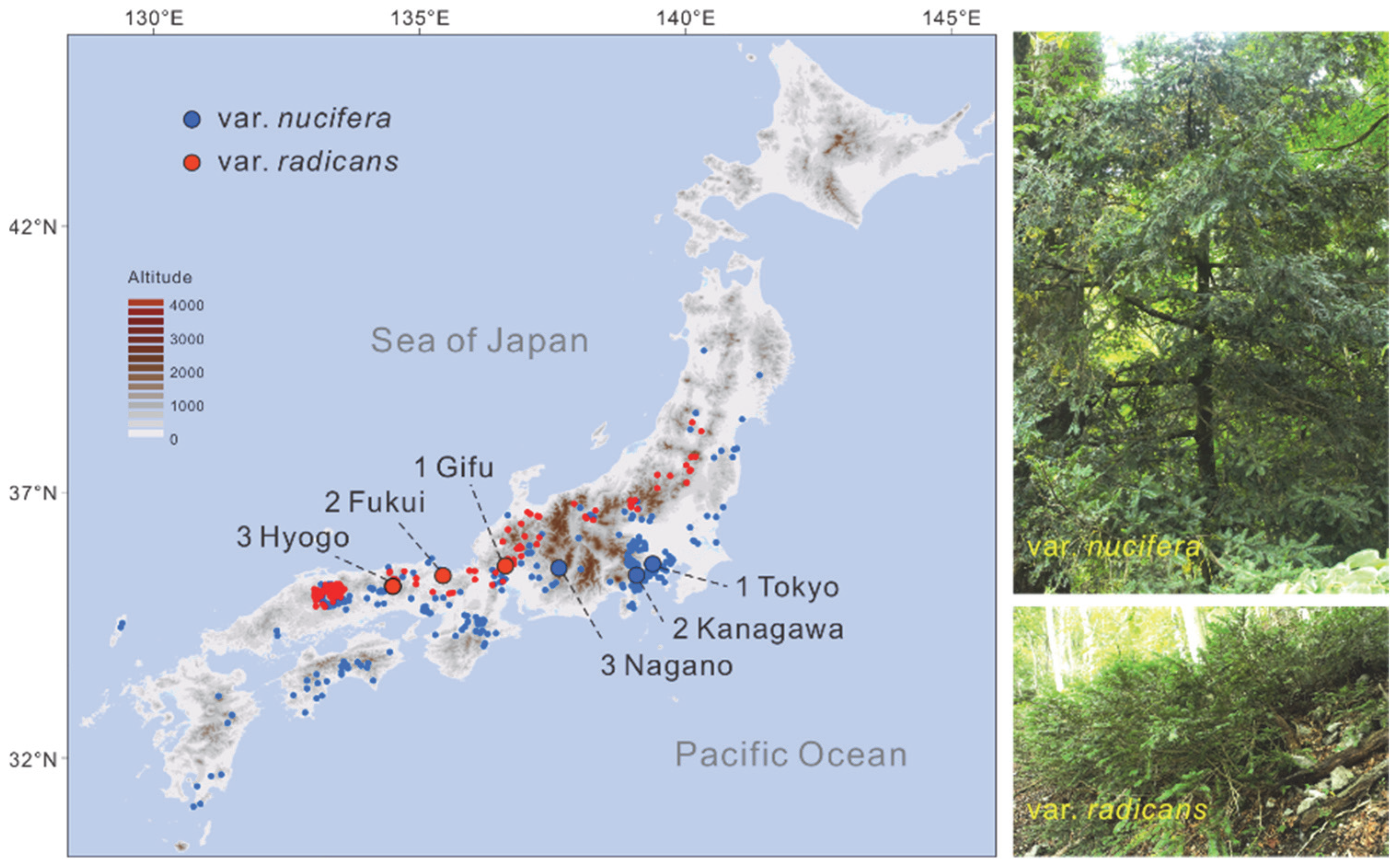
2.2. Population Genetic Structure
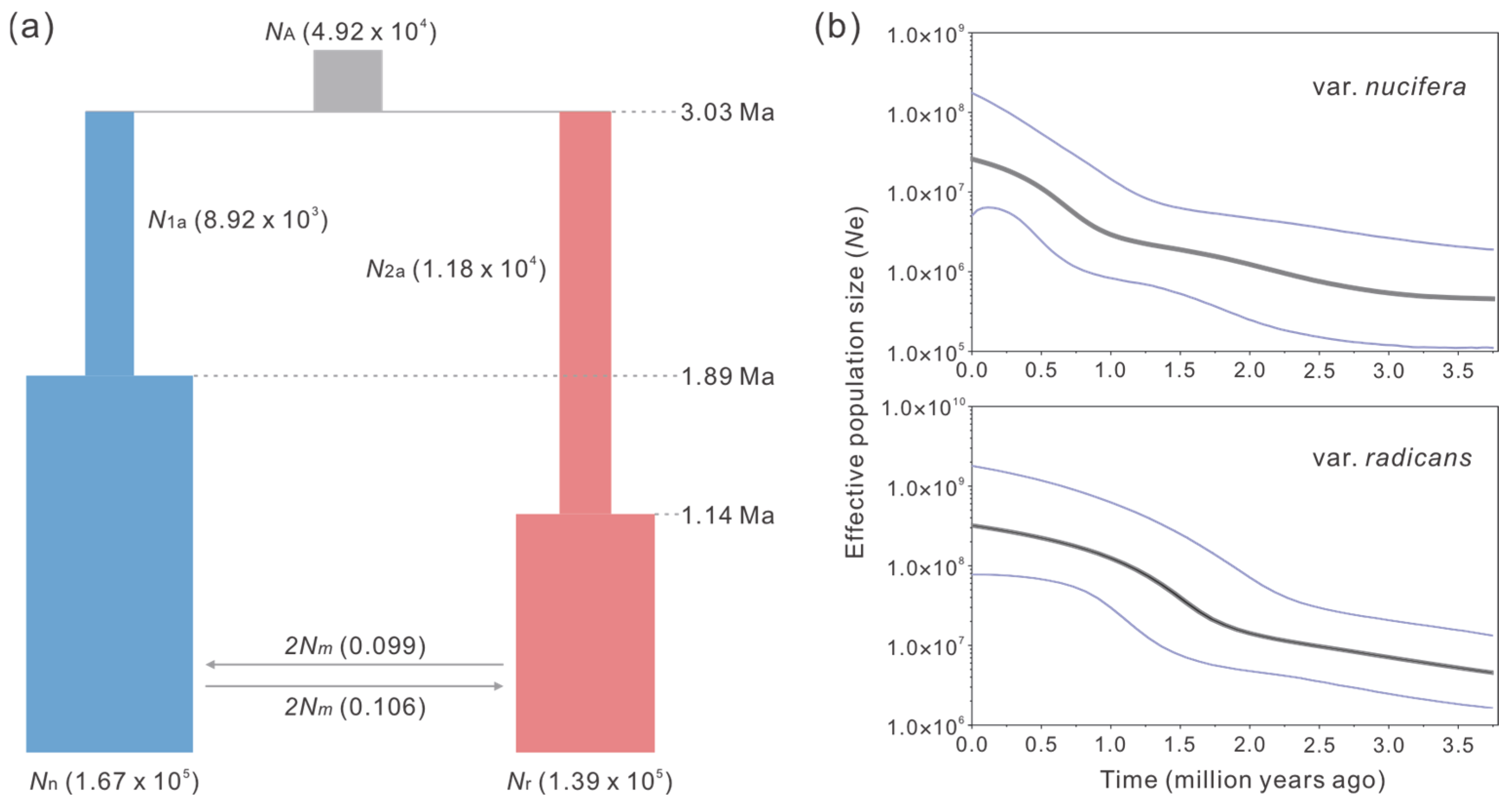
2.3. Divergence and Demographic History
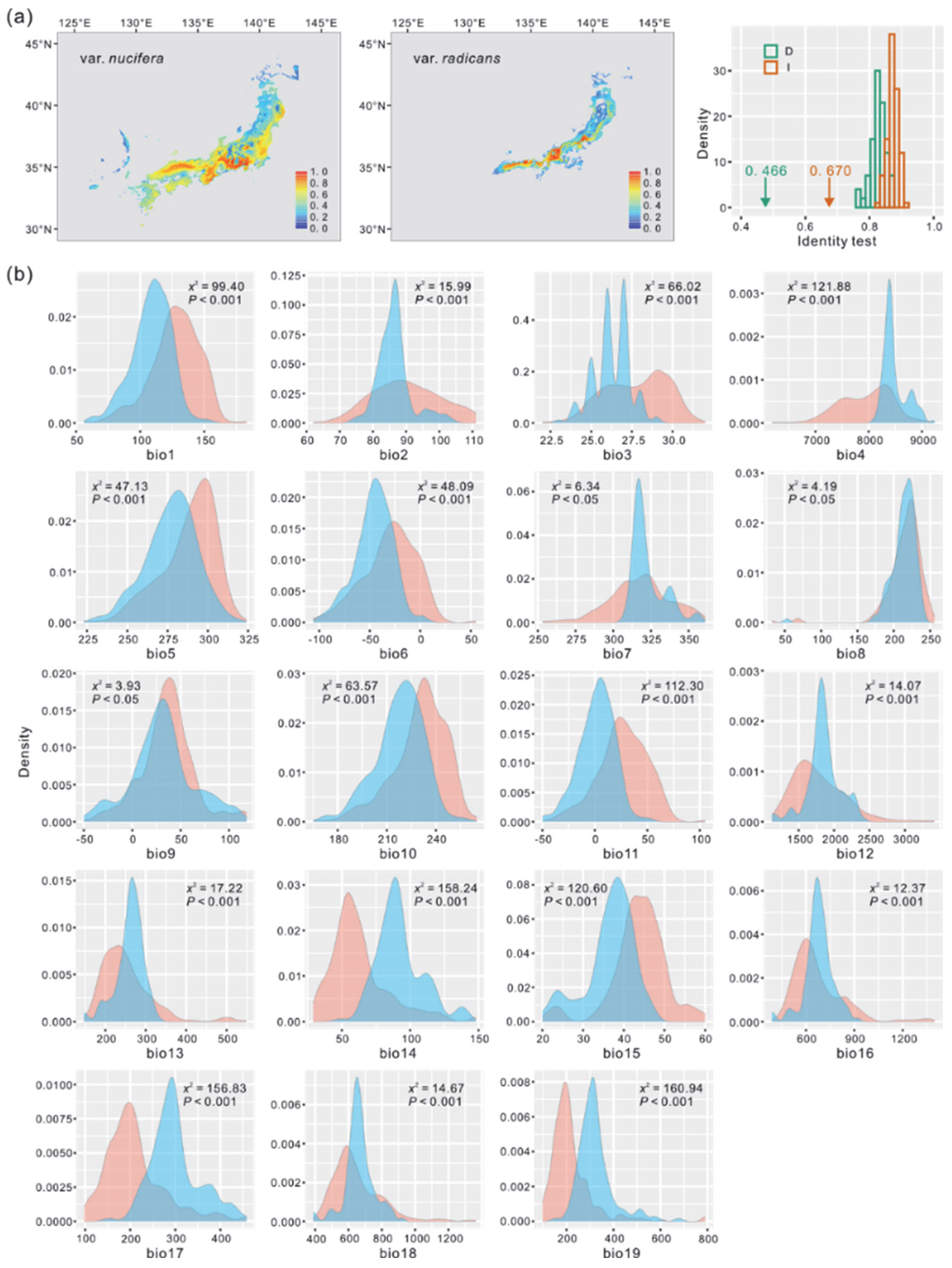
2.4. Climatic Niche Differentiation
3. Discussion
3.1. Recognition of Torreya nucifera var. nucifera and T. nucifera var. radicans as Two Separate Species Is Further Supported by Population Genetic Analyses
3.2. Allopatric Origin Might Be Applicable for T. nucifera var. nucifera and T. nucifera var. radicans
3.3. Climatic Heterogeneity Sustains the Species Boundary Between T. nucifera var. nucifera and T. nucifera var. radicans
4. Materials and Methods
4.1. Sampling and DNA Sequencing
4.2. Genetic Diversity Analyses and Neutrality Tests
4.3. Analyses of Population Genetic Structure
4.4. Inferences of Divergence and Demographic History
4.5. Niche Modeling and Ecological Divergence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.J. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK; New York, NY, USA, 1998. [Google Scholar]
- Weigelt, P.; Steinbauer, M.J.; Cabral, J.S.; Kreft, H. Late Quaternary climate change shapes island biodiversity. Nature 2016, 532, 99–102. [Google Scholar] [CrossRef]
- Comes, H.P.; Tribsch, A.; Bittkau, C. Plant speciation in continental island floras as exemplified by Nigella in the Aegean Archipelago. Philos. Trans. R. Soc. B 2008, 363, 3083–3096. [Google Scholar] [CrossRef]
- Yoichi, W.; Tamaki, I.; Sakaguchi, S.; Song, J.S.; Yamamoto, S.i.; Tomaru, N. Population demographic history of a temperate shrub, Rhododendron weyrichii (Ericaceae), on continental islands of Japan and South Korea. Ecol. Evol. 2016, 6, 8800–8810. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Orr, H.A. Speciation: A Catalogue and Critique of Species Concepts; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Qiu, Y.-X.; Fu, C.-X.; Comes, H.P. Plant molecular phylogeography in China and adjacent regions: Tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenet. Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef]
- Ohsawa, T.; Ide, Y. Phylogeographic patterns of highland and lowland plant species in Japan. Alpine Bot. 2011, 121, 49–61. [Google Scholar] [CrossRef]
- Ikeda, H. Decades-long phylogeographic issues: Complex historical processes and ecological factors on genetic structure of alpine plants in the Japanese Archipelago. J. Plant Res. 2022, 135, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Presgraves, D.C.; Glor, R.E. Evolutionary biology: Speciation on islands. Curr. Biol. 2010, 20, R440–R442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skeels, A.; Cardillo, M. Reconstructing the geography of speciation from contemporary biodiversity data. Am. Nat. 2019, 193, 240–255. [Google Scholar] [CrossRef]
- Schuler, H.; Hood, G.R.; Egan, S.P.; Feder, J.L. Modes and echanisms of speciation. Rev. Cell Biol. Mol. Med. 2016, 2, 60–93. [Google Scholar]
- Wu, Z.; Wu, S. A proposal for a new floristic kingdom (Realm) Á the east Asiatic kingdom, its delineation and characteristics. In Proceedings of the First International Symposium on Floristic Characteristics and Diversity of East Asian Plants; Zhang, A.L., Wu, S.G., Eds.; China Higher Education Press: Beijing, China; Springer: Berlin/Heidelberg, Germany, 1996; pp. 3–42. [Google Scholar]
- Qian, H.; Ricklefs, R.E. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 2000, 407, 180–182. [Google Scholar] [CrossRef]
- Harrison, S.; Yu, G.; Takahara, H.; Prentice, I. Diversity of temperate plants in east Asia. Nature 2001, 413, 129–130. [Google Scholar] [CrossRef]
- Tada, R. Onset and evolution of millennial-scale variability in the Asian Monsoon and its impact on paleoceanography of the Japan Sea. Geophys. Monogr. Ser. 2004, 149, 283–298. [Google Scholar]
- Haq, B.U.; Hardenbol, J.; Vail, P.R. Chronology of fluctuating sea levels since the Triassic. Science 1987, 235, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. Paleogeography of the Ryukyu Islands. Tropics 2000, 10, 5–24. [Google Scholar] [CrossRef]
- Lambeck, K.; Chappell, J. Sea level change through the last glacial cycle. Science 2001, 292, 679–686. [Google Scholar] [CrossRef]
- Aizawa, M.; Worth, J.R. Phylogenetic origin of two Japanese Torreya taxa found in two regions with strongly contrasting snow depth. J. Plant Res. 2021, 134, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Bao, Q.; He, W.; Fan, D.M.; Cheng, S.M.; López-Pujol, J.; Chung, M.G.; Sakaguchi, S.; Sánchez-González, A.; Gedik, A.; et al. Phylogeny and biogeography of Fagus (Fagaceae) based on 28 nuclear single/low-copy loci. J. Syst. Evol. 2022, 60, 759–772. [Google Scholar] [CrossRef]
- Nakai, T. Notulae ad plantas Japoniae et Koreae XXI. Bot. Mag. 1919, 33, 193–216. [Google Scholar] [CrossRef]
- Nakai, T. Indigenous species of conifers and taxads of Korea and Manchuria, and their distribution. Chosen Sanrin Kaiho 1938, 158, 21–49. [Google Scholar]
- Chang, C.-S.; Kim, H.; Chang, K.S. Provisional Checklist of Vascular Plants for the Korea Peninsula Flora (KPF); DESIGNPOST: Köln, Germany, 2014. [Google Scholar]
- Farjon, A. A Handbook of the World’s Conifers; Brill Academic Publishers: Leiden, The Netherlands, 2010. [Google Scholar]
- Li, J.; Davis, C.C.; Donoghue, M.J.; Kelley, S.; Del Tredici, P. Phylogenetic relationships of Torreya (Taxaceae) inferred from sequences of nuclear ribosomal DNA ITS region. Harv. Pap. Bot. 2001, 6, 275–281. [Google Scholar]
- Kou, Y.X.; Xiao, K.; Lai, X.R.; Wang, Y.J.; Zhang, Z.Y. Natural hybridization between Torreya jackii and T. grandis (Taxaceae) in southeast China. J. Syst. Evol. 2017, 55, 25–33. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.J.; Landis, J.B.; Deng, T.; Meng, A.P.; Sun, H.; Peng, Y.S.; Wang, H.C.; Sun, Y.X. Plastome phylogenomic analysis of Torreya (Taxaceae). J. Syst. Evol. 2019, 57, 607–615. [Google Scholar] [CrossRef]
- Zhou, W.; Harris, A.; Xiang, Q.Y. Phylogenomics and biogeography of Torreya (Taxaceae)—Integrating data from three organelle genomes, morphology, and fossils and a practical method for reducing missing data from RAD-seq. J. Syst. Evol. 2022, 60, 1241–1262. [Google Scholar] [CrossRef]
- Mo, Z.-Q.; Wang, J.; Möller, M.; Yang, J.-B.; Gao, L.-M. Phylogenetic relationships and next-generation barcodes in the Genus Torreya reveal a high proportion of misidentified cultivated plants. Int. J. Mol. Sci. 2023, 24, 13216. [Google Scholar] [CrossRef]
- Kou, Y.; Fan, D.; Cheng, S.; Yang, Y.; Wang, M.; Wang, Y.; Zhang, Z. Peripatric speciation within Torreya fargesii (Taxaceae) in the Hengduan Mountains inferred from multi-loci phylogeography. BMC Ecol. Evol. 2023, 23, 74. [Google Scholar] [CrossRef]
- Ikeda, H.; Sakaguchi, S.; Yakubov, V.; Barkalov, V.; Setoguchi, H. Importance of demographic history for phylogeographic inference on the arctic–alpine plant Phyllodoce caerulea in East Asia. Heredity 2016, 116, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, N.; Uchiyama, K.; Miyagi, R.; Moritsuka, E.; Takahashi, A.; Tamura, K.; Tsumura, Y.; Teshima, K.M.; Tachida, H.; Kusumi, J. Inferring the demographic history of Japanese cedar, Cryptomeria japonica, using amplicon sequencing. Heredity 2019, 123, 371–383. [Google Scholar] [CrossRef]
- Hiraoka, K.; Tomaru, N. Genetic divergence in nuclear genomes between populations of Fagus crenata along the Japan Sea and Pacific sides of Japan. J. Plant Res. 2009, 122, 269–282. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Qiu, Y.X.; Liu, Y.H.; Qi, X.S.; Kim, S.H.; Han, J.; Takeuchi, Y.; Worth, J.R.; Yamasaki, M.; Sakurai, S.; et al. Climate oscillation during the Quaternary associated with landscape heterogeneity promoted allopatric lineage divergence of a temperate tree Kalopanax septemlobus (Araliaceae) in East Asia. Mol. Ecol. 2012, 21, 3823–3838. [Google Scholar] [CrossRef]
- Ikeda, H.; Setoguchi, H. A multilocus sequencing approach reveals the cryptic phylogeographical history of Phyllodoce nipponica Makino (Ericaceae). Biol. J. Linn. Soc. 2013, 110, 214–226. [Google Scholar] [CrossRef][Green Version]
- Suda, R.A.; Kubota, S.; Kumar, V.; Castric, V.; Krämer, U.; Morinaga, S.-I.; Tsuchimatsu, T. Population genomics reveals demographic history and climate adaptation in Japanese Arabidopsis halleri. Plant Cell Physiol. 2025, 66, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, L.; He, W.; Fan, D.; Cheng, S.; Yang, Y.; Wang, M.; Tang, S.; Kou, Y.; Zhang, Z. Allopatric speciation and secondary sympatry of Fagus longipetiolata and F. lucida (Fagaceae) in subtropical China. Bot. J. Linn. Soc. 2024, 205, 403–415. [Google Scholar] [CrossRef]
- Milne, R.I.; Abbott, R.J. The origin and evolution of Tertiary relict floras. Adv. Bot. Res. 2002, 38, 281–314. [Google Scholar]
- Donoghue, M.J.; Bell, C.D.; Li, J. Phylogenetic patterns in Northern Hemisphere plant geography. Int. J. Plant Sci. 2001, 162, S41–S52. [Google Scholar] [CrossRef]
- Leslie, A.B.; Beaulieu, J.M.; Rai, H.S.; Crane, P.R.; Donoghue, M.J.; Mathews, S. Hemisphere-scale differences in conifer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 16217–16221. [Google Scholar] [CrossRef]
- Tsumura, Y.; Kimura, M.; Nakao, K.; Uchiyama, K.; Ujino-Ihara, T.; Wen, Y.; Tong, Z.; Han, W. Effects of the last glacial period on genetic diversity and genetic differentiation in Cryptomeria japonica in East Asia. Tree Genet. Genomes 2020, 16, 19. [Google Scholar] [CrossRef]
- Tan, Y.; Ou, Q.; Huang, X.; Wang, Y.; Kou, Y. Alien Species Introduction and Demographic Changes Contributed to the Population Genetic Structure of the Nut-Yielding Conifer Torreya grandis (Taxaceae). Forests 2024, 15, 1451. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, J.; Kou, Y.; Wang, Y. Application of EST-SSR markers developed from the transcriptome of Torreya grandis (Taxaceae), a threatened nut-yielding conifer tree. PeerJ 2018, 6, e5606. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Mogensen, H.L. Invited special paper: The hows and whys of cytoplasmic inheritance in seed plants. Am. J. Bot. 1996, 83, 383–404. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.-X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-X.; Li, W.-H. Statistical tests of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1949, 15, 323–354. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comp. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.A. DISTRUCT: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Cornuet, J.-M.; Pudlo, P.; Veyssier, J.; Dehne-Garcia, A.; Gautier, M.; Leblois, R.; Marin, J.-M.; Estoup, A. DIYABC v2.0: A software to make approximate Bayesian computation inferences about population history using single nucleotide polymorphism, DNA sequence and microsatellite data. Bioinformatics 2014, 30, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Möller, M.; Provan, J.; Gao, L.M.; Poudel, R.C.; Li, D.Z. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytol. 2013, 199, 1093–1108. [Google Scholar] [CrossRef]
- Hey, J. Isolation with Migration Models for More Than Two Populations. Mol. Biol. Evol. 2010, 27, 905–920. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]

| var. nucifera | var. radicans | T. fargesii | T. jackii | |
|---|---|---|---|---|
| var. nucifera | 0.6543 * | 0.7288 * | 0.8089 * | |
| var. radicans | 0.9619 * | 0.7904 * | 0.7944 * | |
| T. fargesii | 0.9760 * | 0.9510 * | 0.8033 * | |
| T. jackii | 0.9866 * | 0.9648 * | 0.9764 * |
| θn | θr | θA | mn>r | mr>n | t | Nn | Nr | NA | 2Nm (n) | 2Nm (r) | T (Years) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLE | 0.152 | 0.511 | 0.862 | 1.307 | 0.415 | 1.692 | 39,668 | 133,667 | 225,702 | 0.106 | 0.099 | 1,772,099 |
| HPD95Lo | 0.100 | 0.345 | 0.070 | 0.562 | 0.153 | 1.204 | 26,157 | 90,202 | 18,328 | 0.026 | 0.028 | 1,260,997 |
| HPD95Hi | 0.263 | 0.695 | 3.818 | 2.354 | 0.827 | 7.996 | 68,889 | 181,844 | 999,686 | 0.287 | 0.310 | 8,374,529 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Q.; Huang, X.; Pan, D.; Wang, S.; Huang, Y.; Lu, S.; Wang, Y.; Kou, Y. The Divergence History of Two Japanese Torreya Taxa (Taxaceae): Implications for Species Diversification in the Japanese Archipelago. Plants 2025, 14, 1537. https://doi.org/10.3390/plants14101537
Ou Q, Huang X, Pan D, Wang S, Huang Y, Lu S, Wang Y, Kou Y. The Divergence History of Two Japanese Torreya Taxa (Taxaceae): Implications for Species Diversification in the Japanese Archipelago. Plants. 2025; 14(10):1537. https://doi.org/10.3390/plants14101537
Chicago/Turabian StyleOu, Qian, Xin Huang, Dingguo Pan, Shulan Wang, Yuting Huang, Sisi Lu, Yujin Wang, and Yixuan Kou. 2025. "The Divergence History of Two Japanese Torreya Taxa (Taxaceae): Implications for Species Diversification in the Japanese Archipelago" Plants 14, no. 10: 1537. https://doi.org/10.3390/plants14101537
APA StyleOu, Q., Huang, X., Pan, D., Wang, S., Huang, Y., Lu, S., Wang, Y., & Kou, Y. (2025). The Divergence History of Two Japanese Torreya Taxa (Taxaceae): Implications for Species Diversification in the Japanese Archipelago. Plants, 14(10), 1537. https://doi.org/10.3390/plants14101537





