More than Just a Shell: Indehiscent Fruits Drive Drought-Tolerant Germination in Invasive Lepidium Species
Abstract
1. Introduction
2. Results
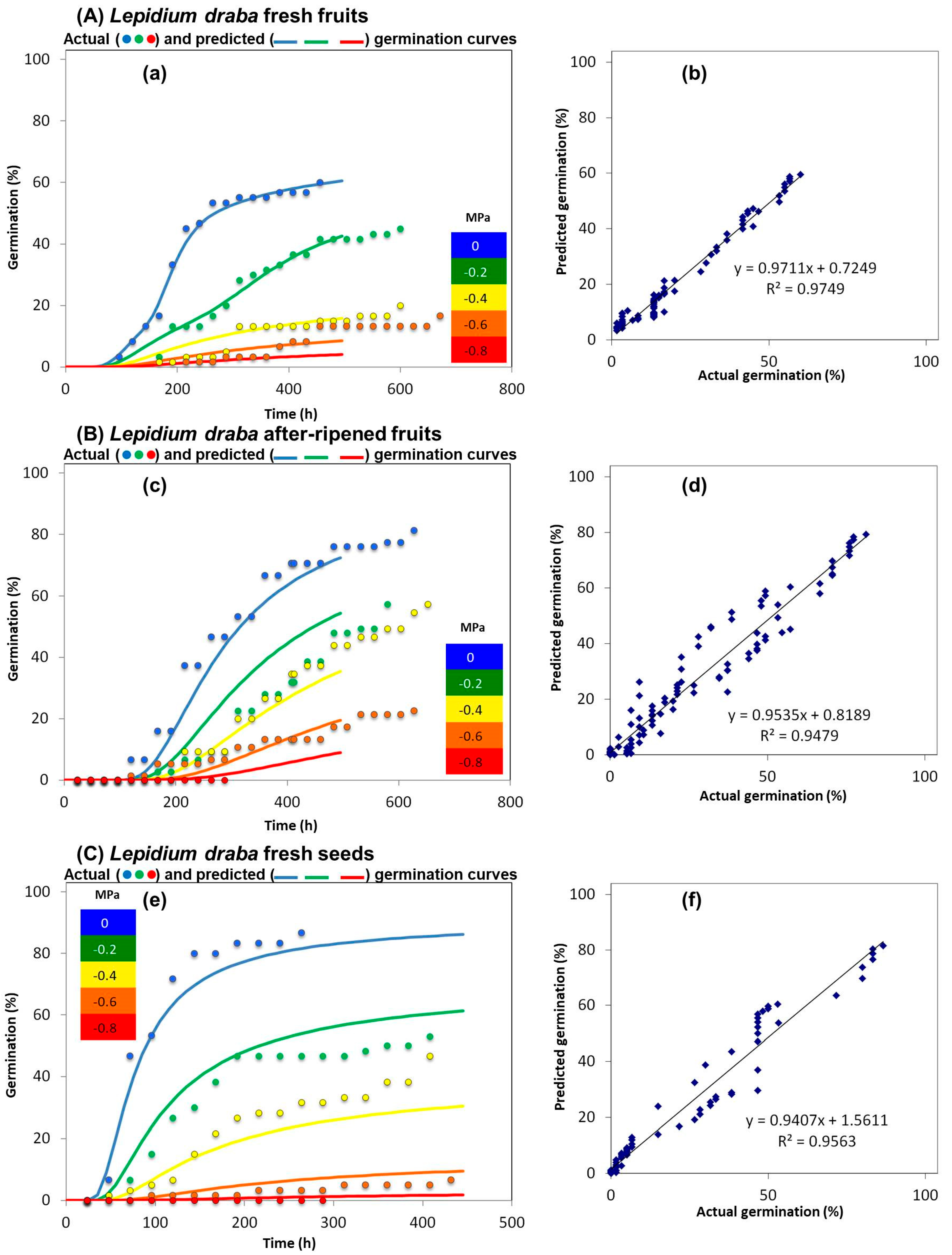
2.1. Indehiscent-Fruited L. appelianum and L. draba Exhibit High Drought Tolerance
2.2. Dehiscent Fruit-Producing L. campestre Exhibit Reduced Drought Stress Tolerance Compared to the Indehiscent Fruit-Producing L. appelianum and L. draba
2.3. Dehiscent vs. Indehiscent: L. appelianum and L. draba’s Indehiscent Fruits Could Benefit from Future Climate Change Relative to Dehiscent Fruit-Producing L. campestre
3. Discussion
3.1. Indehiscent Fruits Drive Enhanced Drought Tolerance and Invasive Potential in Lepidium
3.2. Implications of Indehiscent Fruit Evolution for Drought Stress Adaptation in Lepidium
3.3. Native Distribution Areas of Indehiscent-Fruited Lepidium Species Could Suggest Drought Stress Tolerance
3.4. Global Ranking of the Invasiveness of Indehiscent Fruit-Producing Lepidium Species Indicates Potential Drought Stress Tolerance
3.5. Understanding the Link: How Indehiscent Fruits Facilitate Drought Stress Tolerance and Contribute to Invasiveness
3.6. The Broad Ecological Relevance of PEG in Drought Stress Simulation
4. Materials and Methods
4.1. Seed Sources
4.2. Microscopy of Lepidium Seed and Fruit Morphology and Germinating Units
4.3. Seed and Fruit Material: Collection and Processing
4.4. Dormancy Characterization and Diaspore Types of the Three Lepidium Species
4.5. Dormancy Releasing Treatments
4.6. Drought Stress Experiments
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahedifar, M.; Zohrabi, S. Germination and seedling characteristics of drought-stressed corn seedasinfluenced by seed priming with potassium nano-chelate and sulfate fertilizers. Acta Agric. Slov. 2016, 107, 113–128. [Google Scholar] [CrossRef]
- Dani, A.R.H.; Siswoyo, T.A. Impact of drought stress during germination on antioxidant capacities and antioxidant enzymes activities of Madura local Maize (Zea mays) Seeds. Agric. Sci. 2019, 10, 1506–1516. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Liu, H.; Chen, Y.; Zhang, L.; Kudusi, K.; Song, J. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest China. Front. Ecol. Evol. 2022, 10, 1026095. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.; Liu, K.; Sui, N. Effects of drought stress on seed germination and seedling growth of different Maize varieties. J. Agric. Sci. 2015, 7, 231–240. [Google Scholar] [CrossRef]
- Ahmed, M.; Kheir, A.M.S.; Mehmood, M.Z.; Ahmad, S.; Hasanuzzaman, M. Changes in germination and seedling traits of Sesame under simulated drought. Phyton-Int. J. Exp. Bot. 2022, 91, 713–726. [Google Scholar] [CrossRef]
- Wu, L.-M.; Fang, Y.; Yang, H.-N.; Bai, L.-Y. Effects of drought-stress on seed germination and growth physiology of quincloracresistant Echinochloa crusgalli. PLoS ONE 2019, 14, e0214480. [Google Scholar]
- Majid, A.; Dar, Z.A.; Zaffar, G.; Lone, F.A.; Kumar, I.S.; Sofi, P.A.; Lone, A.A.; ul Islam, N.; Rashid, M. Effect of PEG-6000 induced drought stress on seed germination in Maize (Zea mays). SKUAST J. Res. 2020, 22, 40–44. [Google Scholar]
- Evamoni, F.Z.; Nulit, R.; Yap, C.K.; Ibrahim, M.H.; Sidek, N.B. Assessment of germination performance and early seedling growth of Malaysian indica rice genotypes under drought conditions for strategic cropping during water scarcity. Chil. J. Agric. Res. 2023, 83, 281–292. [Google Scholar] [CrossRef]
- Long, J.; Dong, M.; Wang, C.; Miao, Y. Effects of drought and salt stress on seed germination and seedling growth of Elymus nutans. Peer J. 2023, 11, e15968. [Google Scholar] [CrossRef]
- Hatzig, S.V.; Nuppenau, J.N.; Snowdon, R.J.; Schießl, S. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, D.; Seepaul, R.; George, S.; Groot, J.; Wright, D. Drought tolerance classification of common oilseed species using seed germination assay. J. Oilseed Brassica 2019, 10, 97–105. [Google Scholar]
- Rosa, N.M.; Lin, C.-W.; Kang, Y.J.; Dhondt, S.; Gonzalez, N.; Inzé, D.; Braun, P.F. Drought resistance is mediated by divergent strategies in closely related Brassicaceae. New Phytol. 2019, 223, 783–797. [Google Scholar] [CrossRef]
- Bhatt, A.; Chen, X.; Pompelli, M.F.; Jamal, A.; Mancinelli, R.; Radicetti, E. Characterization of invasiveness, thermotolerance and light requirement of nine invasive species in China. Plants 2023, 12, 1192. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A.; Mummenhoff, K.; Appel, O. Cardaria, Coronopus, and Stroganowia are united with Lepidium (Brassicaceae). Novon 2002, 12, 5–11. [Google Scholar] [CrossRef]
- Darbyshire, S.J.; Favreau, M.; Murray, M. Common and Scientific Names of Weeds in Canada; Publication 1397/B; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2000; p. 132. [Google Scholar]
- Darbyshire, S.J. Inventory of Canadian Agricultural Weeds; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2003; p. 396. [Google Scholar]
- Geleta, M.; Gustafsson, C.; Glaubitz, J.C.; Ortiz, R. High-density genetic linkage mapping of Lepidium based on genotyping-by-sequencing SNPs and segregating contig tag haplotypes. Front. Plant Sci. 2020, 11, 448. [Google Scholar] [CrossRef]
- Francis, A.; Warwick, S.I. The biology of Canadian weeds. 3. Lepidium draba L., L. chalepense L., and L. appelianum Al-Shehbaz (updated). Can. J. Plant Sci. 2008, 88, 379–401. [Google Scholar]
- Gaskin, J.F.; Zhang, D.; Bon, M.C. Invasion of Lepidium draba (Brassicaceae) in the western United States: Distributions and origins of chloroplast DNA haplotypes. Mol. Ecol. 2005, 14, 2331–2341. [Google Scholar] [CrossRef]
- Gaskin, J.F. Clonal structure of invasive hoary cress (Lepidium draba) infestations. Weed Sci. 2006, 54, 428–434. [Google Scholar] [CrossRef]
- Goodwin, K. Biology, Ecology and Management of Whitetop (Cardaria spp.); Montana State University, MSU Extension: Bozeman, MT, USA, 2003. [Google Scholar]
- Hinz, H.L.; Cristofaro, M. Prospects for the Biological Control of Perennial Weeds; CABI Bioscience Switzerland Centre and BBCA: Rome, Italy, 2005; p. 23. [Google Scholar]
- USDA. Hoary Cress Consortium; USDA ARS Northern Plains Agricultural Research Laboratory: Sidney, Australia, 2002. [Google Scholar]
- USDA. The Plants Database; National Plant Data Center: Baton Rouge, LA, USA, 2004. [Google Scholar]
- Rice, P. Invaders Database System. 2017. Available online: http://invader.dbs.umt.edu/ (accessed on 8 February 2025).
- Schroeder, F.G. Lehrbuch der Pflanzengeographie; Quelle and Meyer: Wiesbaden, Germany, 1998. [Google Scholar]
- Partzsch, M. Zur Keimungsbiologie acht ausgewählter kurzlebiger Ruderal und Segetalarten. Hercynia N.F. 2010, 43, 149–166. [Google Scholar]
- Mohammed, S.; Turečková, V.; Tarkowská, D.; Strnad, M.; Mummenhoff, K.; Leubner-Metzger, G. Pericarp-mediated chemical dormancy controls the fruit germination of the invasive hoary cress (Lepidium draba), but not of hairy whitetop (Lepidium appelianum). Weed Sci. 2019, 67, 560–571. [Google Scholar] [CrossRef]
- Mohammed, S.; Bhattacharya, S.; Gesing, M.A.; Klupsch, K.; Theißen, G.; Mummenhoff, K.; Müller, C. Morphologically and physiologically diverse fruits of two Lepidium species differ in allocation of glucosinolates into immature and mature seed and pericarp. PLoS ONE 2020, 15, e0227528. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Steinbrecher, T.; Leubner-Metzger, G.; Mummenhoff, K. Differential Primary Seed and Fruit Dispersal Mechanisms and Dispersal Biomechanics in Invasive Dehiscent and Indehiscent-Fruited Lepidium Species. Plants 2025, 14, 446. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A. The genera of Lepidieae (Cruciferae; Brassicaceae), the southeastern United States. J. Arnold Arbor. 1986, 67, 265–311. [Google Scholar] [CrossRef]
- Mummenhoff, K.; Franzke, A. Gone with the bird: Late tertiary and quaternary intercontinental long-distance dispersal and allopolyploidization in plants. Syst. Biodivers. 2007, 5, 255–260. [Google Scholar] [CrossRef]
- Mohammed, S.; Mummenhoff, K. Germination Under Temperature Stress Facilitates Invasion in Indehiscent Lepidium Species. Agriculture 2025, 15, 1078. [Google Scholar] [CrossRef]
- Mohammed, S.; Mummenhoff, K. Functional divergence exists in mucilage-mediated seed dispersal, but not in germination of myxospermic Lepidium campestre and Lepidium draba (Brassicaceae). Acta Oecol. 2024, 125, 104042. [Google Scholar] [CrossRef]
- Toosi, A.F.; Bakar, B.B.; Azizi, M. Effect of drought stress by using PEG 6000 on germination and early seedling growth of Brassica juncea Var. Ensabi. Agronomy 2014, LVII, 360–363. [Google Scholar]
- Wang, C.; Zhou, L.; Zhang, G.; Xu, Y.; Gao, X.; Jiang, N.; Zhang, L.; Shao, M. Effects of Drought Stress Simulated by Polyethylene Glycol on Seed Germination, Root and Seedling Growth, and Seedling Antioxidant Characteristics in Job’s Tears. Agric. Sci. 2018, 9, 991–1006. [Google Scholar] [CrossRef]
- Sevik, H.; Cetin, M. Effects of water stress on seed germination for select landscape plants. Pol. J. Environ. Stud. 2015, 24, 689–693. [Google Scholar] [CrossRef]
- Channaoui, S.; El Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of some rapeseed (Brassica napus L.) genotypes to different drought stress levels during germination and seedling growth stages. Oilseeds Fats Crops Lipids 2019, 26, 23. [Google Scholar] [CrossRef]
- Michel, B.E. Evaluation of the water potentials of solutions of Polyethylene Glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef]
- Alvarado, V.; Bradford, K.J. A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ. 2022, 25, 1061–1069. [Google Scholar] [CrossRef]
- Kintl, A.; Huňady, I.; Vymyslický, T.; Ondrisková, V.; Hammerschmiedt, T.; Brtnický, M.; Elbl, J. Effect of Seed Coating and PEG-Induced Drought on the Germination Capacity of Five Clover Crops. Plants 2021, 10, 724. [Google Scholar] [CrossRef]
- Mehmandar, M.N.; Rasouli, F.; Giglou, M.T.; Zahedi, S.M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Tajaragh, R.P.; Ryant, P.; Mlcek, J. Polyethylene Glycol and Sorbitol-Mediated In Vitro Screening for Drought Stress as an Efficient and Rapid Tool to Reach the Tolerant Cucumis melo L. Genotypes. Plants 2023, 12, 870. [Google Scholar] [CrossRef]
- Li, S.; Yan, N.; Tanveer, M.; Zhao, Z.; Jiang, L.; Wang, H. Seed Germination Ecology of the Medicinal Plant Peganum harmala (Zygophyllaceae). Plants 2023, 12, 2660. [Google Scholar] [CrossRef]
- Licaj, I.; Fiorillo, A.; Di Meo, M.C.; Varricchio, E.; Rocco, M. Effect of Polyethylene Glycol-Simulated Drought Stress on Stomatal Opening in “Modern” and “Ancient” Wheat Varieties. Plants 2024, 13, 1575. [Google Scholar] [CrossRef]
- Lu, G.; Tian, Z.; Chen, P.; Liang, Z.; Zeng, X.; Zhao, Y.; Li, C.; Yan, T.; Hang, Q.; Jiang, L. Comprehensive Morphological and Molecular Insights into Drought Tolerance Variation at Germination Stage in Brassica napus Accessions. Plants 2024, 13, 3296. [Google Scholar] [CrossRef]
- Eriksson, O.; Friis, E.M.; Löfgren, P. Seed size, fruit size and dispersal systems in angiosperms from the Early Cretaceous to the Late Tertiary. Am. Nat. 2000, 156, 47–58. [Google Scholar] [CrossRef]
- Eriksson, O. Evolution of seed size and biotic seed dispersal in angiosperms: Paleoecological and neoecological evidence. Int. J. Plant Sci. 2008, 169, 863–870. [Google Scholar] [CrossRef]
- Mamut, J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Role of trichomes and pericarp in the seed biology of the desert annual Lachnoloma lehmannii (Brassicaceae). Ecol. Res. 2014, 29, 33–44. [Google Scholar] [CrossRef]
- Mummenhoff, K.; Polster, A.; Mühlhausen, A.; Theißen, G. Lepidium as a model system for studying the evolution of fruit development in Brassicaceae. J. Exp. Bot. 2009, 60, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Mühlhausen, A.; Lenser, T.; Mummenhoff, K.; Theißen, G. Evidence that an evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae) was caused by a change in the control of valve margin identity genes. Plant J. 2013, 73, 824–835. [Google Scholar] [CrossRef]
- Montana Natural Heritage Program. Lepidium campestre (Field Pepper-Grass) Predicted Suitable Habitat Model Created on May 3, 2024; Montana Natural Heritage Program: Helena, MT, USA, 2024; 17p. [Google Scholar]
- Francis, A.; Lujan-Toro, B.E.; Warwick, S.I.; Macklin, J.A.; Martin, S.L. Update on the Brassicaceae species checklist. Biodivers. Data J. 2021, 9, e58773. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.; Smith, L.; Rice, P. Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Sci. 2000, 48, 640–644. [Google Scholar] [CrossRef]
- Mulligan, G.A. Chromosome numbers determined from Canadian and Alaskan material of native and naturalized mustards, Brassicaceae (Cruciferae). Can. Field-Nat. 2002, 116, 611–622. [Google Scholar] [CrossRef]
- Kiemnec, G.; Larson, L. Germination and root growth of two noxious weeds as affected by water and salt stresses. Weed Technol. 1991, 5, 612–615. [Google Scholar] [CrossRef]
- Thiede, D.A.; Augspurger, C.K. Intraspecific variation in seed dispersion of Lepidium campestre (Barassicaceae). Am. J. Bot. 1996, 83, 856–866. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Sami, A.; Zhang, H.; Jin, X.Z.; Zheng, W.Y.; Zhu, Z.Y.; Wu, L.L.; Lei, Y.H.; Chen, Z.P.; Li, Y.; et al. Combined influence of low temperature and drought on different varieties of rapeseed (Brassica napus L.). S. Afr. J. Bot. 2022, 147, 400–414. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Tang, A.J.; Tian, M.H.; Long, C.L. Dormancy and germination in short-lived Lepidium perfoliatum L. (Brassicaceae) seeds. Pak. J. Bot. 2010, 42, 201–211. [Google Scholar]
- Zhou, Y.M.; Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Seed germination ecology of the cold desert annual Isatis violascens (Brassicaceae): Two levels of physiological dormancy and role of the pericarp. PLoS ONE 2015, 10, e0140983. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.; Mummenhoff, K. More than Just a Shell: Indehiscent Fruits Drive Drought-Tolerant Germination in Invasive Lepidium Species. Plants 2025, 14, 1517. https://doi.org/10.3390/plants14101517
Mohammed S, Mummenhoff K. More than Just a Shell: Indehiscent Fruits Drive Drought-Tolerant Germination in Invasive Lepidium Species. Plants. 2025; 14(10):1517. https://doi.org/10.3390/plants14101517
Chicago/Turabian StyleMohammed, Said, and Klaus Mummenhoff. 2025. "More than Just a Shell: Indehiscent Fruits Drive Drought-Tolerant Germination in Invasive Lepidium Species" Plants 14, no. 10: 1517. https://doi.org/10.3390/plants14101517
APA StyleMohammed, S., & Mummenhoff, K. (2025). More than Just a Shell: Indehiscent Fruits Drive Drought-Tolerant Germination in Invasive Lepidium Species. Plants, 14(10), 1517. https://doi.org/10.3390/plants14101517





