Nutritional Efficiency of Coffea canephora: The Role of Genetic Variability and Nutrient Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Fruits Samples and Nutrient Accumulation
2.3. Statistical Analyses
3. Results
3.1. Nutrient Accumulation
3.2. Genetic Variability
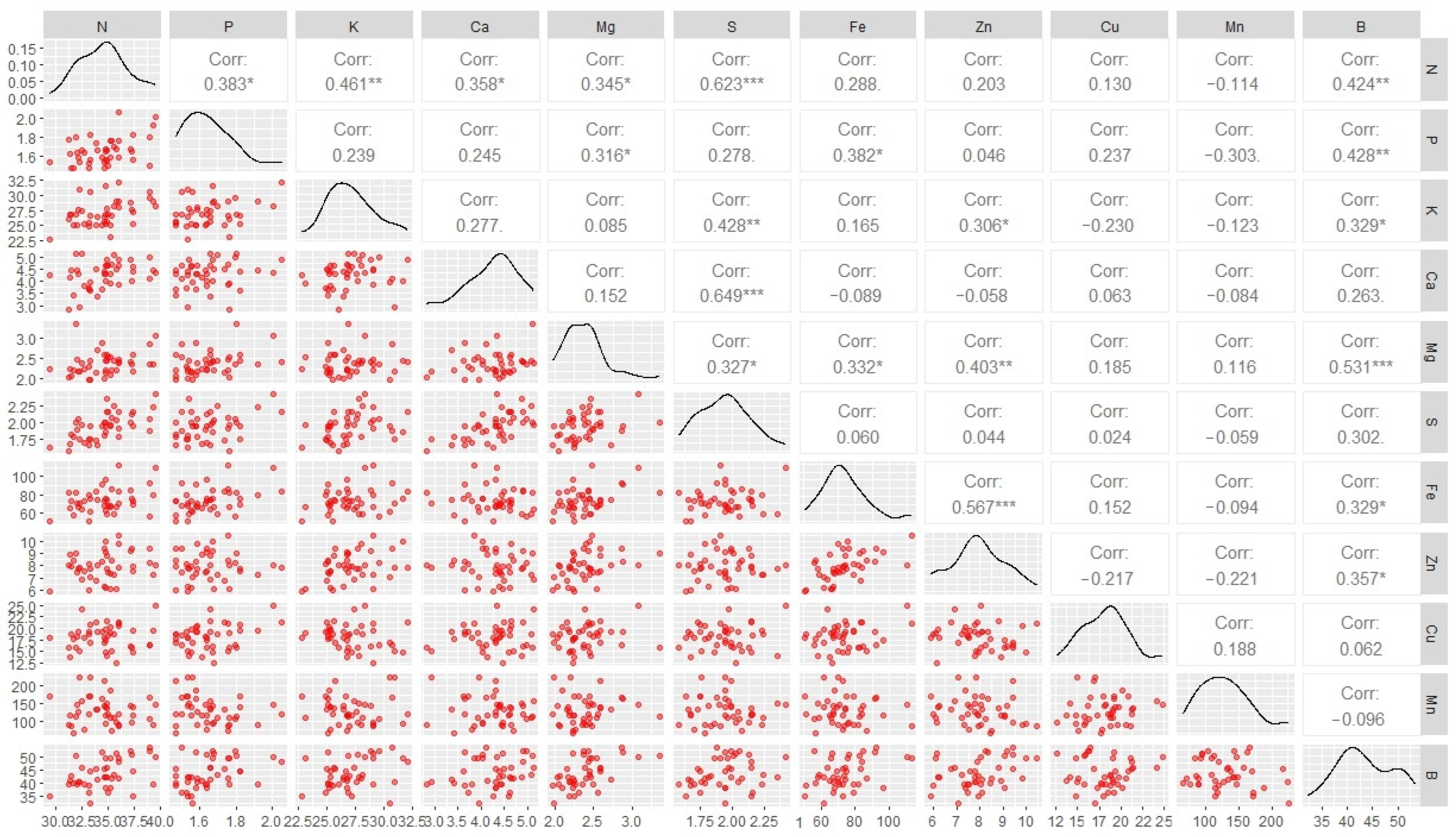
3.3. Correlation Among Nutrient Accumulations in the Fruit
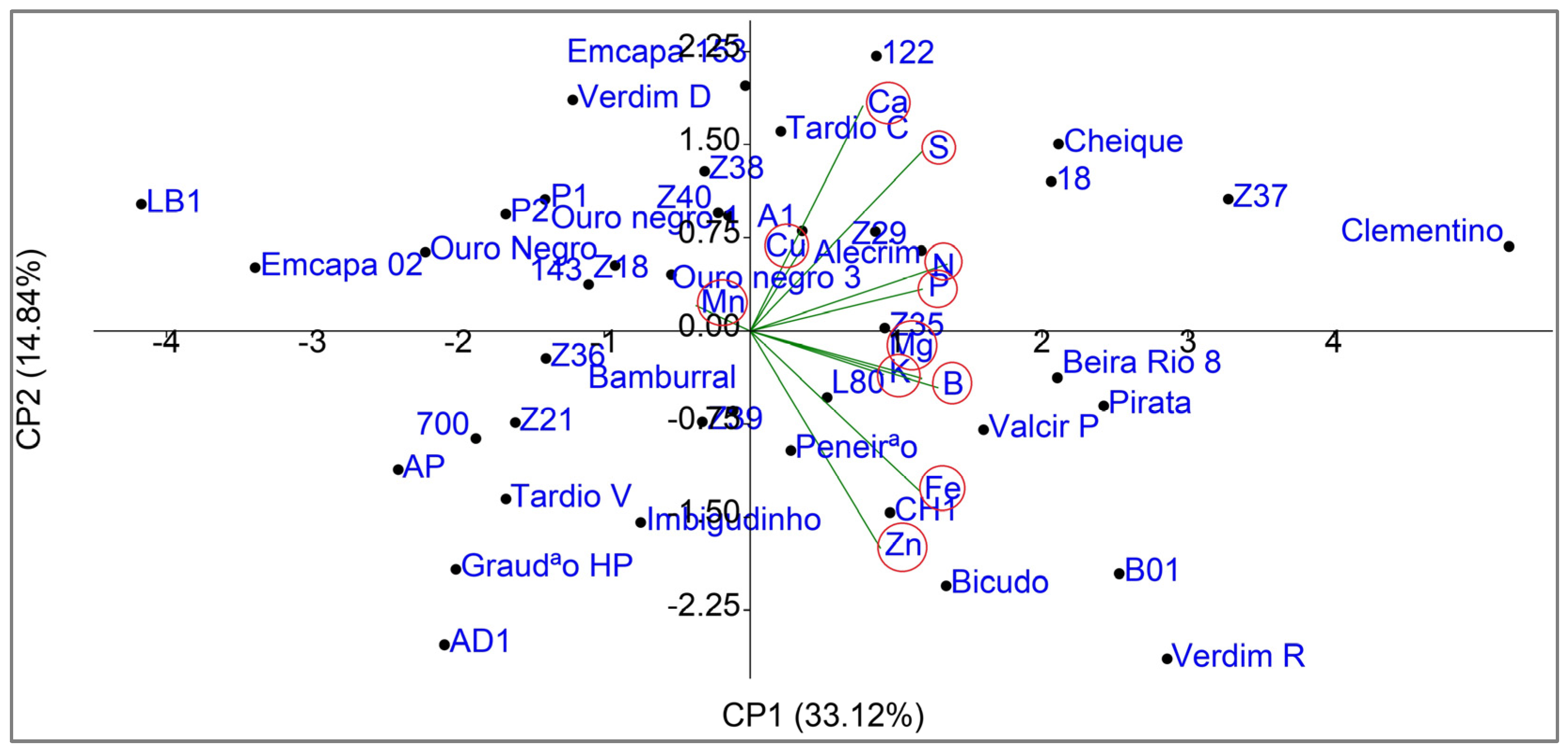
3.4. Multivariate Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- Depolo, R.P.; Rocha, R.B.; de Souza, C.A.; dos Santos, M.R.A.; Espindula, M.C.; Teixeira, A.L. Expression of self-incompatibility in Coffea canephora genotypes grown in the western Amazon. Pesq. Agropec. Bras. 2022, 57, e03031. [Google Scholar] [CrossRef]
- Moraes, M.S.; Castro, P.H.; Freitas, M.S.; Amaral, J.F.; Martins, M.Q.; Ramalho, J.C. Characterization of gametophytic self-incompatibility of superior clones of Coffea canephora. Genet. Mol. Res. 2018, 17, gmr16039876. [Google Scholar] [CrossRef]
- Dubberstein, D.; Oliveira, M.G.; Aoyama, E.M.; Guilhen, J.H.; Ferreira, A.; Marques, I.; Ramalho, J.C.; Partelli, F.L. Diversity of leaf stomatal traits among Coffea canephora Pierre ex A. Froehner genotypes. Agronomy 2021, 11, 1126. [Google Scholar] [CrossRef]
- de Araújo, L.F.B.; Espindula, M.C.; Rocha, R.B.; Torres, J.D.; Campanharo, M.; Pego, W.F.O.; de Souza Rosa, S.E. Divergência genética com base em características vegetativas e anatômicas foliares de clones de Coffea canephora. Semina Ciênc. Agrár. 2021, 42, 2717–2734. [Google Scholar] [CrossRef]
- Santos, M.M.; Oliveira, M.G.; Cassol, D.; Rodrigues, W.P.; Falqueto, A.R.; Ramalho, J.C.; Partelli, F.L. Genotypic Diversity of Coffea canephora cv. Conilon Identified through Leaf Morpho- and Eco-Physiological Traits. Sci. Hortic. 2024, 324, 112603. [Google Scholar] [CrossRef]
- Silva, C.A.; Partelli, F.L.; Aoyama, E.M.; Bonomo, R.; Vieira, H.D.; Ramalho, J.C.; Ribeiro-Barros, A.I. Floral Morphology of Robusta Coffee Genotypes. Agron. J. 2021, 113, 3080–3088. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Rodrigues, M.J.L.; Almeida, R.N.; Semedo, J.N.; Rakocevic, M.; Partelli, F.L. Towards a minimum number of key flower traits in studies of Coffea spp. phenotype variability. Sci. Hortic. 2024, 337, 113513. [Google Scholar] [CrossRef]
- Sousa, P.; Vieira, H.; Santos, E.; Viana, A.; Boaechat, M.; Partelli, F. Coffea canephora: Heterotic Crosses Indicated by Molecular Approach. Plants 2022, 11, 3023. [Google Scholar] [CrossRef]
- Dubberstein, D.; Partelli, F.L.; Espindula, M.C.; Dias, J.R.M. Concentration and accumulation of micronutrients in robust coffee. Acta Sci. Agron. 2019, 41, e42685. [Google Scholar] [CrossRef]
- Silva, C.A.; Santos, E.A.; Viana, A.P.; Dias, J.R.M.; Partelli, F.L. Genetic diversity in Coffea canephora genotypes for leaf nutrient concentration. Rev. FCA UNCuyo 2021, 53, 22–34. [Google Scholar] [CrossRef]
- Rodrigues, M.J.L.; da Silva, C.A.; Braun, H.; Partelli, F.L. Nutritional Balance and Genetic Diversity of Coffea canephora Genotypes. Plants 2023, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; da Silva, C.A.; Silva, L.O.E.; Espindula, M.C.; Rodrigues, W.P.; Vieira, H.D.; Tomaz, M.A.; Partelli, F.L. Accumulation of Nutrients and the Relation between Fruit, Grain, and Husk of Coffee Robusta Cultivated in Brazilian Amazon. Plants 2023, 12, 3476. [Google Scholar] [CrossRef]
- Abeele, V.S.; Janssens, S.B.; Anio, J.A.; Bawin, Y.; Depecker, J.; Kambale, B.; Mwanga, I.M.; Ntore, S.; Ebele, T.; Stoffelen, P.; et al. Genetic diversity of wild and cultivated Coffea canephora in northeastern DR Congo and the implications for conservation. Am. J. Bot. 2021, 108, 2425–2434. [Google Scholar] [CrossRef]
- Bollen, R.; Verleysen, L.; Katshela, B.N.; Kambale, J.-L.; Ebele, T.; Ruttink, T.; Vandelook, F.; Honnay, O.; Stoffelen, P. Sensory profiles of Robusta coffee (Coffea canephora) genetic resources from the Democratic Republic of the Congo. Front. Sustain. Food Syst. 2024, 8, 1382976. [Google Scholar] [CrossRef]
- Aquino, S.O.; Kiwuka, C.; Tournebize, R.; Gain, C.; Marraccini, P.; Mariac, C.; Bethune, K.; Couderc, M.; Cubry, P.; Andrade, A.C.; et al. Adaptive Potential of Coffea canephora from Uganda in Response to Climate Change. Mol. Ecol. 2022, 31, 1800–1819. [Google Scholar] [CrossRef]
- Kiwuka, C.; Goudsmit, E.; Tournebize, R.; de Aquino, S.O.; Douma, J.C.; Bellanger, L.; Crouzillat, D.; Stoffelen, P.; Sumirat, U.; Legnaté, H.; et al. Genetic diversity of native and cultivated Ugandan Robusta coffee (Coffea canephora Pierre ex A. Froehner): Climate influences, breeding potential, and diversity conservation. PLoS ONE 2021, 16, e0245965. [Google Scholar] [CrossRef]
- Akpertey, A.; Anim-Kwapong, E.; Adu-Gyamfi, P.K.K.; Ofori, A. Genetic Variability for Vigor and Yield of Robusta Coffee (Coffea canephora) Clones in Ghana. Heliyon 2022, 8, e10192. [Google Scholar] [CrossRef]
- Mishra, M.K.; Huded, A.K.C.; Jingade, P.; Bychappa, M. Molecular characterization and genetic structure analysis of Coffea arabica and Coffea canephora cultivars from India using SCoT markers. Ecol. Genet. Genom. 2022, 23, 100117. [Google Scholar] [CrossRef]
- Mihai, R.A.; Ortiz-Pillajo, D.C.; Iturralde-Proaño, K.M.; Vinueza-Pullotasig, M.Y.; Sisa-Tolagasí, L.A.; Villares-Ledesma, M.L.; Melo-Heras, E.J.; Cubi-Insuaste, N.S.; Catana, R.D. Comprehensive Assessment of Coffee Varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador: A Holistic Evaluation of Metabolism, Antioxidant Capacity and Sensory Attributes. Horticulturae 2024, 10, 200. [Google Scholar] [CrossRef]
- Martins, L.D.; Machado, L.D.S.; Tomaz, M.A.; do Amaral, J.F.T. A eficiência nutricional de Coffea spp.: Uma revisão. Afr. J. Biotechnol. 2015, 14, 728–734. [Google Scholar] [CrossRef]
- Moura, W.d.M.; Soares, Y.J.B.; Júnior, A.T.d.A.; de Lima, P.C.; Martinez, H.E.P.; Gravina, G.d.A. Genetic Diversity in Arabica Coffee Grown in Potassium-Constrained Environment. Ciênc. Agrotec. 2015, 39, 23–31. [Google Scholar] [CrossRef]
- Partelli, F.L.; Silva, F.A.; Covre, A.M.; Oliosi, G.; Correa, C.C.G.; Viana, A.P. Adaptability and stability of Coffea canephora to dynamic environments using the Bayesian approach. Sci. Rep. 2022, 12, 12768. [Google Scholar] [CrossRef]
- Alvares, A.C.; Stape, J.; Sentelhas, P.; Gonçalves, J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Partelli, F.L. Café Conilon: Tecnologias na Produção e na Qualidade; CAUFES: Alegre, ES, Brazil, 2022; 136p. [Google Scholar]
- Bragança, S.M.; Carvalho, C.H.S.; Fonseca, A.F.A.; Ferrão, R.G. Variedades clonais de café Conilon para o estado do Espírito Santo. Pesq. Agropec. Bras. 2001, 36, 765–770. [Google Scholar] [CrossRef]
- Partelli, F.L.; Pereira, L.L.; Oliosi, G.; Campanharo, A.; Covre, A.M.; Alberto, N.J.; Salvador, H.P. Research and Development in Conilon and Robusta Coffee; Khas Editora: São Mateus, ES, Brazil, 2024. [Google Scholar]
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; pp. 191–233. [Google Scholar]
- Mojena, R. Hierarchical grouping method and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant. Breed 1981, 41, 237–245. [Google Scholar]
- Cruz, C.D. Programa Genes—Ampliado e integrado aos aplicativos R, Matlab e Selegen. Acta Sci. Agron. 2016, 38, 547–552. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: New York, NY, USA, 2015; p. 858. [Google Scholar]
- Clemente, J.M.; Martinez, H.E.P.; Alves, L.C.; Finger, F.L.; Cecon, P.R. Effects of nitrogen and potassium on the chemical composition of coffee beans and on beverage quality. Acta Sci. Agron. 2015, 37, 297–305. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Silva, L.O.E.; Schmidt, R.; Valani, G.P.; Ferreira, A.; Ribeiro-Barros, A.I.; Partelli, F.L. Root trait variability in Coffea canephora genotypes and its relation to plant height and crop yield. Agronomy 2020, 10, 1394. [Google Scholar] [CrossRef]
- Schmidt, R.; Silva, L.O.E.; Ferreira, A.; Rodrigues, W.P.; Vieira, H.D.; Tomaz, M.A.; Partelli, F.L. Root system of Coffea canephora cv. Robusta genotypes cultivated in the Brazilian Amazon. Discov. Agric. 2024, 2, 32. [Google Scholar] [CrossRef]
- Partelli, F.L.; Oliosi, G.; Dalazen, J.R.; Silva, C.A.; Vieira, H.D.; Espindula, M.C. Proportion of ripe fruit weight and volume to green coffee: Differences in 43 genotypes of Coffea canephora. Agron. J. 2021, 113, 1050–1057. [Google Scholar] [CrossRef]
- Gabelman, W.H.; Gerloff, G.C. The search for and interpretation of genetic controls that enhance plant growth under deficiency levels of a macronutrient. Plant Soil 1983, 72, 335–350. [Google Scholar] [CrossRef]
- Amaral, J.F.T.; Martinez, H.E.P.; Laviola, B.G.; Fernandes Filho, E.I.; Cruz, C.D. Eficiência de utilização de nutrientes por cultivares de cafeeiro. Ciênc. Rural 2011, 41, 621–629. [Google Scholar] [CrossRef]
- Oliveira, L.N.L.; Rocha, R.B.; Ferreira, F.M.; Spinelli, V.M.; Ramalho, A.R.; Teixeira, A.L. Selection of Coffea canephora parents from the botanical varieties Conilon and Robusta for the production of intervarietal hybrids. Cienc Rural 2018, 48, e20170444. [Google Scholar] [CrossRef]
- Gomes, W.R.; Rodrigues, W.P.; Vieira, H.D.; Oliveira, M.G.; Dias, J.R.M.; Partelli, F.L. Genetic Diversity of Standard Leaf Nutrients in Coffea canephora Genotypes during Phenological Phases. Genet. Mol. Res. 2016, 15, gmr.15048839. [Google Scholar] [CrossRef]
- Martins, M.Q.; Partelli, F.L.; Ferreira, A.; Bernardes, C.O.; Golynski, A.; Vieira, H.D.; Freitas, M.S.M.; Ramalho, J.C. Genetic Variability on Nutrient Contents in Coffea canephora Genotypes Cultivated at 850 Meters of Altitude in Two Crop Seasons. Funct. Plant Breed. J. 2019, 1, 59–71. [Google Scholar] [CrossRef]
- Rodrigues, W.N.; Tomaz, M.A.; Ferrão, R.G.; Ferrão, M.A.G.; da Fonseca, A.F.A.; de Miranda, F.D. Genetic Parameters Estimation in Groups of Conilon Coffee Clones. Coffee Sci. 2012, 7, 177–186. [Google Scholar]
- Santos, M.M.d.; Silva, C.A.d.; Oza, E.F.; Gontijo, I.; Amaral, J.F.T.d.; Partelli, F.L. Concentration of Nutrients in Leaves, Flowers, and Fruits of Genotypes of Coffea canephora. Plants 2021, 10, 2661. [Google Scholar] [CrossRef]

| Chemical Attributes | Depth (cm) | |||||
|---|---|---|---|---|---|---|
| 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | |
| K (mg dm−3) | 110.0 | 95.0 | 74.0 | 57.0 | 52.0 | 46.0 |
| S (mg dm−3) | 15.0 | 11.0 | 29.0 | 15.0 | 15.0 | 17.0 |
| Ca (cmol dm−3) | 3.8 | 3.4 | 1.9 | 1.0 | 0.7 | 0.6 |
| Mg (cmol dm3) | 1.0 | 0.9 | 0.4 | 0.3 | 0.1 | 0.1 |
| Al (cmol dm−3) | 0.0 | 0.0 | 0.3 | 0.7 | 0.8 | 0.8 |
| H + Al | 1.6 | 1.8 | 2.4 | 2.9 | 3.1 | 3.1 |
| pH-H2O | 6.6 | 6.5 | 5.3 | 4.8 | 4.8 | 4.8 |
| Organic matter (dag dm−3) | 2.1 | 1.7 | 1.1 | 0.8 | 0.7 | 0.5 |
| Fe (mg dm−3) | 140.0 | 138.0 | 126.0 | 94.0 | 88.0 | 87.0 |
| Zn (mg dm−3) | 10.2 | 4.5 | 2.9 | 1.1 | 0.6 | 0.5 |
| Cu (mg dm−3) | 3.4 | 4.3 | 3.0 | 1.9 | 1.2 | 1.0 |
| Mn (mg dm−3) | 207.0 | 174.0 | 104.0 | 46.0 | 44.0 | 40.0 |
| B (mg dm−3) | 0.8 | 0.8 | 0.6 | 0.6 | 0.6 | 0.6 |
| Na (mg dm−3) | 11.0 | 37.0 | 8.0 | 6.0 | 5.0 | 4.0 |
| Granulometry (g kg−1) | ||||||
| Sand | 434.0 | 352.0 | 188.0 | 368.0 | 366.0 | 376.0 |
| Silt | 86.0 | 168.0 | 212.0 | 32.0 | 74.0 | 124.0. |
| Clay | 480.0 | 480.0 | 600.0 | 600.0 | 560.0 | 500.0 |
| Identification | Name | Identification | Name | Identification | Name |
|---|---|---|---|---|---|
| 1 | Verdim R | 15 | Bamburral | 29 | Tardio C |
| 2 | B01 | 16 | Pirata | 30 | A1 |
| 3 | Bicudo | 17 | Peneirão | 31 | Cheique |
| 4 | Alecrim | 18 | Z39 | 32 | P2 |
| 5 | 700 | 19 | Z35 | 33 | Emcapa 02 |
| 6 | CH1 | 20 | Z40 | 34 | Emcapa 153 |
| 7 | Imbigudinho | 21 | Z29 | 35 | P1 |
| 8 | AD1 | 22 | Z38 | 36 | LB1 |
| 9 | Graudão HP | 23 | Z18 | 37 | 122 |
| 10 | Valcir P | 24 | Z37 | 38 | Verdim D |
| 11 | Beira Rio 8 | 25 | Z21 | 39 | Emcapa 143 |
| 12 | Tardio V | 26 | Z36 | 40 | Ouro negro 1 |
| 13 | AP | 27 | Ouro Negro | 41 | Ouro negro 2 |
| 14 | L80 | 28 | 18 | 42 | Clementino |
| Genotypes | N | P | K | Ca | Mg | S | Fe | Zn | Cu | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| —————— kg·ton−1 —————— | ————— g·ton−1 ————— | ||||||||||
| Verdim R | 36.1 c | 1.8 e | 29.1 b | 3.8 c | 2.5 f | 1.9 f | 112.8 a | 10.5 a | 21.2 b | 84.0 i | 49.4 b |
| B01 | 39.0 a | 1.5 j | 30.7 a | 4.1 c | 2.9 c | 1.9 f | 92.2 c | 9.4 c | 16.4 g | 166.8 c | 53.4 a |
| Bicudo | 34.8 d | 1.7 f | 31.6 a | 4.0 c | 2.4 f | 1.9 f | 75.3 g | 10.0 b | 14.8 h | 92.2 i | 49.2 b |
| Alecrim | 34.9 d | 1.7 f | 25.6 d | 4.7 b | 2.6 e | 2.1 c | 74.4 g | 8.8 d | 21.5 b | 134.8 f | 45.9 c |
| 700 | 33.2 e | 1.5 i | 24.9 d | 3.7 d | 2.3 g | 1.8 h | 79.0 f | 8.1 f | 19.2 c | 171.6 c | 36.4 f |
| CH1 | 34.4 d | 1.6 h | 25.1 d | 3.6 d | 2.9 c | 1.9 e | 91.2 c | 8.3 e | 19.2 c | 163.4 d | 51.7 a |
| Imbigudinho | 34.0 e | 1.7 f | 26.4 c | 3.4 e | 2.7 d | 1.7 i | 74.1 g | 8.1 f | 19.1 c | 133.4 f | 41.1 d |
| AD1 | 31.3 f | 1.8 e | 26.0 c | 2.8 f | 2.1 i | 1.6 i | 81.9 e | 8.0 f | 15.8 g | 88.8 i | 39.5 e |
| Graudão HP | 32.3 f | 1.5 i | 30.9 a | 2.9 f | 2.2 h | 1.8 h | 70.0 i | 7.8 f | 15.0 h | 224.1 a | 40.6 e |
| Valcir P | 37.3 b | 1.6 g | 27.7 c | 4.0 c | 2.4 g | 2.1 c | 76.0 g | 9.4 c | 15.9 g | 94.8 i | 50.6 b |
| Beira Rio 8 | 37.1 b | 1.7 f | 28.8 b | 4.9 a | 2.4 f | 2.0 e | 84.5 d | 9.2 d | 16.2 g | 142.4 e | 51.8 a |
| Tardio V | 31.4 f | 1.6 g | 26.8 c | 4.1 b | 2.2 h | 1.7 i | 65.5 j | 8.9 d | 13.7 i | 114.4 g | 40.8 e |
| AP | 31.8 f | 1.5 j | 25.1 d | 3.4 e | 2.2 h | 1.8 g | 57.0 l | 8.4 e | 17.8 e | 64.6 j | 39.9 e |
| L80 | 36.0 c | 1.6 h | 28.0 b | 4.6 b | 2.4 g | 1.8 g | 69.8 i | 9.8 b | 20.6 b | 89.3 i | 41.9 d |
| Bamburral | 34.7 d | 1.5 j | 25.8 d | 4.3 b | 2.6 e | 2.0 e | 72.7 h | 9.4 c | 16.8 f | 216.4 a | 45.3 c |
| Pirata | 31.9 f | 1.8 d | 26.8 c | 5.1 a | 3.4 a | 2.0 e | 82.6 e | 9.0 d | 15.8 g | 113.1 g | 50.4 b |
| Peneirão | 34.8 d | 1.6 g | 26.8 c | 4.3 b | 2.2 h | 1.9 e | 96.5 b | 9.1 d | 17.3 f | 115.0 g | 38.3 f |
| Z39 | 33.3 e | 1.8 d | 26.6 c | 3.6 d | 2.4 f | 1.7 h | 75.1 g | 7.8 f | 20.8 b | 170.1 c | 44.6 c |
| Z35 | 35.4 d | 1.8 e | 26.7 c | 4.7 b | 2.2 h | 2.1 d | 86.5 d | 8.5 e | 15.1 h | 71.4 j | 41.9 d |
| Z40 | 37.4 b | 1.6 i | 27.2 c | 4.6 b | 2.2 h | 2.2 c | 72.8 h | 7.9 f | 14.8 h | 114.9 g | 35.0 g |
| Z29 | 37.4 b | 1.8 d | 25.4 d | 4.4 b | 2.6 e | 1.9 e | 69.5 i | 7.6 g | 19.4 c | 138.0 e | 44.4 c |
| Z38 | 35.1 d | 1.5 j | 26.9 c | 4.6 b | 2.6 e | 2.2 c | 67.1 j | 7.5 g | 18.6 d | 117.6 g | 36.3 f |
| Z18 | 32.2 f | 1.6 h | 27.6 c | 4.3 b | 2.2 h | 2.0 d | 66.9 j | 7.8 f | 19.1 c | 126.8 f | 40.7 e |
| Z37 | 36.1 c | 2.1 a | 32.1 a | 4.9 a | 2.4 f | 2.2 c | 83.6 e | 7.9 f | 21.3 b | 120.2 g | 48.1 b |
| Z21 | 32.7 e | 1.6 g | 25.3 d | 3.8 d | 2.3 h | 1.7 h | 72.9 h | 7.5 g | 17.9 e | 75.9 j | 39.4 e |
| Z36 | 31.5 f | 1.5 j | 26.9 c | 4.5 b | 2.1 i | 1.8 g | 73.4 h | 7.9 f | 18.7 d | 84.9 i | 42.8 d |
| Ouro Negro | 33.4 e | 1.6 i | 30.5 a | 4.0 c | 2.0 i | 1.9 e | 61.4 k | 7.1 g | 16.9 f | 226.4 a | 32.3 g |
| 18 | 39.0 a | 1.8 d | 29.5 b | 5.0 a | 2.4 g | 2.1 d | 55.9 l | 8.2 f | 16.4 g | 103.9 h | 52.0 a |
| Tardio C | 34.9 d | 1.5 i | 27.8 b | 4.9 a | 2.5 f | 2.4 a | 58.5 l | 7.8 f | 14.2 h | 149.1 e | 42.3 d |
| A1 | 35.8 c | 1.7 f | 29.0 b | 4.5 b | 2.2 h | 2.0 d | 66.8 j | 6.1 i | 12.6 j | 94.0 i | 51.0 a |
| Cheique | 39.3 a | 1.9 c | 29.1 b | 4.4 b | 2.4 g | 2.2 b | 78.9 f | 7.3 g | 19.5 c | 89.2 i | 39.6 e |
| P2 | 34.7 d | 1.5 i | 26.4 c | 4.4 b | 2.0 i | 1.8 g | 67.2 j | 6.3 i | 18.1 e | 143.2 e | 42.1 d |
| Emcapa 02 | 33.2 e | 1.5 j | 25.6 d | 3.7 d | 2.0 i | 1.7 i | 51.1 m | 6.0 i | 18.7 d | 119.4 g | 39.1 e |
| Emcapa 153 | 32.6 e | 1.7 f | 27.4 c | 5.1 a | 2.4 g | 2.0 e | 63.8 k | 6.9 h | 24.2 a | 157.1 d | 45.7 c |
| P1 | 35.2 d | 1.6 h | 25.2 d | 4.3 b | 2.1 i | 1.9 e | 67.7 j | 7.4 g | 20.2 c | 188.2 b | 38.1 f |
| LB1 | 29.5 g | 1.5 i | 22.8 e | 4.3 b | 2.2 h | 1.6 i | 50.5 m | 5.9 i | 17.9 e | 171.6 c | 35.2 g |
| 122 | 35.6 c | 1.7 f | 28.2 b | 5.1 a | 2.4 f | 2.2 b | 59.1 l | 7.2 g | 18.7 d | 108.9 h | 43.2 d |
| Verdim D | 34.8 d | 1.6 i | 25.4 d | 4.6 b | 2.2 h | 2.0 e | 62.0 k | 6.2 i | 19.7 c | 140.8 e | 42.4 d |
| 143 | 32.1 f | 1.6 g | 25.2 d | 4.4 b | 2.5 e | 1.9 f | 71.0 h | 7.5 g | 18.2 e | 146.2 e | 39.4 e |
| Ouro negro 1 | 35.2 d | 1.8 e | 23.2 e | 3.9 c | 2.5 e | 2.0 e | 65.9 j | 6.1 i | 19.1 c | 108.1 h | 49.3 b |
| Ouro negro 3 | 33.9 e | 1.7 e | 25.0 d | 4.5 b | 2.1 h | 1.7 h | 83.0 e | 6.8 h | 21.2 b | 131.5 f | 45.1 c |
| Clementino | 39.6 a | 2.0 b | 28.3 b | 4.3 b | 3.1 b | 2.4 a | 110.2 a | 8.1 f | 24.9 a | 146.0 e | 50.0 b |
| Summary of variance analysis | |||||||||||
| Genotypes | 47.6 ** | 84.6 ** | 18.4 ** | 20.9 ** | 57.9 ** | 35.2 ** | 175.4 ** | 77.9 ** | 87.8 ** | 144.9 ** | 27.4 ** |
| CVe (%) | 1.9 | 1.9 | 3.7 | 5.7 | 3.2 | 3.4 | 2.8 | 3.2 | 3.1 | 5.1 | 4.8 |
| Média | 34.6 | 1.7 | 27.1 | 4.3 | 2.4 | 1.9 | 73.7 | 7.9 | 18.2 | 129.8 | 43.5 |
| Nutrients | CVe (%) | CVg (%) | H2 (%) |
|---|---|---|---|
| N | 1.98 | 6.75 | 97.90 |
| P | 1.86 | 8.53 | 98.83 |
| K | 3.67 | 7.64 | 94.55 |
| Ca | 5.69 | 12.70 | 95.22 |
| Mg | 3.17 | 11.95 | 98.28 |
| S | 3.44 | 10.04 | 97.15 |
| Cu | 3.12 | 14.54 | 98.86 |
| Fe | 2.79 | 18.40 | 99.43 |
| Mn | 5.11 | 30.66 | 99.31 |
| Zn | 3.24 | 14.21 | 98.72 |
| B | 4.76 | 12.23 | 96.35 |
| Nutrients | S.j. 1 | Contribution % | Accumulated Value |
|---|---|---|---|
| Mn | 2,747,064.9 | 87.2 | 87.2 |
| Fe | 318,796.0 | 10.1 | 97.3 |
| B | 50,556.6 | 1.6 | 98.9 |
| Cu | 12,128.1 | 0.4 | 99.3 |
| N | 9627.1 | 0.3 | 99.6 |
| K | 7824.8 | 0.3 | 99.9 |
| Zn | 2215.8 | 0.1 | 99.9 |
| Ca | 528.1 | 0.1 | 99.9 |
| Mg | 14.2 | 0.0 | 100.0 |
| S | 67.1 | 0.0 | 100.0 |
| P | 34.6 | 0.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.A.d.; Dalazen, J.R.; Rodrigues, W.P.; Rocha, R.B.; Partelli, F.L. Nutritional Efficiency of Coffea canephora: The Role of Genetic Variability and Nutrient Accumulation. Plants 2025, 14, 1509. https://doi.org/10.3390/plants14101509
Silva CAd, Dalazen JR, Rodrigues WP, Rocha RB, Partelli FL. Nutritional Efficiency of Coffea canephora: The Role of Genetic Variability and Nutrient Accumulation. Plants. 2025; 14(10):1509. https://doi.org/10.3390/plants14101509
Chicago/Turabian StyleSilva, Cleidson Alves da, Jéssica Rodrigues Dalazen, Weverton Pereira Rodrigues, Rodrigo Barros Rocha, and Fábio Luiz Partelli. 2025. "Nutritional Efficiency of Coffea canephora: The Role of Genetic Variability and Nutrient Accumulation" Plants 14, no. 10: 1509. https://doi.org/10.3390/plants14101509
APA StyleSilva, C. A. d., Dalazen, J. R., Rodrigues, W. P., Rocha, R. B., & Partelli, F. L. (2025). Nutritional Efficiency of Coffea canephora: The Role of Genetic Variability and Nutrient Accumulation. Plants, 14(10), 1509. https://doi.org/10.3390/plants14101509








