Performance, Agro-Morphological, and Quality Traits of Durum Wheat (Triticum turgidum L. ssp. durum Desf.) Germplasm: A Case Study in Jemâa Shaïm, Morocco
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics of Agro-Morphological and Quality Traits Measured Across All Wheat Lines
Correlation Analysis of Agro-Morphological and Quality Parameters Across All Lines
2.2. Principal Component Analysis
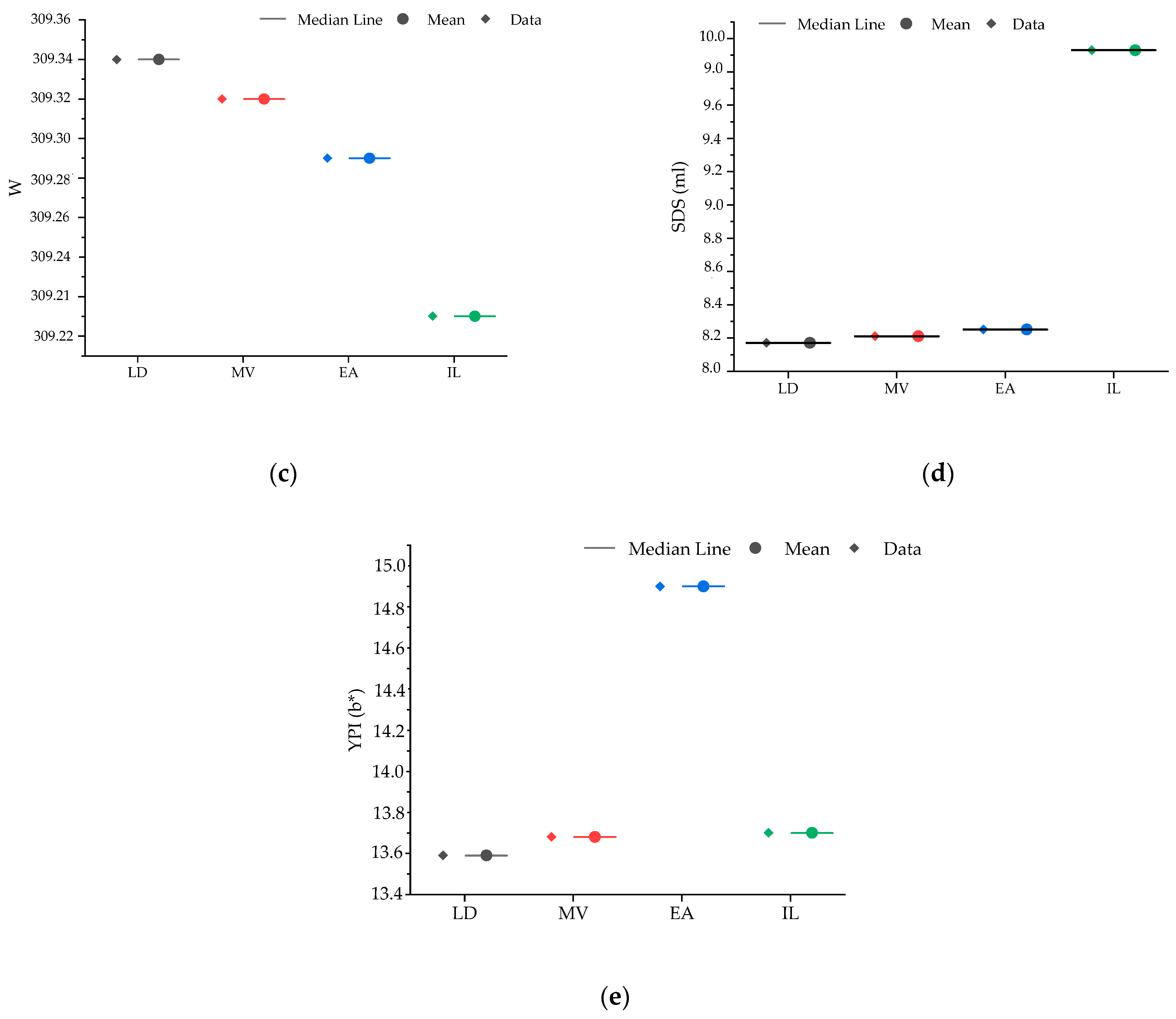
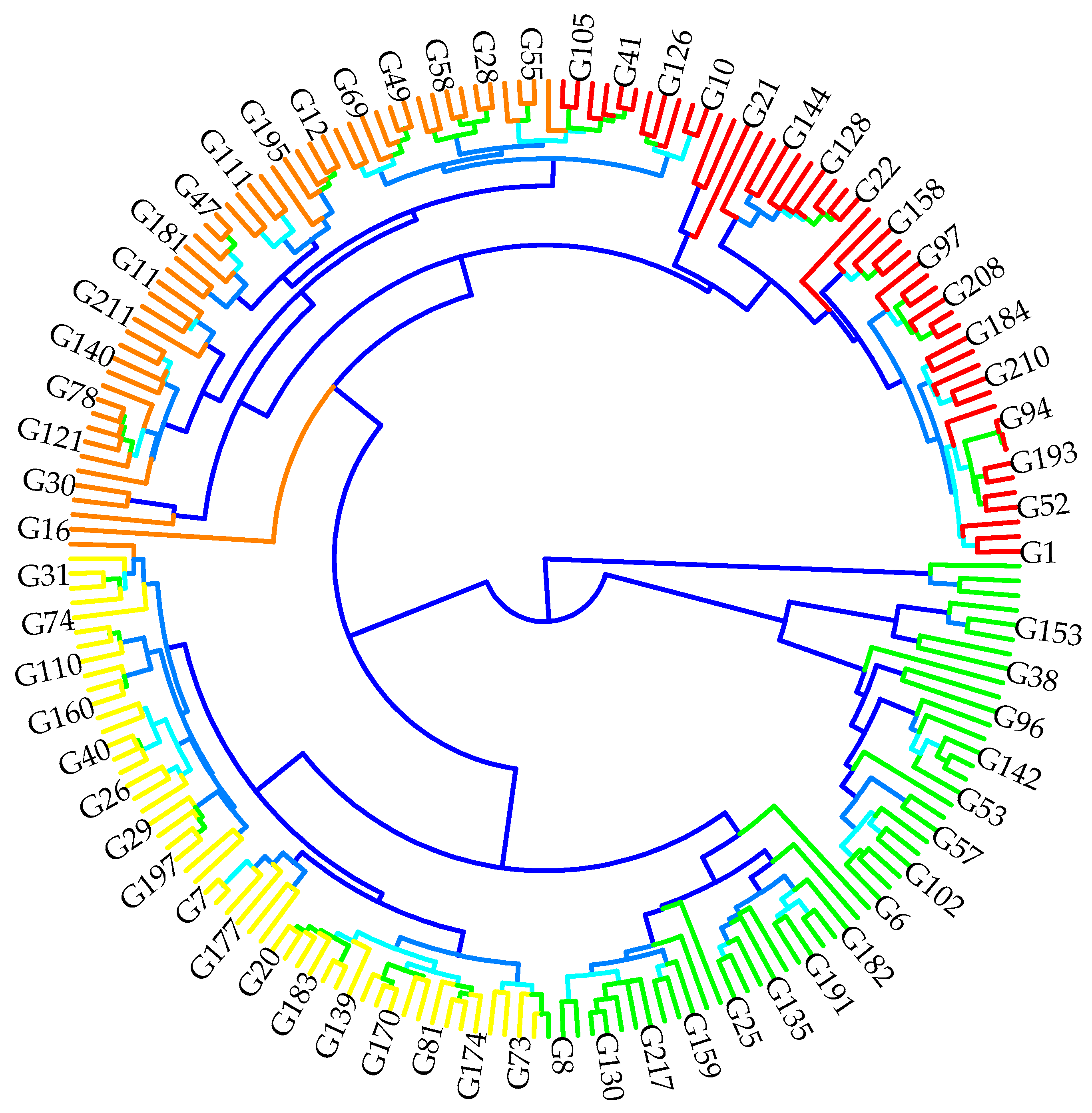
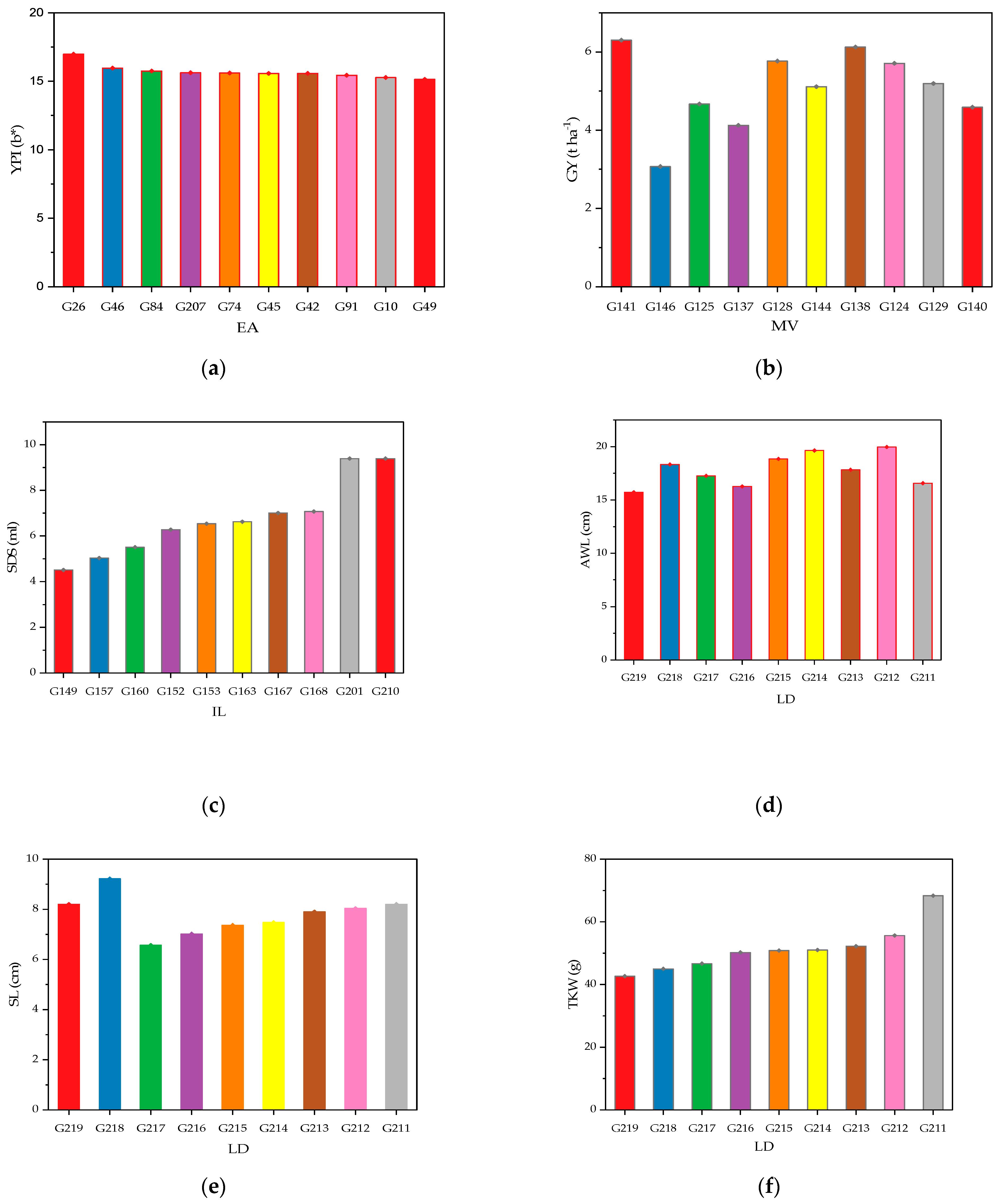
2.3. Genotypic Classification According to Agro-Morphological and Quality Performances
3. Materials and Methods
3.1. Experimental Site, Experimental Design, and Management Practices
3.2. Plant Material
3.3. Agro-Morphological Characterization
3.4. Quality Parameters Characterization
3.4.1. Whole Grain-Based Parameters
3.4.2. Flour-Based Parameters
3.4.3. Data Analysis
4. Discussion
4.1. Variability in Agro-Morphological Parameters and Grain Quality Traits
4.2. Correlations Between Agro-Morphological Traits in the Context of Different Durum Wheat Genotypes
4.3. Principal Component Analysis and Hierarchical Classification Analyses According to Agro-Morphological and Quality Performances
4.4. Screening and Performance of High-Performing Genotypes in Durum Wheat Lines
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smutka, L.; Steininger, M.; Miffek, O. World Agricultural Production and Consumption. AGRIS-Line Pap. Econ. Inform. 2009, 1, 3–12. [Google Scholar]
- Smutka, L.; Steininger, M.; Maitah, M.; Škubna, O. The Czech Agrarian Foreign Trade—Ten Years after the EU Accession. In Agrarian Perspectives XXIV: Proceedings of the 24th International Scientific Conference; Czech University of Life Sciences Prague, Faculty of Economics and Management: Prague, Czech Republic, 2015; pp. 385–392. [Google Scholar]
- Wegren, S.K.; Elvestad, C. Russia’s Food Self-Sufficiency and Food Security: An Assessment. Post-Communist Econ. 2018, 30, 565–587. [Google Scholar] [CrossRef]
- Cook, D.C.; Fraser, R.W.; Paini, D.R.; Warden, A.C.; Lonsdale, W.M.; De Barro, P.J. Biosecurity and Yield Improvement Technologies Are Strategic Complements in the Fight against Food Insecurity. PLoS ONE 2011, 6, e26084. [Google Scholar] [CrossRef] [PubMed]
- Stocking, M.A. Tropical Soils and Food Security: The Next 50 Years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Liu, M.; Jin, M.; Sun, X.; Pang, Y.; Yan, Q.; Liu, C.; Liu, S. QTL Mapping for Agronomic Important Traits in Well-Adapted Wheat Cultivar Ningchun 4. Agronomy 2024, 14, 940. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Yang, X.; Zhang, A.; Liu, D. Molecular Markers and Their Applications in Marker-Assisted Selection in Wheat Breeding. Agriculture 2023, 13, 642. [Google Scholar] [CrossRef]
- Pinstrup-Andersen, P.; Pandya-Lorch, R. Food Security and Sustainable Use of Natural Resources: A 2020 Vision. Ecol. Econ. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Grosse-Heilmann, M.; Cristiano, E.; Deidda, R.; Viola, F. Durum Wheat Productivity Today and Tomorrow: A Review of Influencing Factors and Climate Change Effects. Res. Environ. 2024, 17, 100170. [Google Scholar] [CrossRef]
- Anderson, T.R.; Slotkin, T.A. Maturation of the Adrenal Medulla—IV. Effects of Morphine. Biochem. Pharmacol. 1975, 24, 1469–1474. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil. Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Chab, L.; Biagini, L.; Severini, S. Towards an Effective Risk Management in Durum Wheat Production: A Systematic Review and Bibliometric Analysis of Factors Influencing Quality and Yield. Agriculture 2024, 14, 2266. [Google Scholar] [CrossRef]
- Yen, C.; Yang, J. Biosystematics of Triticeae: Volume I. Triticum-Aegilops Complex; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Taylor, R.D.; Koo, W.W. 2015 Outlook of the U.S. and World Wheat Industries, 2015–2024. Agribus. Appl. Econ. 2015, 738, 201310. [Google Scholar] [CrossRef]
- Mcfadden, E.S.; Sears, E.R. The origin of triticum spelta and its free-threshing hexaploid relatives. J. Hered. 1946, 37, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, F.; Ammar, K.; Solís, I. Global Changes in Cultivated Area and Breeding Activities of Durum Wheat from 1800 to Date: A Historical Review. Agronomy 2022, 12, 1135. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum Wheat Breeding in the Mediterranean Region: Current Status and Future Prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Graziano, S.; Marando, S.; Prandi, B.; Boukid, F.; Marmiroli, N.; Francia, E.; Pecchioni, N.; Sforza, S.; Visioli, G.; Gullì, M. Technological Quality and Nutritional Value of Two Durum Wheat Varieties Depend on Both Genetic and Environmental Factors. J. Agric. Food Chem. 2019, 67, 2384–2395. [Google Scholar] [CrossRef]
- Tidiane Sall, A.; Chiari, T.; Legesse, W.; Seid-Ahmed, K.; Ortiz, R.; Van Ginkel, M.; Bassi, F.M. Durum Wheat (Triticum durum Desf.): Origin, Cultivation and Potential Expansion in Sub-Saharan Africa. Agronomy 2019, 9, 263. [Google Scholar] [CrossRef]
- Urbanavičiūtė, I.; Bonfiglioli, L.; Pagnotta, M.A. Diversity in Root Architecture of Durum Wheat at Stem Elongation under Drought Stress. Agronomy 2022, 12, 1329. [Google Scholar] [CrossRef]
- Rezzouk, F.Z.; Gracia-Romero, A.; Kefauver, S.C.; Nieto-Taladriz, M.T.; Serret, M.D.; Araus, J.L. Durum Wheat Ideotypes in Mediterranean Environments Differing in Water and Temperature Conditions. Agric. Water Manag. 2022, 259, 107257. [Google Scholar] [CrossRef]
- Taghouti, M.; Bassi, F.M.; Nasrellah, N.; Amri, A.; Motawaj, J.; Nachit, M. ‘Nachit’, a Wild-relative-derived Durum Wheat Resilient to Climate Change in Morocco. J. Plant Regist. 2023, 17, 529–535. [Google Scholar] [CrossRef]
- Manhou, K.; Moussadek, R.; Yachou, H.; Zouahri, A.; Douaik, A.; Hilal, I.; Ghanimi, A.; Hmouni, D.; Dakak, H. Assessing the Impact of Saline Irrigation Water on Durum Wheat (cv. Faraj) Grown on Sandy and Clay Soils. Agronomy 2024, 14, 2865. [Google Scholar] [CrossRef]
- Hannachi, S.; Steppe, K.; Eloudi, M.; Mechi, L.; Bahrini, I.; Van Labeke, M.-C. Salt Stress Induced Changes in Photosynthesis and Metabolic Profiles of One Tolerant (‘Bonica’) and One Sensitive (‘Black Beauty’) Eggplant Cultivars (Solanum melongena L.). Plants 2022, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Rabti, A.; Mekaoussi, R.; Fellahi, Z.E.A.; Hannachi, A.; Benbelkacem, A.; Benmahammed, A.; Bouzerzour, H. Characterization of Old and Recent Durum Wheat [Triticum turgidum (L.) Tell. Convar. durum (Desf.) Mackey] Varieties Assessed under South Mediterranean Conditions. Egypt. J. Agron. 2020, 42, 307–320. [Google Scholar] [CrossRef]
- Fellahi, Z.E.A.; Hannachi, A.; Bouzerzour, H. Analysis of Direct and Indirect Selection and Indices in Bread Wheat (Triticum aestivum L.) Segregating Progeny. Int. J. Agron. 2018, 2018, 8312857. [Google Scholar] [CrossRef]
- Casagrande, C.R.; Mezzomo, H.C.; Silva, C.M.; Lima, G.W.; Souza, D.J.P.; Borém, A.; Nardino, M. Selection Indexes Based on Genotypic Values Applied to Brazilian Tropical Wheat Breeding. Agron. Sci. Biotechnol. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Galal, A.A.; Safhi, F.A.; El-Hity, M.A.; Kamara, M.M.; Gamal El-Din, E.M.; Rehan, M.; Farid, M.; Behiry, S.I.; El-Soda, M.; Mansour, E. Molecular Genetic Diversity of Local and Exotic Durum Wheat Genotypes and Their Combining Ability for Agronomic Traits under Water Deficit and Well-Watered Conditions. Life 2023, 13, 2293. [Google Scholar] [CrossRef]
- Kroupin, P.Y.; Bespalova, L.A.; Kroupina, A.Y.; Yanovsky, A.S.; Korobkova, V.A.; Ulyanov, D.S.; Karlov, G.I.; Divashuk, M.G. Association of High-Molecular-Weight Glutenin Subunits with Grain and Pasta Quality in Spring Durum Wheat (Triticum turgidum spp. durum L.). Agronomy 2023, 13, 1510. [Google Scholar] [CrossRef]
- Rham, I.; Bouchiha, F.; Kharbouche, H.A.; El Maataoui, S.; Radi, H.; Choukrane, B.; Karama, M.; Ouhboun, R.; Hmouni, D.; Mazri, M.A. Effects of medium components on cactus pear (Opuntia ficus-indica (L.) Mill. genotype ‘M2’) organogenesis, and assessment of transplanting techniques and vitroplant fruit quality. Plant Cell Tissue Organ Cult. 2025, 160. [Google Scholar] [CrossRef]
- ICARDA (International Center for Agricultural Research in the Dry Areas). Morocco and ICARDA: Collaboration Since 1977; ICARDA: Aleppo, Syria, 2005. [Google Scholar]
- International Center for Agricultural Research in the Dry Areas (ICARDA). ICARDA Annual Report; ICARDA: Aleppo, Syria, 1989. [Google Scholar]
- International Center for Agricultural Research in the Dry Areas (ICARDA). Landraces in Crop Breeding; ICARDA: Aleppo, Syria, 1996. [Google Scholar]
- Taghouti, M.; Nsarellah, N.; Gaboun, F.; Rochdi, A. Multi-Environment Assessment of the Impact of Genetic Improvement on Agronomic Performance and on Grain Quality Traits in Moroccan Durum Wheat Varieties of 1949 to 2017. Glob. J. Plant Breed. Genet. 2017, 4, 394–404. [Google Scholar]
- Nsarellah, N.; Amamou, A.; Mona, T.; Annicchiarico, P. Adaptation of Moroccan Durum Wheat Varieties from Different Breeding Eras. J. Plant Breed. Crop Sci. 2011, 3, 34–40. [Google Scholar]
- International Board for Plant Genetic Resources (IBPGR). Descriptors for Wheat (Revised); International Board for Plant Genetic Resources: Wageningen, The Netherlands, 1985; p. 12. [Google Scholar]
- Johnson, M.; Kumar, A.; Oladzad-Abbasabadi, A.; Salsman, E.; Aoun, M.; Manthey, F.A.; Elias, E.M. Association Mapping for 24 Traits Related to Protein Content, Gluten Strength, Color, Cooking, and Milling Quality Using Balanced and Unbalanced Data in Durum Wheat [Triticum turgidum L. Var. durum (Desf).]. Front. Genet. 2019, 10, 717. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International; Benjamin Franklin Station: Washington, DC, USA, 2016. [Google Scholar]
- ISO 712:2009; International Organization for Standardization Cereals and Cereal Products—Determination of Moisture Content (Reference Method). ISO: Geneva, Switzerland, 2009.
- ISO 21415-2:2015; International Organization for Standardization Cereals and Cereal Products—Determination of the Gluten Content—Part 2: Spectrometric Method. ISO: Geneva, Switzerland, 2015.
- American Association of Cereal Chemists International (AACC). Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting Genetic Diversity from Landraces in Wheat Breeding for Adaptation to Climate Change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef]
- Santra, M.; Rao, V.S.; Tamhankar, S.A. Modification of AACC Procedure for Measuring β-Carotene in Early Generation Durum Wheat. Cereal Chem. 2003, 80, 130–131. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, J. Applying statistical methods to library data analysis. Ser. Libr. 2019, 76, 195–200. [Google Scholar] [CrossRef]
- Tonk, F.A.; Ilker, E.; Tosun, M. Evaluation of genotype x environment interactions in maize hybrids using GGE biplot analysis. Crop Breed. Appl. Biotechnol. 2011, 11, 1–9. [Google Scholar] [CrossRef]
- Pantha, S.; Kilian, B.; Özkan, H.; Zeibig, F.; Frei, M. Physiological and Biochemical Changes Induced by Drought Stress during the Stem Elongation and Anthesis Stages in the Triticum Genus. Environ. Exp. Bot. 2024, 228, 106047. [Google Scholar] [CrossRef]
- Lazaridi, E.; Kapazoglou, A.; Gerakari, M.; Kleftogianni, K.; Passa, K.; Sarri, E.; Papasotiropoulos, V.; Tani, E.; Bebeli, P.J. Crop Landraces and Indigenous Varieties: A Valuable Source of Genes for Plant Breeding. Plants 2024, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lai, X.; Ren, Y.; Yang, H.; Wang, H.; Wang, C.; Xia, J.; Wang, Z.; Yang, Z.; Geng, H.; et al. Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat. Agronomy 2023, 13, 2968. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Carranza-Gallego, G.; Guzmán, G.I.; Garcia-Ruíz, R.; González De Molina, M.; Aguilera, E. Addressing the Role of Landraces in the Sustainability of Mediterranean Agroecosystems. Sustainability 2019, 11, 6029. [Google Scholar] [CrossRef]
- Dagnaw, T.; Mulugeta, B.; Haileselassie, T.; Geleta, M.; Ortiz, R.; Tesfaye, K. Genetic Diversity of Durum Wheat (Triticum turgidum L. ssp. durum, Desf.) Germplasm as Revealed by Morphological and SSR Markers. Genes 2023, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, A.; Corinzia, S.A.; Caruso, P.; Cosentino, S.L.; Testa, G. Productivity of Wheat Landraces in Rainfed and Irrigated Conditions under Conventional and Organic Input in a Semiarid Mediterranean Environment. Agronomy 2024, 14, 2338. [Google Scholar] [CrossRef]
- Corinzia, S.A.; Caruso, P.; Scandurra, A.; Anastasi, U.; Cosentino, S.L.; Testa, G. Yield Response and Leaf Gas Exchange of Sicilian Wheat Landraces. Agronomy 2024, 14, 1038. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, B.; He, Y. Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes. Biology 2022, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Ren, X.; Wei, S.; Zhu, Z.; Zhang, F.; Hu, S.; Ding, Y.; Sun, F.; Han, D.; et al. Mapping and validation of QTkw.cau-3DL, a major QTL controlling thousand-kernel weight in wheat. Theor. Appl. Genet. 2025, 138, 46. [Google Scholar] [CrossRef]
- Frantová, N.; Rábek, M.; Elzner, P.; Středa, T.; Jovanović, I.; Holková, L.; Martinek, P.; Smutná, P.; Prášil, I.T. Different Drought Tolerance Strategy of Wheat Varieties in Spike Architecture. Agronomy 2022, 12, 2328. [Google Scholar] [CrossRef]
- Khalid, A.; Hameed, A.; Tahir, M.F. Wheat Quality: A Review on Chemical Composition, Nutritional Attributes, Grain Anatomy, Types, Classification, and Function of Seed Storage Proteins in Bread Making Quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef]
- Blanco, A.; Mangini, G.; Giancaspro, A.; Giove, S.; Colasuonno, P.; Simeone, R.; Signorile, A.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L.; et al. Relationships between Grain Protein Content and Grain Yield Components through Quantitative Trait Locus Analyses in a Recombinant Inbred Line Population Derived from Two Elite Durum Wheat Cultivars. Mol. Breed. 2012, 30, 79–92. [Google Scholar] [CrossRef]
- Mercier, S.; Moresoli, C.; Mondor, M.; Villeneuve, S.; Marcos, B. A Meta-Analysis of Enriched Pasta: What Are the Effects of Enrichment and Process Specifications on the Quality Attributes of Pasta? Compr. Rev. Food Sci. Food Saf. 2016, 15, 685–704. [Google Scholar] [CrossRef]
- Dexter, J.E.; Matsuo, R.R. Relationship between Durum Wheat Protein Properties and Pasta Dough Rheology and Spaghetti Cooking Quality. J. Agric. Food Chem. 1980, 28, 899–902. [Google Scholar] [CrossRef]
- Beltrão Martins, R.; Nunes, M.C.; Ferreira, L.M.M.; Peres, J.A.; Barros, A.I.R.N.A.; Raymundo, A. Impact of Acorn Flour on Gluten-Free Dough Rheology Properties. Foods 2020, 9, 560. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat Gluten Functionality as a Quality Determinant in Cereal-Based Food Products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Mamat, H.; Hill, S.E. Effect of Fat Types on the Structural and Textural Properties of Dough and Semi-Sweet Biscuit. J. Food Sci. Technol. 2014, 51, 1998–2005. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Mastrangelo, A.M.; Trono, D.; Borrelli, G.M.; De Vita, P.; Fares, C.; Beleggia, R.; Platani, C.; Papa, R. The Colours of Durum Wheat: A Review. Crop Pasture Sci. 2014, 65, 1. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage (CIE). Colorimetry, 2nd ed.; Commission Internationale de l’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- Sharma, C.; Kamle, M.; Kumar, P. Microbial-Derived Carotenoids and Their Health Benefits. Microbiol. Res. 2024, 15, 1670–1689. [Google Scholar] [CrossRef]
- Nazco, R.; Villegas, D.; Ammar, K.; Peña, R.J.; Moragues, M.; Royo, C. Can Mediterranean Durum Wheat Landraces Contribute to Improved Grain Quality Attributes in Modern Cultivars? Euphytica 2012, 185, 1–17. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of Drought Tolerance of Some Wheat (Triticum aestivum L.) Genotypes through Phenology, Growth, and Physiological Indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Zhang, Z.; Pan, H.; Lan, L.; Huang, R.; Deng, X.; Peng, Y. Effects of Exponential N Application on Soil Exchangeable Base Cations and the Growth and Nutrient Contents of Clonal Chinese Fir Seedlings. Plants 2023, 12, 851. [Google Scholar] [CrossRef]
- Aktaş, H. drought tolerance indices of selected landraces and bread wheat (Triticum aestivum L.) genotypes derived from synthetic wheats. Appl. Ecol. Env. Res. 2016, 14, 177–189. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z.; et al. Wheat Adaptation to Environmental Stresses under Climate Change: Molecular Basis and Genetic Improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef] [PubMed]
- Robles-Zazueta, C.A.; Crespo-Herrera, L.A.; Piñera-Chavez, F.J.; Rivera-Amado, C.; Aradottir, G.I. Climate Change Impacts on Crop Breeding: Targeting Interacting Biotic and Abiotic Stresses for Wheat Improvement. Plant Genome 2024, 17, e20365. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Kaur, S.; Katyal, M.; Virdi, A.S.; Kaur, D.; Ahlawat, A.K.; Singh, A.M. Relationship of Various Flour Properties with Noodle Making Characteristics among Durum Wheat Varieties. Food Chem. 2015, 188, 517–526. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; Machet, J.-M.; Jeuffroy, M.-H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the Grain Protein Concentration-Grain Yield Negative Relationship Is Highly Correlated to Post-Anthesis N Uptake in Winter Wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mantovani, E.E.; Seetan, R.; Soltani, A.; Echeverry-Solarte, M.; Jain, S.; Simsek, S.; Doehlert, D.; Alamri, M.S.; Elias, E.M.; et al. Dissection of Genetic Factors Underlying Wheat Kernel Shape and Size in an Elite × Nonadapted Cross Using a High-Density SNP Linkage Map. Plant Genome 2016, 9, eplantgenome2015.09.0081. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Valizadeh, M.; Shahbazy, H.; Zaefizadeh, M.; Habibpor, M. Morphological Diversity and Interrelationships Traits in Durum Wheat Landraces under Normal Irrigation and Drought Stress Conditions. Adv. Environ. Biol. 2011, 5, 1934–1940. [Google Scholar]
- (PDF) Dissecting Genotype by Environment Interactions in Moroccan Wheat: An Advanced Biplot and Heatmap Analysis Unveiling Agronomic, Quality Traits, and Genotypic Stability for Tailored Breeding Strategies. Available online: https://www.researchgate.net/publication/379722871_Dissecting_Genotype_by_Environment_Interactions_in_Moroccan_Wheat_An_Advanced_Biplot_and_Heatmap_Analysis_Unveiling_Agronomic_Quality_Traits_and_Genotypic_Stability_for_Tailored_Breeding_Strategies (accessed on 19 March 2025).
- Broccanello, C.; Bellin, D.; DalCorso, G.; Furini, A.; Taranto, F. Genetic Approaches to Exploit Landraces for Improvement of Triticum turgidum ssp. durum in the Age of Climate Change. Front. Plant Sci. 2023, 14, 1101271. [Google Scholar] [CrossRef]
- Sourour, A.; Chahine, K.; Youssef, T.; Olfa, S.; Hajer, S. Phenotypic Diversity of Tunisian Durum Wheat Landraces. Afr. Crop Sci. J. 2010, 18, 54197. [Google Scholar] [CrossRef]
- Panghal, A.; Chhikara, N.; Khatkar, B.S. Characterisation of Indian Wheat Varieties for Chapatti (Flat Bread) Quality. J. Saudi Soc. Agric. Sci. 2019, 18, 107–111. [Google Scholar] [CrossRef]
- Sanad, H.; Oueld Lhaj, M.; Zouahri, A.; Saafadi, L.; Dakak, H. Groundwater Pollution by Nitrate and Salinization in Morocco: A Comprehensive Review. J. Water Health 2024, 22, 1756–1773. [Google Scholar] [CrossRef]
- Raun, W.; Johnson, G. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Hailu, F.; Merker, A.; Singh, H.; Belay, G.; Johansson, E. Multivariate Analysis of Diversity of Tetraploid Wheat Germplasm from Ethiopia. Genet. Resour. Crop Evol. 2006, 53, 1089–1098. [Google Scholar] [CrossRef]
- Oueld Lhaj, M.; Moussadek, R.; Mouhir, L.; Mdarhri Alaoui, M.; Sanad, H.; Iben Halima, O.; Zouahri, A. Assessing the Evolution of Stability and Maturity in Co-Composting Sheep Manure with Green Waste Using Physico-Chemical and Biological Properties and Statistical Analyses: A Case Study of Botanique Garden in Rabat, Morocco. Agronomy 2024, 14, 1573. [Google Scholar] [CrossRef]
- Soriano, J.M.; Villegas, D.; Sorrells, M.E.; Royo, C. Durum Wheat Landraces from East and West Regions of the Mediterranean Basin Are Genetically Distinct for Yield Components and Phenology. Front. Plant Sci. 2018, 9, 80. [Google Scholar] [CrossRef]
- López-Bellido, L.; López-Bellido, R.J.; Redondo, R. Nitrogen Efficiency in Wheat under Rainfed Mediterranean Conditions as Affected by Split Nitrogen Application. Field Crops Res. 2005, 94, 86–97. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of Landraces in Cereal Breeding for Stress Tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef]
- Nazco, R.; Peña, R.J.; Ammar, K.; Villegas, D.; Crossa, J.; Moragues, M.; Royo, C. Variability in Glutenin Subunit Composition of Mediterranean Durum Wheat Germplasm and Its Relationship with Gluten strength. J. Agric. Sci. 2014, 152, 379–393. [Google Scholar] [CrossRef]
- Eltaher, S.; Sallam, A.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Börner, A.; Baenziger, P.S.; Mourad, A.M.I. Genome-Wide Association Mapping Revealed SNP Alleles Associated with Spike Traits in Wheat. Agronomy 2022, 12, 1469. [Google Scholar] [CrossRef]
- Alemu, Y.A.; Anley, A.M.; Abebe, T.D. Genetic Variability and Association of Traits in Ethiopian Durum Wheat (Triticum turgidium L. Var. durum) Landraces at Dabat Research Station, North Gondar. Cogent Food Agric. 2020, 6, 1778604. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Bustos, D.V.; Hasan, A.K.; Reynolds, M.P.; Calderini, D.F. Combining High Grain Number and Weight through a DH-Population to Improve Grain Yield Potential of Wheat in High-Yielding Environments. Field Crops Res. 2013, 145, 106–115. [Google Scholar] [CrossRef]
- Oueld Lhaj, M.; Moussadek, R.; Zouahri, A.; Sanad, H.; Saafadi, L.; Mdarhri Alaoui, M.; Mouhir, L. Sustainable Agriculture Through Agricultural Waste Management: A Comprehensive Review of Composting’s Impact on Soil Health in Moroccan Agricultural Ecosystems. Agriculture 2024, 14, 2356. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sharifi, P.; Karimizadeh, R.; Shefazadeh, M.K. Relationships between Grain Yield and Yield Components in Bread Wheat under Different Water Availability (Dryland and Supplemental Irrigation Conditions). Not. Bot. Hort. Agrobot. Cluj-Napoca 2012, 40, 195. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative Study on Gluten Protein Composition of Ancient (Einkorn, Emmer and Spelt) and Modern Wheat Species (Durum and Common Wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Sliwinski, E.L.; Kolster, P.; Prins, A.; Vliet, T.v. On the Relationship between Gluten Protein Composition of Wheat Flours and Large-Deformation Properties of Their Doughs. J. Cereal Sci. 2004, 39, 247–264. [Google Scholar] [CrossRef]
- Marchetti, L.; Cardós, M.; Campaña, L.; Ferrero, C. Effect of Glutens of Different Quality on Dough Characteristics and Breadmaking Performance. LWT—Food Sci. Technol. 2012, 46, 224–231. [Google Scholar] [CrossRef]
- Carrillo, J.; Vazquez, J.; Orkellana, J. Relationship Between Gluten Strength and Glutenin Proteins in Durum Wheat Cultivars. Plant Breed. 2006, 104, 325–333. [Google Scholar] [CrossRef]
- Ayed, S.; Bouhaouel, I.; Othmani, A. Genome-Wide Association Studies of Agronomic and Quality Traits in Durum Wheat. Agronomy 2019, 9, 440. [Google Scholar] [CrossRef]
- Ayed, S.; Bouhaouel, I.; Othmani, A. Screening of Durum Wheat Cultivars for Selenium Response under Contrasting Environments, Based on Grain Yield and Quality Attributes. Agriculture 2023, 14, 1743. [Google Scholar] [CrossRef]




| Parameter | Df | Sum Sq | Mean Sq | p-Value | Mean ± SE | Mean | Minimum | Maximum | CV (%) | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| AWL (cm) | 4 | 5.58 | 9.93 | <0.01 | 18.90 ab ± 0.56 | 17.22 | 11.34 | 23.10 | 6.60 | 1.16 |
| SL (cm) | 4 | 6.10 | 1.55 | <0.01 | 6.89 ab ± 0.22 | 8.45 | 4.85 | 12.05 | 12.10 | 3.57 |
| SPS | 4 | 1.92 | 0.48 | >0.05 | 18.55 a ± 0.21 | 18.88 | 12.05 | 25.70 | 6.60 | 2.18 |
| TKW (g) | 4 | 559.12 | 136.56 | <0.001 | 49.39 abcd ± 0.35 | 27.18 | 23.70 | 30.65 | 12.00 | 2.77 |
| GNS | 4 | 379.35 | 97.13 | >0.05 | 52.25 ± 0.07 | 24.64 | 19.02 | 30.25 | 4.80 | 1.17 |
| GY (t haࢤ1) | 4 | 13.67 | 3.58 | <0.001 | 5.81 abcde ± 0.11 | 5.37 | 1.38 | 9.35 | 19.70 | 2.21 |
| GC (%) | 4 | 64.92 | 16.23 | <0.01 | 15.90 abc ± 0.44 | 37.08 | 2.05 | 48.10 | 10.40 | 1.76 |
| PC (%) | 4 | 9.56 | 2.37 | <0.01 | 35.97 abc ± 0.35 | 15.53 | 12.10 | 18.95 | 7.40 | 0.93 |
| W | 4 | 12,686.25 | 3174.06 | >0.05 | 309.32 a ± 0.04 | 297.70 | 184.20 | 411.20 | 13.20 | 2.17 |
| SDS (mL) | 4 | 21.22 | 5.59 | <0.05 | 8.51 ab ± 0.26 | 8.55 | 4.55 | 12.55 | 6.80 | 1.16 |
| YPI (b) | 4 | 5.37 | 1.39 | <0.01 | 13.97 ab ± 0.33 | 13.93 | 11.06 | 16.80 | 8.50 | 0.38 |
| AWL | SL | SPS | GNS | TKW | GY | PC | GC | W | SDS | YPI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AWL | 1 | ||||||||||
| SL | 0.379 * | 1 | |||||||||
| SPS | 0.414 ** | 0.950 *** | 1 | ||||||||
| GNS | 0.259 * | 0.166 | 0.161 | 1 | |||||||
| TKW | 0.189 | 0.195 | 0.287 | −0.498 ** | 1 | ||||||
| GY | 0.526 ** | 0.769 *** | 0.646 *** | −0.791 *** | 0.530 ** | 1 | |||||
| PC | −0.020 | 0.030 | 0.080 | 0.010 | 0.420 ** | 0.020 | 1 | ||||
| GC | 0.030 | 0.020 | 0.060 | 0.070 | −0.010 | −0.020 | 0.863 *** | 1 | |||
| W | −0.100 | −0.110 | −0.050 | −0.040 | 0.050 | 0.020 | 0.438 ** | 0.410 ** | 1 | ||
| SDS | −0.060 | −0.050 | −0.050 | −0.050 | −0.010 | 0.010 | 0.125 | 0.143 | −0.112 | 1 | |
| YPI | −0.060 | −0.060 | −0.050 | −0.050 | −0.010 | 0.430 ** | 0.143 | 0.825 *** | −0.079 | 0.831 *** | 1 |
| Month | Precipitation (mm) | Air Temperature [(°C)] | Growth Stage | BCCH | ||
|---|---|---|---|---|---|---|
| Mean | Min | Max | ||||
| November 2017 | 41.20 | 11.75 | 3.00 | 20.50 | Sowing | 00 |
| December 2017 | 42.20 | 12.35 | 3.30 | 24.90 | Tillering | 21 |
| January 2018 | 46.80 | 11.98 | −0.10 | 24.50 | Tillering | 21 |
| February 2018 | 62.80 | 11.58 | 0.60 | 24.40 | 1st node 1 | 31 |
| March 2018 | 52.60 | 14.75 | 2.80 | 26.30 | 2nd node 2 | 32 |
| April 2018 | 19.60 | 16.71 | 5.40 | 36.50 | Full Flowering | 65 |
| May 2018 | 8.40 | 18.05 | 5.50 | 29.40 | Maturity | 99 |
| June 2018 | 0.00 | 21.04 | 10.00 | 31.10 | Maturity | 99 |
| Code | Wheat Variety | The Official Year of Inscription | Origin/Breeder | Adaptability | Yield Potential (t ha−1) | Quality Traits |
|---|---|---|---|---|---|---|
| G121 | Anouar | 1993 | Crossbreeding and selection of Moroccan varieties on introduced material | Large adaptation, irrigated | 7.5 | PC: 13.5%; Mt: Moderately Resistant BK: Good; SQ: Good |
| G122 | Jawhar | 1993 | Selection through hybridization with introduced material | Large adaptation, irrigated | 7.5 | PC: 13.1%; Mt: Moderately Resistant BK: Good; SQ: Good |
| G123 | Amjad | 1995 | Moroccan crossbreeding with introduced material | Large adaptation, irrigated | 7.5 | PC: 13.0%; MT: Moderately Resistant BK: Good; SQ: Good |
| G124 | Tarek | 1995 | Moroccan crossbreeding with introduced material | Large adaptation | 7.5 | PC: 13.1%; MT: Moderately Resistant BK: Good; SQ: Good |
| G125 | Tomouh | 1997 | Moroccan selection on introduced material (Oum Rabia 6 from ICARDA) | Arid areas and plateaus | 6.0 | PC: 14.0%; MT: Moderately Resistant BK: Good; SQ: Good |
| G126 | Karim | 1985 | Moroccan crossbreeding with CIMMYT material | Large adaptation except in high altitude | 7.5 | PC: 13.2%; MT: Moderately Resistant BK: Average to Good; SQ: Good |
| G127 | Marzak | 1984 | Moroccan crossbreeding with CIMMYT material | Large adaptation except in high altitude | 7.5 | PC: 13.2%; MT: Moderately Resistant BK: Average to Good; SQ: Average |
| G128 | Amria | 2003 | Moroccan crossbreeding and selection | Semi-arid areas | 5.0 | PC: 13.4%; MT: Moderately Resistant BK: Good; SQ: Good |
| G129 | Chaoui | 2003 | Moroccan crossbreeding and selection | Semi-arid areas | 6.0 | PC: 13.5%; MT: Moderately Resistant BK: Good; SQ: Good |
| G130 | IRDEN | 2003 | Moroccan crossbreeding and selection | Semi-arid areas | 6.0 | PC: 13.5%; MT: Moderately Resistant; BK: Good; SQ: Good |
| G131 | Nassira | 2003 | INRA Morocco | Semi-arid areas | 4.5 | PC: 15.0%; BK: Good; SQ: Good |
| G144 | Prospero | 2007 | Florimond Desprez | Favorable areas Semi-arid areas | high | BK: High |
| G145 | Boniduro | 2012 | Semillas Battle | Favorable areas Semi-arid areas | High | PC: High; Vit.: Superior; GQ: High; YPI: Medium-High |
| G146 | Marjana | 1996 | INRA selection from CGIAR material | Large adaptation | 4.8 | PC: 13.2%; MT: Moderately Resistant BK: Good; SQ: Good |
| G147 | Carioca | 2005 | Serasem-France | Irrigated areas | high | BK: Good |
| G132 | Marouane | 2003 | Moroccan crossbreeding and selection | Semi-arid areas | 6.0 | PC: 13.5%; MT: Moderately Resistant BK: Good; SQ: Good |
| G132 | Nassira | 2003 | INRA Morocco | Semi-arid areas | 4.5 | PC: 15.0%; BK: Good; SQ: Good |
| G133 | Yasmine | 1993 | Moroccan crossbreeding on introduced material | Large adaptation | 7.5 | PC: 13.2%; MT: Moderately Resistant BK: Good; SQ: Good |
| G134 | Ourgh | 1995 | Moroccan crossbreeding on introduced material | Large adaptation | 7.5 | PC: 13.5%; MT: Moderately Resistant BK: Good; SQ: Good |
| G135 | Louiza | 2011 | INRA Morocco | Large adaptation | 6.0 | PC: 14.8%; BK: Good; SQ: Good |
| G136 | Oued Znati | 1949 | Selection from a former Moroccan local population | North and mountainous areas | 4.0 | PC: 14.7%; MT: Moderately Resistant BK: Low; SQ: Medium |
| G137 | Kyperounda | 1956 | Selection from a former local Moroccan population | Favorable plains, northern regions, and mountainous areas | 4.0 | PC: 14.0%; Mt: Moderately Resistant BK: Good; SQ: Good |
| G138 | Nachit | 2018 | INRA Morocco | Favorable areas Semi-arid areas | 7.1 | PC: 15.0%; BK: Good; SQ: Good |
| G139 | BD 15-13 | Seeds of Provence | - | - | - | - |
| G140 | Jabal | Delivered in 2021 | JLIBEN CONSULTING | Semi-arid areas | 3.0 | PC: 13%; BK: Good; SQ: Good |
| G141 | Faraj | 2007 | INRA Morocco | Favorable areas Semi-arid areas | 6.8 | PC: 15.3%; BK: Good; SQ: Good |
| G142 | Itri | 2016 | INRA Morocco | Favorable areas Semi-arid areas | 7.0 | PC: 14.1%; BK: Good; SQ: Good |
| G143 | Kanakis | 2009 | Florimond Desprez | Favorable areas Semi-arid areas | High | BK: High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manhou, K.; Taghouti, M.; Moussadek, R.; Elyacoubi, H.; Bennani, S.; Zouahri, A.; Ghanimi, A.; Sanad, H.; Oueld Lhaj, M.; Hmouni, D.; et al. Performance, Agro-Morphological, and Quality Traits of Durum Wheat (Triticum turgidum L. ssp. durum Desf.) Germplasm: A Case Study in Jemâa Shaïm, Morocco. Plants 2025, 14, 1508. https://doi.org/10.3390/plants14101508
Manhou K, Taghouti M, Moussadek R, Elyacoubi H, Bennani S, Zouahri A, Ghanimi A, Sanad H, Oueld Lhaj M, Hmouni D, et al. Performance, Agro-Morphological, and Quality Traits of Durum Wheat (Triticum turgidum L. ssp. durum Desf.) Germplasm: A Case Study in Jemâa Shaïm, Morocco. Plants. 2025; 14(10):1508. https://doi.org/10.3390/plants14101508
Chicago/Turabian StyleManhou, Khadija, Mona Taghouti, Rachid Moussadek, Houda Elyacoubi, Sahar Bennani, Abdelmjid Zouahri, Ahmed Ghanimi, Hatim Sanad, Majda Oueld Lhaj, Driss Hmouni, and et al. 2025. "Performance, Agro-Morphological, and Quality Traits of Durum Wheat (Triticum turgidum L. ssp. durum Desf.) Germplasm: A Case Study in Jemâa Shaïm, Morocco" Plants 14, no. 10: 1508. https://doi.org/10.3390/plants14101508
APA StyleManhou, K., Taghouti, M., Moussadek, R., Elyacoubi, H., Bennani, S., Zouahri, A., Ghanimi, A., Sanad, H., Oueld Lhaj, M., Hmouni, D., & Dakak, H. (2025). Performance, Agro-Morphological, and Quality Traits of Durum Wheat (Triticum turgidum L. ssp. durum Desf.) Germplasm: A Case Study in Jemâa Shaïm, Morocco. Plants, 14(10), 1508. https://doi.org/10.3390/plants14101508








