Identification of a Specific Role of Dihydrozeatin in the Regulation of the Cell Differentiation Activity in Arabidopsis Roots
Abstract
1. Introduction
2. Results
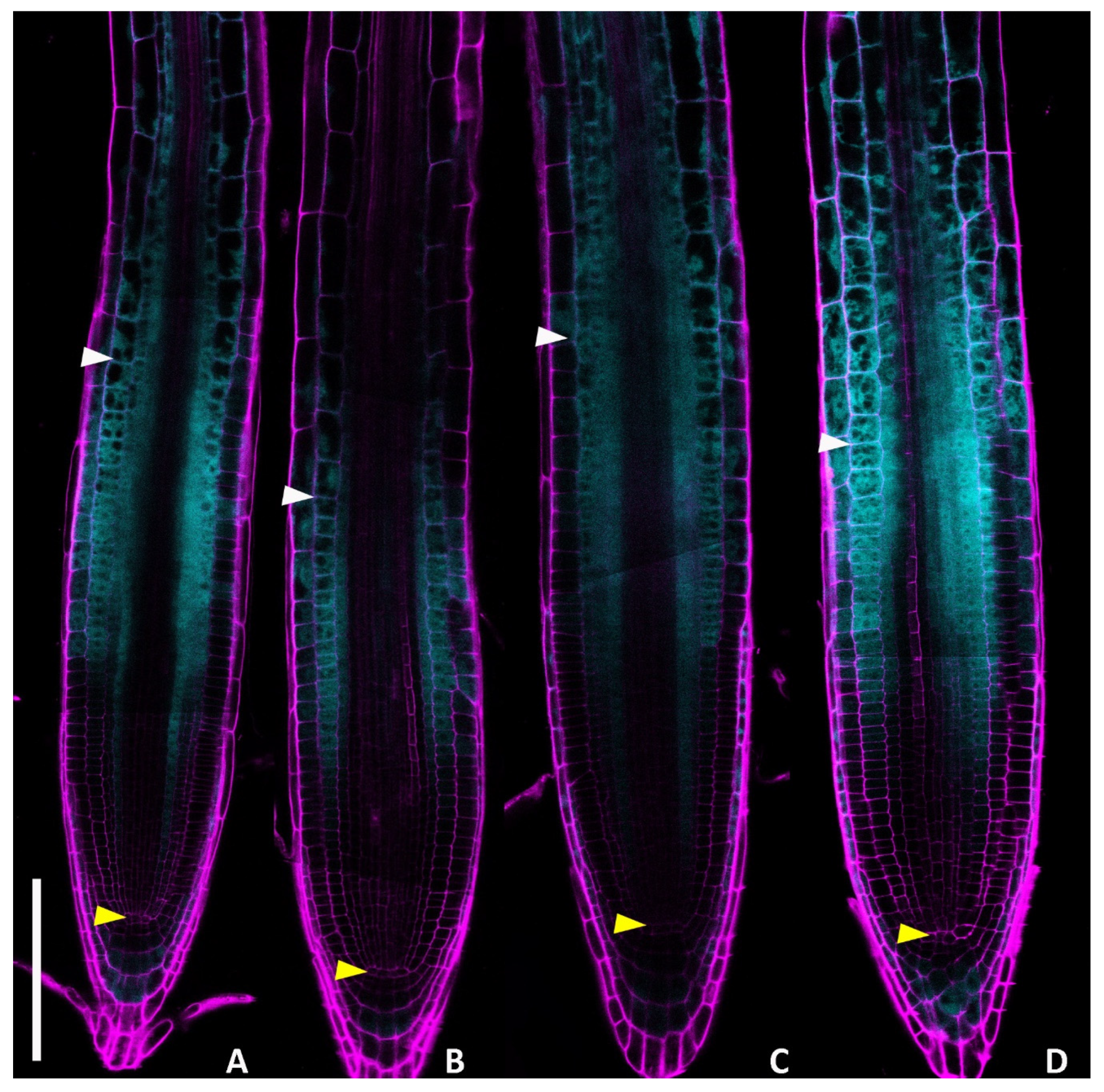
2.1. DHZ Promotes Cell Differentiation in Arabidopsis Root
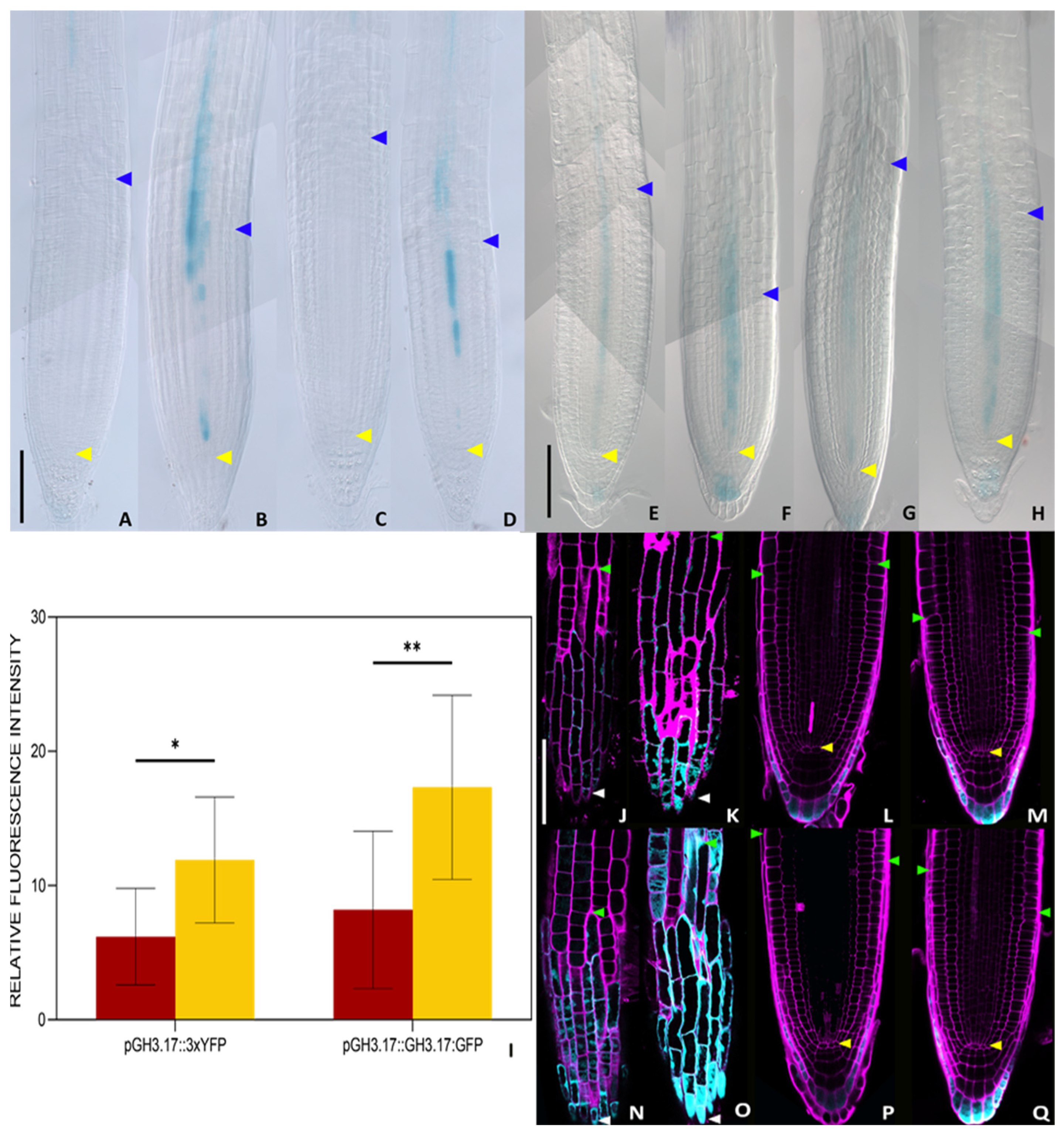
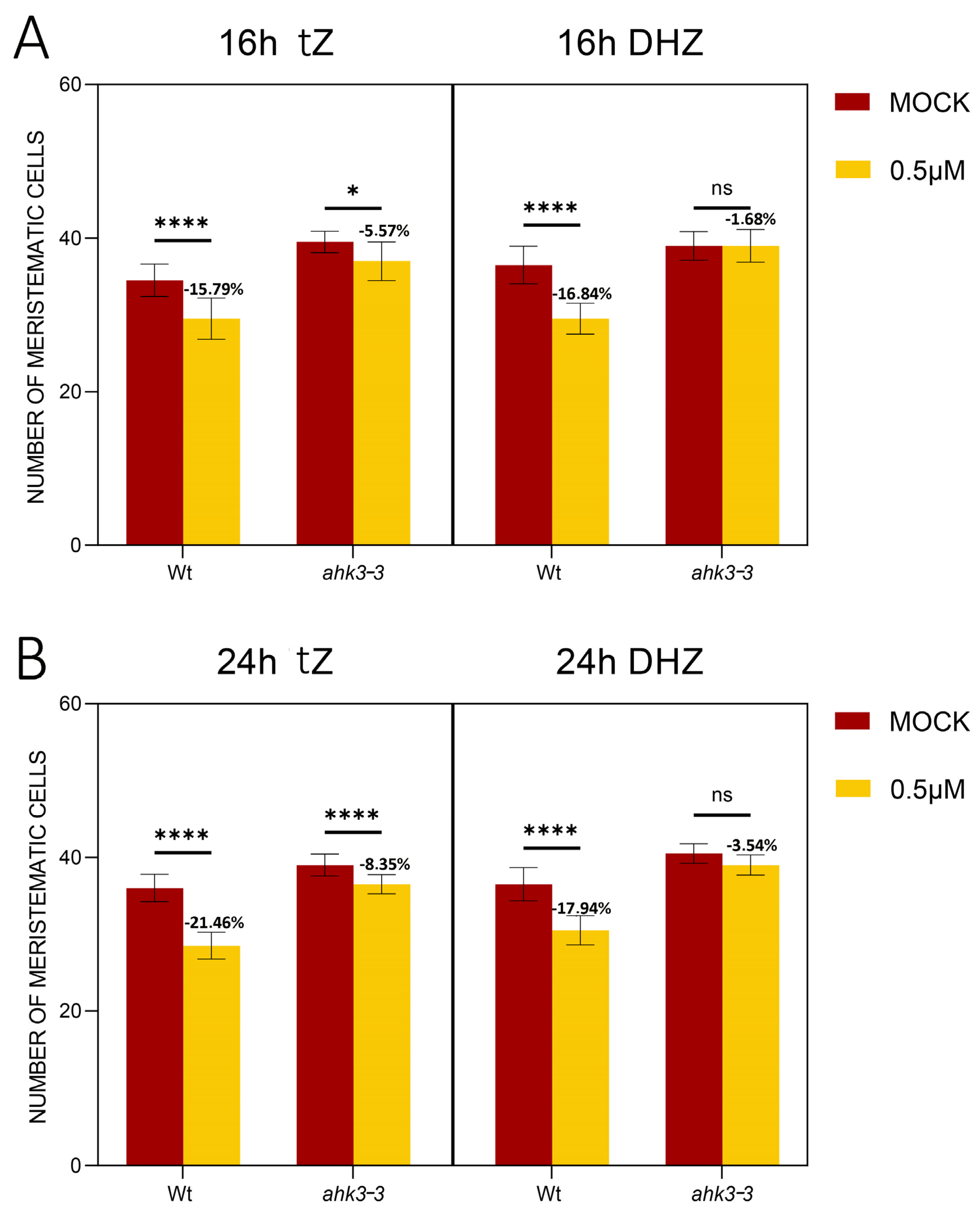
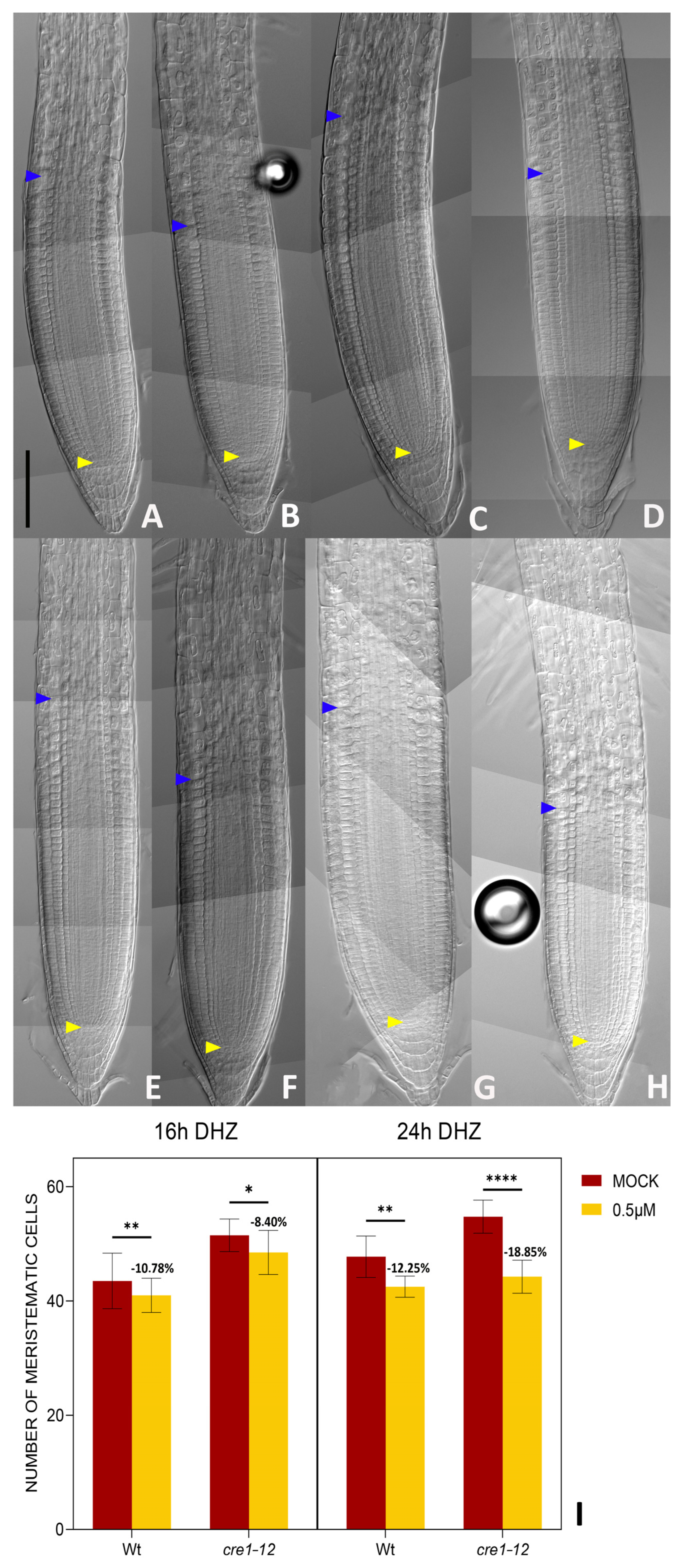
2.2. DHZ Is Perceived at the TZ by AHK3, but Not by CRE1/AHK4
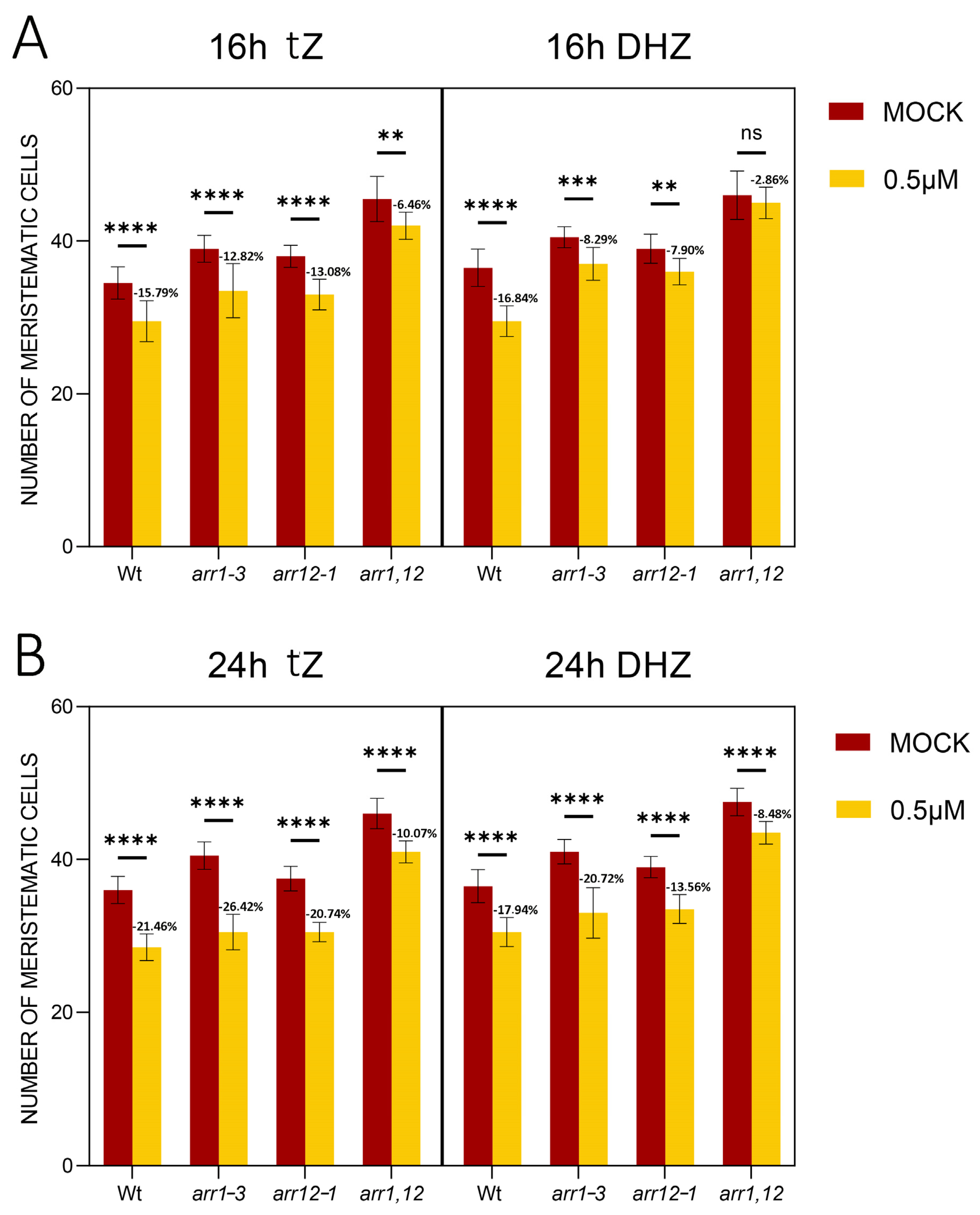
2.3. DHZ Regulates TZ Positioning via ARR1, 11 and 12
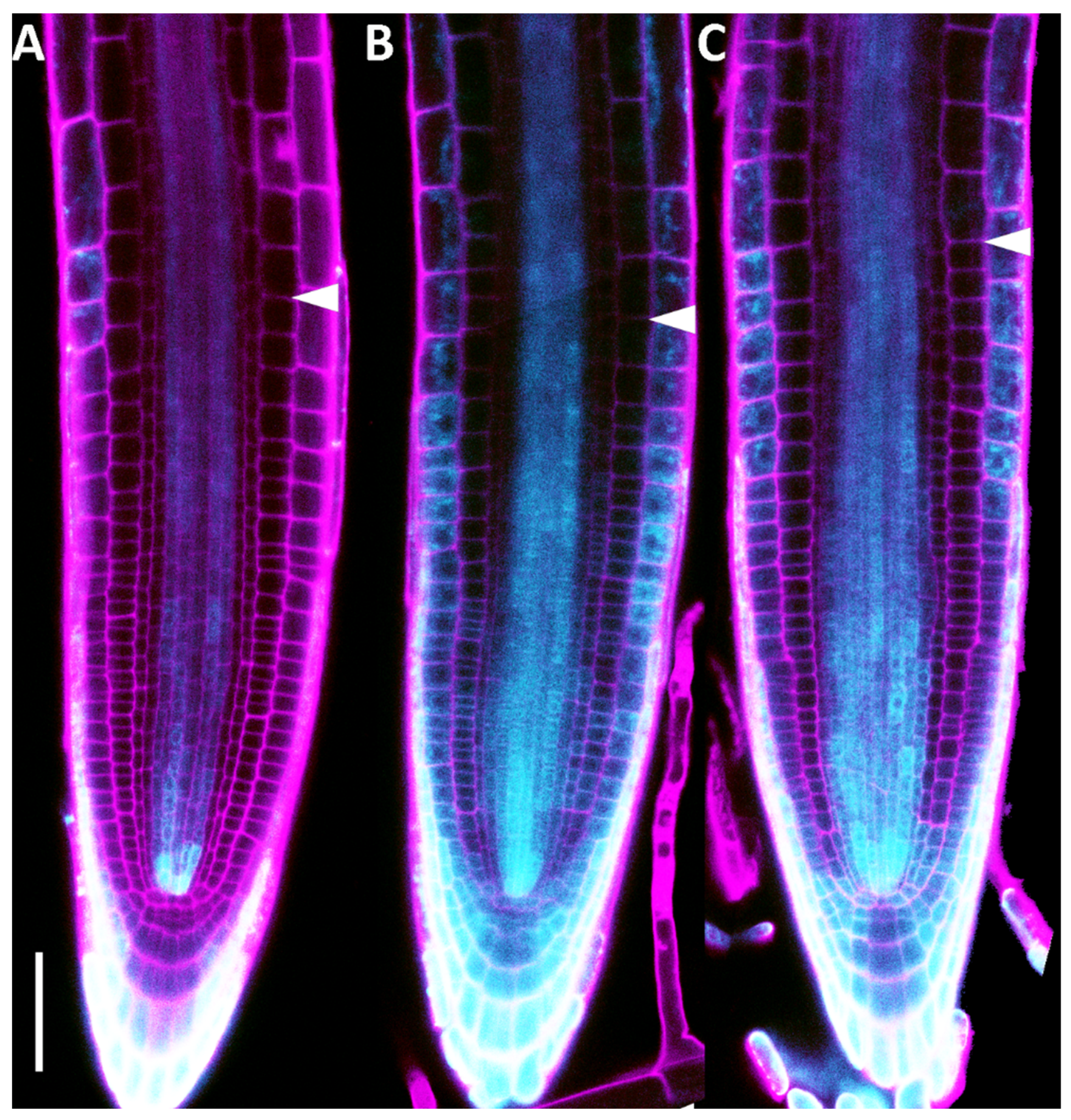
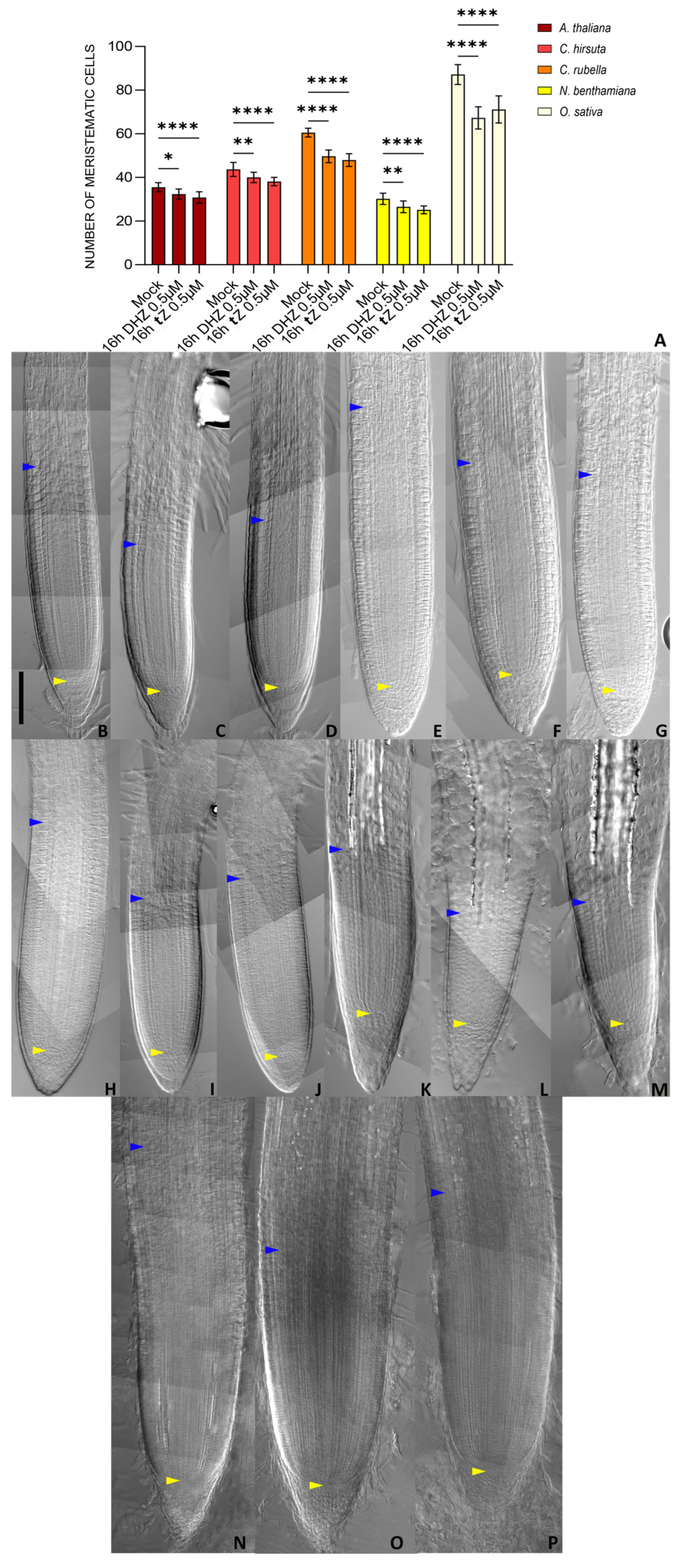
2.4. Root Meristem Size Regulation Operated by DHZ Is Potentially Conserved Among Plants
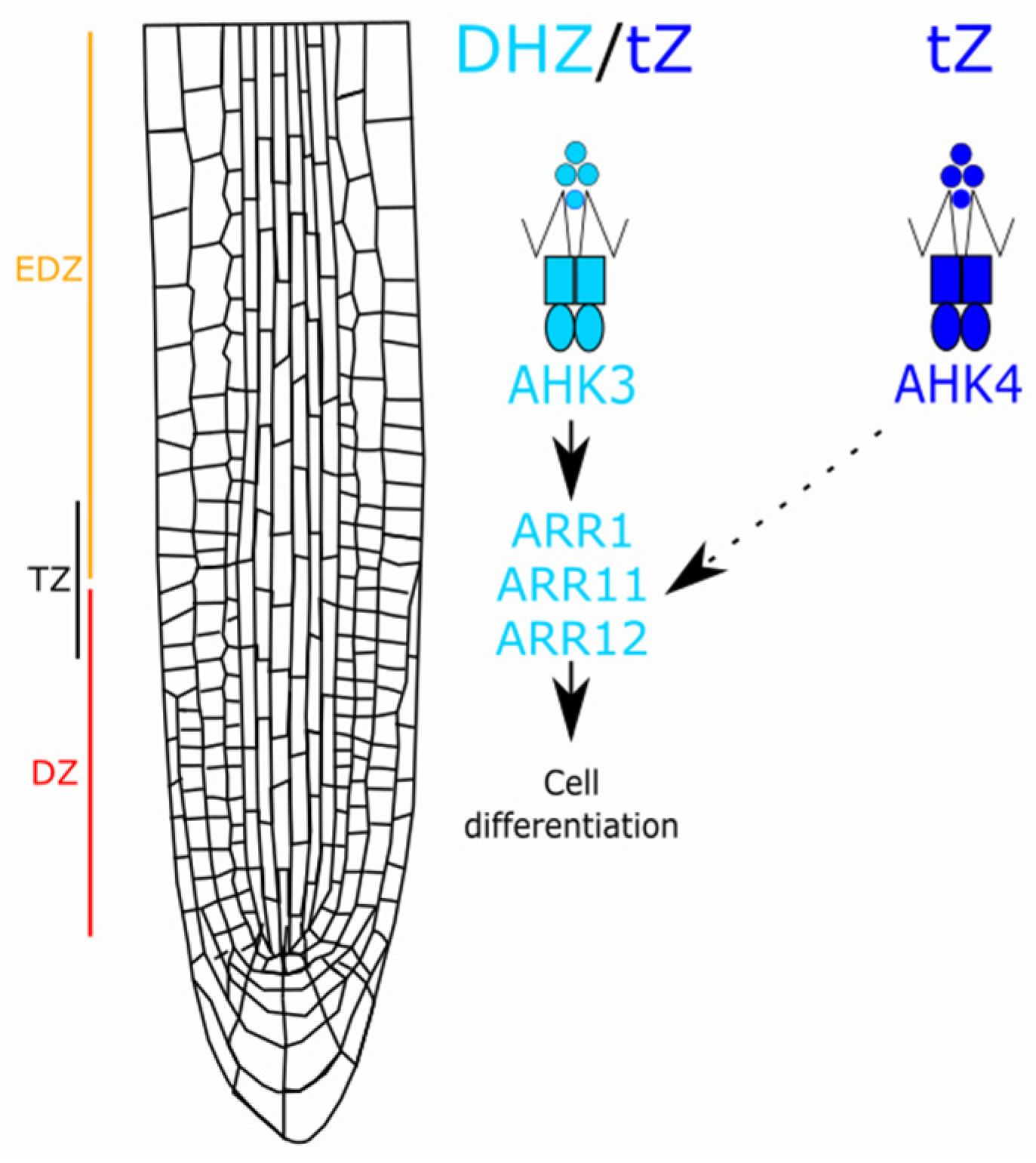
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Growth Conditions
5.2. Arabidopsis Locus IDs from This Article
5.3. Meristem-Size Analysis
5.4. Analysis of Expression Patterns
5.5. Hormonal Treatments
5.6. Image Processing and Assembly
5.7. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frugier, F.; Kosuta, S.; Murray, J.D.; Crespi, M.; Szczyglowski, K. Cytokinin: Secret Agent of Symbiosis. Trends Plant Sci. 2008, 13, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Raines, T.; Kieber, J.J. Cytokinin Signaling and Transcriptional Networks. Curr. Opin. Plant Biol. 2010, 13, 533–539. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Mughal, N.; Shoaib, N.; Chen, J.; Li, Y.; He, Y.; Fu, M.; Li, X.; He, Y.; Guo, J.; Deng, J.; et al. Adaptive Roles of Cytokinins in Enhancing Plant Resilience and Yield against Environmental Stressors. Chemosphere 2024, 364, 143189. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, Q.; Jiang, W.; Xiao, N.; Zeng, F.; Chen, G.; Mak, M.; Chen, Z.-H.; Deng, F. Molecular Regulation and Evolution of Cytokinin Signaling in Plant Abiotic Stresses. Plant Cell Physiol. 2023, 63, 1787–1805. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the Crossroads of Abiotic Stress Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Wang, Q.; Zhang, G. Roles of Cytokinins in Root Growth and Abiotic Stress Response of Arabidopsis Thaliana. Plant Growth Regul. 2021, 94, 151–160. [Google Scholar] [CrossRef]
- Chang, L.; Ramireddy, E.; Schmülling, T. Cytokinin as a Positional Cue Regulating Lateral Root Spacing in Arabidopsis. J. Exp. Bot. 2015, 66, 4759–4768. [Google Scholar] [CrossRef]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B.; et al. Cytokinins Act Directly on Lateral Root Founder Cells to Inhibit Root Initiation. Plant Cell 2007, 19, 3889–3900. [Google Scholar] [CrossRef]
- Scintu, D.; Scacchi, E.; Cazzaniga, F.; Vinciarelli, F.; De Vivo, M.; Shtin, M.; Svolacchia, N.; Bertolotti, G.; Unterholzner, S.J.; Del Bianco, M.; et al. microRNA165 and 166 Modulate Response of the Arabidopsis Root Apical Meristem to Salt Stress. Commun. Biol. 2023, 6, 834. [Google Scholar] [CrossRef]
- Antoniadi, I.; Plačková, L.; Simonovik, B.; Doležal, K.; Turnbull, C.; Ljung, K.; Novák, O. Cell-Type-Specific Cytokinin Distribution within the Arabidopsis Primary Root Apex. Plant Cell 2015, 27, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Takei, K.; Kojima, M.; Sakakibara, H. Side-Chain Modification of Cytokinins Controls Shoot Growth in Arabidopsis. Dev. Cell 2013, 27, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Mok, D.W.; Mok, M.C. CYTOKININ METABOLISM AND ACTION. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef]
- Sakakibara, H. CYTOKININS: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Perilli, S.; Perez-Perez, J.M.; Di Mambro, R.; Peris, C.L.; Díaz-Triviño, S.; Del Bianco, M.; Pierdonati, E.; Moubayidin, L.; Cruz-Ramírez, A.; Costantino, P.; et al. RETINOBLASTOMA-RELATED Protein Stimulates Cell Differentiation in the Arabidopsis Root Meristem by Interacting with Cytokinin Signaling. Plant Cell 2013, 25, 4469–4478. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef]
- Vylíčilová, H.; Bryksová, M.; Matušková, V.; Doležal, K.; Plíhalová, L.; Strnad, M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules 2020, 10, 832. [Google Scholar] [CrossRef]
- Nedvěd, D.; Hošek, P.; Klíma, P.; Hoyerová, K. Differential Subcellular Distribution of Cytokinins: How Does Membrane Transport Fit into the Big Picture? Int. J. Mol. Sci. 2021, 22, 3428. [Google Scholar] [CrossRef]
- Brenner, W.G.; Schmülling, T. Transcript Profiling of Cytokinin Action in Arabidopsis Roots and Shoots Discovers Largely Similar but Also Organ-Specific Responses. BMC Plant Biol. 2012, 12, 112. [Google Scholar] [CrossRef]
- Martin, R.C.; Mok, M.C.; Mok, D.W.S. A Gene Encoding the Cytokinin Enzyme Zeatin O -Xylosyltransferase of Phaseolus Vulgaris 1. Plant Physiol. 1999, 120, 553–558. [Google Scholar] [CrossRef]
- Offringa, R.; Hooykaas, P. Molecular Approaches to Study Plant Hormone Signalling. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 1999; Volume 33, pp. 391–410. ISBN 978-0-444-89825-8. [Google Scholar]
- Kakimoto, T. Perception and Signal Transduction of Cytokinins. Annu. Rev. Plant Biol. 2003, 54, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Higuchi, M.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Kato, T.; Tabata, S.; Shinozaki, K.; Kakimoto, T. Identification of CRE1 as a Cytokinin Receptor from Arabidopsis. Nature 2001, 409, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.K.; Huang, G.; Bhosale, R.; Hartman, S.; Sturrock, C.J.; Jose, L.; Martin, O.C.; Karady, M.; Voesenek, L.A.C.J.; Ljung, K.; et al. Plant Roots Sense Soil Compaction through Restricted Ethylene Diffusion. Science 2021, 371, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of Cytokinin Mutants and Regulation of Cytokinin Metabolic Genes Reveals Important Regulatory Roles of Cytokinins in Drought, Salt and Abscisic Acid Responses, and Abscisic Acid Biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmülling, T. Arabidopsis Cytokinin Receptor Mutants Reveal Functions in Shoot Growth, Leaf Senescence, Seed Size, Germination, Root Development, and Cytokinin Metabolism. Plant Cell 2005, 18, 40–54. [Google Scholar] [CrossRef]
- Hothorn, M.; Dabi, T.; Chory, J. Structural Basis for Cytokinin Recognition by Arabidopsis Thaliana Histidine Kinase 4. Nat. Chem. Biol. 2011, 7, 766–768. [Google Scholar] [CrossRef]
- Ohashi-Ito, K.; Saegusa, M.; Iwamoto, K.; Oda, Y.; Katayama, H.; Kojima, M.; Sakakibara, H.; Fukuda, H. A bHLH Complex Activates Vascular Cell Division via Cytokinin Action in Root Apical Meristem. Curr. Biol. 2014, 24, 2053–2058. [Google Scholar] [CrossRef]
- Romanov, G.A.; Lomin, S.N.; Schmülling, T. Biochemical Characteristics and Ligand-Binding Properties of Arabidopsis Cytokinin Receptor AHK3 Compared to CRE1/AHK4 as Revealed by a Direct Binding Assay. J. Exp. Bot. 2006, 57, 4051–4058. [Google Scholar] [CrossRef]
- Di Mambro, R.; Svolacchia, N.; Dello Ioio, R.; Pierdonati, E.; Salvi, E.; Pedrazzini, E.; Vitale, A.; Perilli, S.; Sozzani, R.; Benfey, P.N.; et al. The Lateral Root Cap Acts as an Auxin Sink That Controls Meristem Size. Curr. Biol. 2019, 29, 1199–1205.e4. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef]
- Shahan, R.; Hsu, C.-W.; Nolan, T.M.; Cole, B.J.; Taylor, I.W.; Greenstreet, L.; Zhang, S.; Afanassiev, A.; Vlot, A.H.C.; Schiebinger, G.; et al. A Single-Cell Arabidopsis Root Atlas Reveals Developmental Trajectories in Wild-Type and Cell Identity Mutants. Dev. Cell 2022, 57, 543–560.e9. [Google Scholar] [CrossRef]
- Li, S.; Yamada, M.; Han, X.; Ohler, U.; Benfey, P.N. High-Resolution Expression Map of the Arabidopsis Root Reveals Alternative Splicing and lincRNA Regulation. Dev. Cell 2016, 39, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Di Mambro, R.; De Ruvo, M.; Pacifici, E.; Salvi, E.; Sozzani, R.; Benfey, P.N.; Busch, W.; Novak, O.; Ljung, K.; Di Paola, L.; et al. Auxin Minimum Triggers the Developmental Switch from Cell Division to Cell Differentiation in the Arabidopsis Root. Proc. Natl. Acad. Sci. USA 2017, 114, E7641–E7649. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Liu, J.; Ding, Z. The Root Transition Zone: A Hot Spot for Signal Crosstalk. Trends Plant Sci. 2018, 23, 403–409. [Google Scholar] [CrossRef]
- Baluška, F.; Mancuso, S. Root Apex Transition Zone As Oscillatory Zone. Front. Plant Sci. 2013, 4, 354. [Google Scholar] [CrossRef] [PubMed]
- Bai, B. Root Zonation: New Molecular Insights. Mol. Plant 2020, 13, 1236. [Google Scholar] [CrossRef]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A Genetic Framework for the Control of Cell Division and Differentiation in the Root Meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Uhlir, N.J.; Reed, J.W. Arabidopsis SHY2/IAA3 Inhibits Auxin-Regulated Gene Expression. Plant Cell 2002, 14, 301–319. [Google Scholar] [CrossRef]
- Li, T.; Kang, X.; Lei, W.; Yao, X.; Zou, L.; Zhang, D.; Lin, H. SHY2 as a Node in the Regulation of Root Meristem Development by Auxin, Brassinosteroids, and Cytokinin. J. Integr. Plant Biol. 2020, 62, 1500–1517. [Google Scholar] [CrossRef]
- Goh, T.; Kasahara, H.; Mimura, T.; Kamiya, Y.; Fukaki, H. Multiple AUX/IAA–ARF Modules Regulate Lateral Root Formation: The Role of Arabidopsis SHY2/IAA3-Mediated Auxin Signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1461–1468. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J. Two-Component Circuitry in Arabidopsis Cytokinin Signal Transduction. Nature 2001, 413, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Pierdonati, E.; Unterholzner, S.J.; Salvi, E.; Svolacchia, N.; Bertolotti, G.; Dello Ioio, R.; Sabatini, S.; Di Mambro, R. Cytokinin-Dependent Control of GH3 Group II Family Genes in the Arabidopsis Root. Plants 2019, 8, 94. [Google Scholar] [CrossRef]
- Salvi, E.; Rutten, J.P.; Di Mambro, R.; Polverari, L.; Licursi, V.; Negri, R.; Dello Ioio, R.; Sabatini, S.; Ten Tusscher, K. A Self-Organized PLT/Auxin/ARR-B Network Controls the Dynamics of Root Zonation Development in Arabidopsis Thaliana. Dev. Cell 2020, 53, 431–443.e23. [Google Scholar] [CrossRef] [PubMed]
- Boudolf, V.; Barrôco, R.; Engler, J.D.; Verkest, A.; Beeckman, T.; Naudts, M.; Inzé, D.; De Veylder, L. B1-Type Cyclin-Dependent Kinases Are Essential for the Formation of Stomatal Complexes in Arabidopsis Thaliana. Plant Cell 2004, 16, 945–955. [Google Scholar] [CrossRef]
- Pacifici, E.; Di Mambro, R.; Dello Ioio, R.; Costantino, P.; Sabatini, S. Acidic Cell Elongation Drives Cell Differentiation in the Arabidopsis Root. EMBO J. 2018, 37, e99134. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Clabaugh, I.; To, J.P.; Maxwell, B.B.; Chiang, Y.-H.; Schaller, G.E.; Loraine, A.; Kieber, J.J. Identification of Cytokinin-Responsive Genes Using Microarray Meta-Analysis and RNA-Seq in Arabidopsis. Plant Physiol. 2013, 162, 272–294. [Google Scholar] [CrossRef]
- Samalova, M.; Melnikava, A.; Elsayad, K.; Peaucelle, A.; Gahurova, E.; Gumulec, J.; Spyroglou, I.; Zemlyanskaya, E.V.; Ubogoeva, E.V.; Balkova, D.; et al. Hormone-Regulated Expansins: Expression, Localization, and Cell Wall Biomechanics in Arabidopsis Root Growth. Plant Physiol. 2024, 194, 209–228. [Google Scholar] [CrossRef]
- Ramakrishna, P.; Ruiz Duarte, P.; Rance, G.A.; Schubert, M.; Vordermaier, V.; Vu, L.D.; Murphy, E.; Vilches Barro, A.; Swarup, K.; Moirangthem, K.; et al. EXPANSIN A1-Mediated Radial Swelling of Pericycle Cells Positions Anticlinal Cell Divisions during Lateral Root Initiation. Proc. Natl. Acad. Sci. USA 2019, 116, 8597–8602. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant Cell Wall Loosening by Expansins. Annu. Rev. Cell Dev. Biol. 2024, 40, 329–352. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Li, L.C.; Cho, H.-T.; Hoffmann-Benning, S.; Moore, R.C.; Blecker, D. The Growing World of Expansins. Plant Cell Physiol. 2002, 43, 1436–1444. [Google Scholar] [CrossRef]
- Cho, H.-T.; Cosgrove, D.J. Regulation of Root Hair Initiation and Expansin Gene Expression in Arabidopsis. Plant Cell 2002, 14, 3237–3253. [Google Scholar] [CrossRef]
- Gruel, J.; Landrein, B.; Tarr, P.; Schuster, C.; Refahi, Y.; Sampathkumar, A.; Hamant, O.; Meyerowitz, E.M.; Jönsson, H. An Epidermis-Driven Mechanism Positions and Scales Stem Cell Niches in Plants. Sci. Adv. 2016, 2, e1500989. [Google Scholar] [CrossRef] [PubMed]
- Fridman, Y.; Elkouby, L.; Holland, N.; Vragović, K.; Elbaum, R.; Savaldi-Goldstein, S. Root Growth Is Modulated by Differential Hormonal Sensitivity in Neighboring Cells. Genes. Dev. 2014, 28, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid Perception in the Epidermis Controls Root Meristem Size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins Determine Arabidopsis Root-Meristem Size by Controlling Cell Differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Di Ruocco, G.; Bertolotti, G.; Pacifici, E.; Polverari, L.; Tsiantis, M.; Sabatini, S.; Costantino, P.; Dello Ioio, R. Differential Spatial Distribution of miR165/6 Determines Variability in Plant Root Anatomy. Development 2017, 145, dev153858. [Google Scholar] [CrossRef]
- Pasternak, T.; Haser, T.; Falk, T.; Ronneberger, O.; Palme, K.; Otten, L. A 3D Digital Atlas of the Nicotiana Tabacum Root Tip and Its Use to Investigate Changes in the Root Apical Meristem Induced by the Agrobacterium 6b Oncogene. Plant J. 2017, 92, 31–42. [Google Scholar] [CrossRef]
- Kirschner, G.K.; Stahl, Y.; Von Korff, M.; Simon, R. Unique and Conserved Features of the Barley Root Meristem. Front. Plant Sci. 2017, 8, 1240. [Google Scholar] [CrossRef]
- Jantapo, K.; Wimonchaijit, W.; Wang, W.; Chaiwanon, J. Supraoptimal Brassinosteroid Levels Inhibit Root Growth by Reducing Root Meristem and Cell Elongation in Rice. Plants 2021, 10, 1962. [Google Scholar] [CrossRef]
- Wang, L.; Chu, H.; Li, Z.; Wang, J.; Li, J.; Qiao, Y.; Fu, Y.; Mou, T.; Chen, C.; Xu, J. Origin and Development of the Root Cap in Rice1 [W] [OPEN]. Plant Physiol. 2014, 166, 603–613. [Google Scholar] [CrossRef]
- Ding, W.; Tong, H.; Zheng, W.; Ye, J.; Pan, Z.; Zhang, B.; Zhu, S. Isolation, Characterization and Transcriptome Analysis of a Cytokinin Receptor Mutant Osckt1 in Rice. Front. Plant Sci. 2017, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z. Supraoptimal Cytokinin Content Inhibits Rice Seminal Root Growth by Reducing Root Meristem Size and Cell Length via Increased Ethylene Content. Int. J. Mol. Sci. 2018, 19, 4051. [Google Scholar]
- Rebouillat, J.; Jantapo, K. Molecular Genetics of Rice Root Development. Rice 2009, 2, 15–34. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Weir, N.R.; Hill, K.; Zhang, W.; Kim, H.J.; Shiu, S.-H.; Schaller, G.E.; Kieber, J.J. Characterization of Genes Involved in Cytokinin Signaling and Metabolism from Rice. Plant Physiol. 2012, 158, 1666–1684. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K.; et al. A PHABULOSA/Cytokinin Feedback Loop Controls Root Growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Vatén, A.; Dettmer, J.; Wu, S.; Stierhof, Y.-D.; Miyashima, S.; Yadav, S.R.; Roberts, C.J.; Campilho, A.; Bulone, V.; Lichtenberger, R.; et al. Callose Biosynthesis Regulates Symplastic Trafficking during Root Development. Dev. Cell 2011, 21, 1144–1155. [Google Scholar] [CrossRef]
- Hluska, T.; Hlusková, L.; Emery, R.J.N. The Hulks and the Deadpools of the Cytokinin Universe: A Dual Strategy for Cytokinin Production, Translocation, and Signal Transduction. Biomolecules 2021, 11, 209. [Google Scholar] [CrossRef]
- Vyroubalová, S.; Václavíková, K.; Turečková, V.; Novák, O.; Šmehilová, M.; Hluska, T.; Ohnoutková, L.; Frébort, I.; Galuszka, P. Characterization of New Maize Genes Putatively Involved in Cytokinin Metabolism and Their Expression during Osmotic Stress in Relation to Cytokinin Levels. Plant Physiol. 2009, 151, 433–447. [Google Scholar] [CrossRef]
- Bilyeu, K.D.; Cole, J.L.; Laskey, J.G.; Riekhof, W.R.; Esparza, T.J.; Kramer, M.D.; Morris, R.O. Molecular and Biochemical Characterization of a Cytokinin Oxidase from Maize. Plant Physiol. 2001, 125, 378–386. [Google Scholar] [CrossRef]
- Galuszka, P.; Popelková, H.; Werner, T.; Frébortová, J.; Pospíšilová, H.; Mik, V.; Köllmer, I.; Schmülling, T.; Frébort, I. Biochemical Characterization of Cytokinin Oxidases/Dehydrogenases from Arabidopsis thaliana Expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Arkhipov, D.V.; Savelieva, E.M.; Osolodkin, D.I.; Romanov, G.A. Cytokinin Perception in Potato: New Features of Canonical Players. J. Exp. Bot. 2018, 69, 3839–3853. [Google Scholar] [CrossRef] [PubMed]
- Lomin, S.N.; Krivosheev, D.M.; Steklov, M.Y.; Arkhipov, D.V.; Osolodkin, D.I.; Schmülling, T.; Romanov, G.A. Plant Membrane Assays with Cytokinin Receptors Underpin the Unique Role of Free Cytokinin Bases as Biologically Active Ligands. J. Exp. Bot. 2015, 66, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Kuderová, A.; Gallová, L.; Kuricová, K.; Nejedlá, E.; Čurdová, A.; Micenková, L.; Plíhal, O.; Šmajs, D.; Spíchal, L.; Hejátko, J. Identification of AHK2- and AHK3-like Cytokinin Receptors in Brassica Napus Reveals Two Subfamilies of AHK2 Orthologues. J. Exp. Bot. 2015, 66, 339–353. [Google Scholar] [CrossRef]
- Antoniadi, I.; Mateo-Bonmatí, E.; Pernisová, M.; Brunoni, F.; Antoniadi, M.; Villalonga, M.G.-A.; Ament, A.; Karády, M.; Turnbull, C.; Doležal, K.; et al. IPT9, a Cis-Zeatin Cytokinin Biosynthesis Gene, Promotes Root Growth. Front. Plant Sci. 2022, 13, 932008. [Google Scholar] [CrossRef]
- Köllmer, I.; Novák, O.; Strnad, M.; Schmülling, T.; Werner, T. Overexpression of the Cytosolic Cytokinin Oxidase/Dehydrogenase (CKX 7) from A Rabidopsis Causes Specific Changes in Root Growth and Xylem Differentiation. Plant J. 2014, 78, 359–371. [Google Scholar] [CrossRef]
- Mason, M.G.; Mathews, D.E.; Argyros, D.A.; Maxwell, B.B.; Kieber, J.J.; Alonso, J.M.; Ecker, J.R.; Schaller, G.E. Multiple Type-B Response Regulators Mediate Cytokinin Signal Transduction in Arabidopsis. Plant Cell 2005, 17, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, E.; Tavor-Deslex, D.; Lituiev, D.; Enkerli, K.; Tarr, P.T.; Müller, B. A Robust and Sensitive Synthetic Sensor to Monitor the Transcriptional Output of the Cytokinin Signaling Network in Planta. Plant Physiol. 2013, 161, 1066–1075. [Google Scholar] [CrossRef]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW Is Involved in Positioning the Stem Cell Niche in the Arabidopsis Root Meristem. Genes. Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef]
- Di Mambro, R.; Sabatini, S. Developmental Analysis of Arabidopsis Root Meristem. In Root Development: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 33–45. [Google Scholar]
- Casamitjana-Martínez, E.; Hofhuis, H.F.; Xu, J.; Liu, C.-M.; Heidstra, R.; Scheres, B. Root-Specific CLE19 Overexpression and the Sol1/2 Suppressors Implicate a CLV-like Pathway in the Control of Arabidopsis Root Meristem Maintenance. Curr. Biol. 2003, 13, 1435–1441. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinciarelli, F.; De Vivo, M.; Terenzi, A.; Cazzaniga, F.; Amati, S.; Damato, P.; Salvi, E.; Del Bianco, M.; Di Mambro, R.; Costantino, P.; et al. Identification of a Specific Role of Dihydrozeatin in the Regulation of the Cell Differentiation Activity in Arabidopsis Roots. Plants 2025, 14, 1501. https://doi.org/10.3390/plants14101501
Vinciarelli F, De Vivo M, Terenzi A, Cazzaniga F, Amati S, Damato P, Salvi E, Del Bianco M, Di Mambro R, Costantino P, et al. Identification of a Specific Role of Dihydrozeatin in the Regulation of the Cell Differentiation Activity in Arabidopsis Roots. Plants. 2025; 14(10):1501. https://doi.org/10.3390/plants14101501
Chicago/Turabian StyleVinciarelli, Federico, Mirko De Vivo, Alessio Terenzi, Francesca Cazzaniga, Samuele Amati, Pierpaolo Damato, Elena Salvi, Marta Del Bianco, Riccardo Di Mambro, Paolo Costantino, and et al. 2025. "Identification of a Specific Role of Dihydrozeatin in the Regulation of the Cell Differentiation Activity in Arabidopsis Roots" Plants 14, no. 10: 1501. https://doi.org/10.3390/plants14101501
APA StyleVinciarelli, F., De Vivo, M., Terenzi, A., Cazzaniga, F., Amati, S., Damato, P., Salvi, E., Del Bianco, M., Di Mambro, R., Costantino, P., Sabatini, S., & Dello Ioio, R. (2025). Identification of a Specific Role of Dihydrozeatin in the Regulation of the Cell Differentiation Activity in Arabidopsis Roots. Plants, 14(10), 1501. https://doi.org/10.3390/plants14101501











