Phosphite Compounds Suppress Anthracnose in Soybean Seeds Infected by Colletotrichum truncatum and Stimulate Growth and Defense Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Sources and Doses of Phosphites and Fungicide
2.3. Inoculation of Soybean Seeds with C. truncatum
2.4. Seed Treatments
2.5. Effects of Phosphites on Seed Health and Germination
2.6. Emergence and Development of Soybean Seedlings Under Greenhouse Conditions
2.7. Fungitoxic Activity of Phosphites
2.8. Activation of Biochemical Defense Mechanisms
2.9. Statistical Analysis
3. Results
3.1. Effects of Phosphites on Seed Health and Germination
3.2. Emergence and Development of Soybean Seedlings Under Greenhouse Conditions
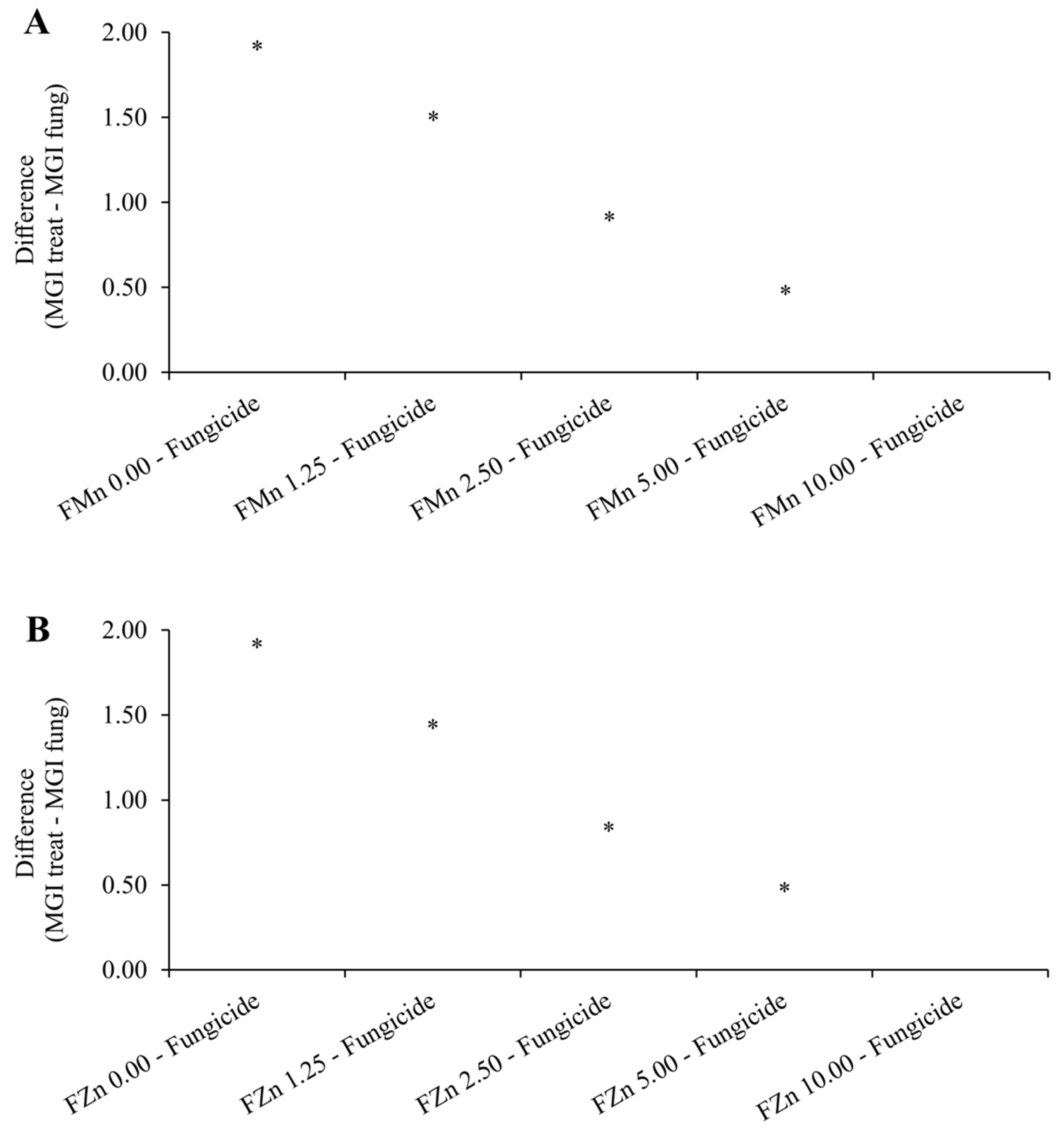
3.3. Fungitoxic Activity of Phosphites
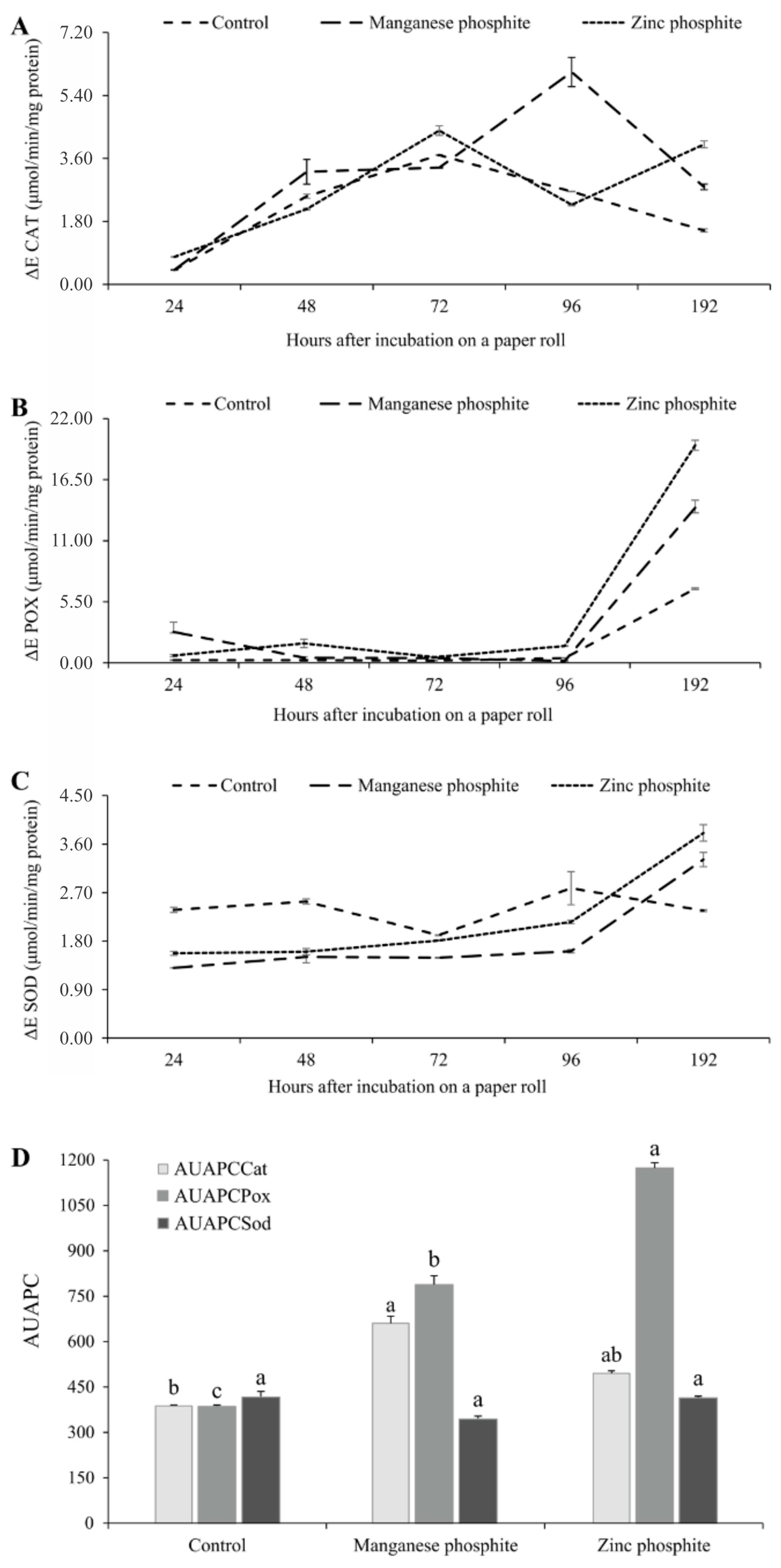
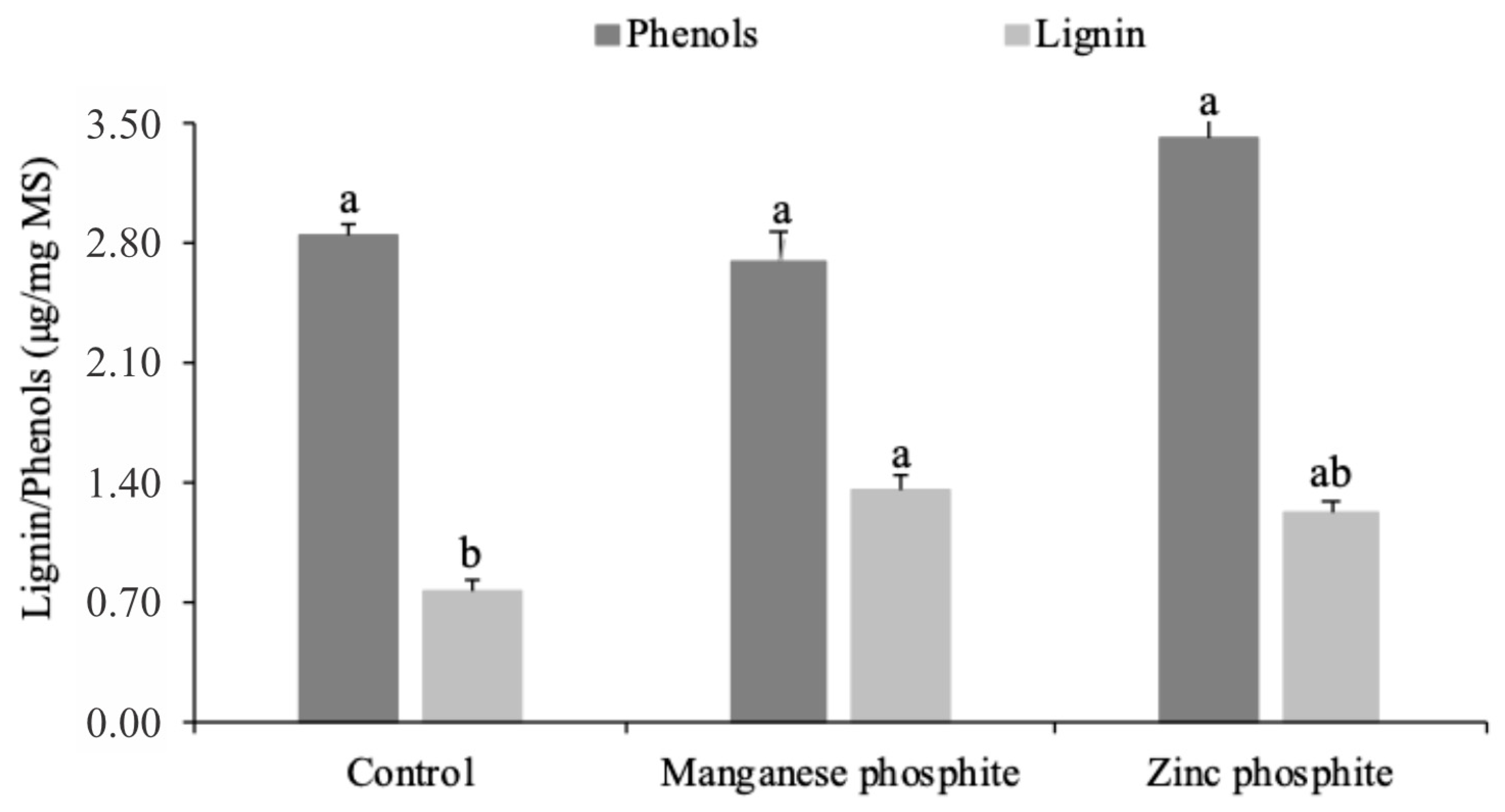
3.4. Activation of Biochemical Defense Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Li, H.; Liu, Z.; Zhang, C.; Zhang, S.; Atkinson, P.M. A novel Greenness and Water Content Composite Index (GWCCI) for soybean mapping from single remotely sensed multispectral images. Remote Sens. Environ. 2023, 295, 113679. [Google Scholar] [CrossRef]
- Liu, K. Soybean: Overview. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Matton, N.; Canto, G.S.; Waldner, F.; Valero, S.; Morin, D.; Inglada, J.; Arias, M.; Bontemps, S.; Koetz, B.; Defourny, P. An automated method for annual cropland mapping along the season for various globally-distributed agrosystems using high spatial and temporal resolution time series. Remote Sens. 2015, 7, 13208–13232. [Google Scholar] [CrossRef]
- Rogério, F.; de Castro, R.R.L.; Júnior, N.S.M.; Boufleur, T.R.; dos Santos, R.F. Multiple resistance of Colletotrichum truncatum from soybean to QoI and MBC fungicides in Brazil. J. Phytopathol. 2024, 172, 13341. [Google Scholar] [CrossRef]
- Hartman, G.L.; Sinclair, J.B.; Rupe, J.C. Compendium of Soybean Diseases, 4th ed.; APS Press: St. Paul, MN, USA, 1999. [Google Scholar] [CrossRef]
- Billores, S.D.O.; Joshi, P.; Bhatia, V.S.; Ramesh, A. Soybean Based Intercropping Systems in India—A Review. Soybean Res. 2011, 9, 1–30. [Google Scholar]
- Dias, M.D.; Pinheiro, V.F.; Café-Filho, A.C. Impact of anthracnose on the yield of soybean subjected to chemical control in the north region of Brazil. Summa Phytopathol. 2016, 42, 18–23. [Google Scholar] [CrossRef]
- Dias, M.D.; Dias-Neto, J.J.; Santos, M.D.; Formento, A.N.; Bizerra, L.V.; Fonseca, M.E.N.; Boiteux, L.S.; Café-Filho, A.C. Current Status of Soybean Anthracnose Associated with Colletotrichum truncatum in Brazil and Argentina. Plants 2019, 8, 459. [Google Scholar] [CrossRef]
- da Silva, A.C.; de Souza, P.E.; Machado, J.d.C.; da Silva, B.M.; Pinto, J.E.B.P. Effectiveness of essential oils in the treatment of Colletotrichum truncatum-infected soybean seeds. Trop. Plant Pathol. 2012, 37, 305–313. [Google Scholar] [CrossRef]
- Galli, J.; Panizzi, R.d.C.; Vieira, R. Resistência de variedades de soja à morte de plântulas causada por Colletotrichum truncatum. Arq. Inst. Biológico 2007, 74, 163–165. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gupta, G.K.; Ramteke, R. Colletotrichum truncatum [(Schw.) Andrus and W.D. Moore], the causal agent of anthracnose of soybean [Glycine max (L.) Merrill.]—A review. Soybean Res. 2011, 9, 31–52. [Google Scholar]
- Júnior, M.B.d.S.; Resende, M.L.V.; Pozza, E.A.; Machado, J.d.C.; de Resende, A.R.M.; Cardoso, A.M.S.; Guimarães, S.d.S.C.; Botelho, D.M.d.S. Effect of temperature on Colletotrichum truncatum growth, and evaluation of its inoculum potential in soybean seed germination. Eur. J. Plant Pathol. 2021, 160, 999–1004. [Google Scholar] [CrossRef]
- Boufleur, T.R.; Ciampi-Guillardi, M.; Tikami, Í.; Rogério, F.; Thon, M.R.; Sukno, S.A.; Júnior, N.S.M.; Baroncelli, R. Soybean anthracnose caused by Colletotrichum species: Current status and future prospects. Mol. Plant Pathol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Pereira, C.E.; Oliveira, J.A.; Rosa, M.C.M.; Oliveira, G.E.; Neto, J.C. Tratamento fungicida de sementes de soja inoculadas com Colletotrichum truncatum. Cienc. Rural. 2009, 39, 2390–2395. [Google Scholar] [CrossRef]
- Yang, H.-C.; Hartman, G.L. Methods and Evaluation of Soybean Genotypes for Resistance to Colletotrichum truncatum. Plant Dis. 2015, 99, 143–148. [Google Scholar] [CrossRef]
- Poti, T.; Mahawan, K.; Cheewangkoon, R.; Arunothayanan, H.; Akimitsu, K.; Nalumpang, S. Detection and molecular characterization of carbendazim-resistant Colletotrichum truncatum Isolates causing anthracnose of soybean in Thailand. J. Phytopathol. 2020, 168, 267–278. [Google Scholar] [CrossRef]
- de Mello, F.E.; Mathioni, S.M.; Matos, V.O.R.L.; da Silva, M.A.; Marques, D.B.; Rambach, O.; Torriani, S.F.F.; Deuner, C.C.; Antunes, R.F.D. Sensitivity of Colletotrichum plurivorum and C. truncatum isolated from soybean in Brazil to SDHIs and DMIs fungicides. Trop. Plant Pathol. 2024, 49, 83–92. [Google Scholar] [CrossRef]
- Begum, M.; Sariah, M.; Puteh, A.; Abidin, M.Z.; Rahman, M.; Siddiqui, Y. Field performance of bio-primed seeds to suppress Colletotrichum truncatum causing damping-off and seedling stand of soybean. Biol. Control. 2010, 53, 18–23. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Ribeiro Júnior, P.M.; Resende, M.L.V.; Silva, A.C.; Blumer, S.; Pereira, V.F.; Osswald, W.; Pascholati, S.F. O triplo modo de ação dos fosfitos em plantas. Revisão Anu. Patol. Plantas 2012, 20, 206–242. [Google Scholar]
- Buffara, C.R.S.; Angelotti, F.; Tessmann, D.J.; de Souza, C.D.; Vida, J.B. Atividade de fosfito de potássio na pré e pós-infecção de Phakopsora euvitis em folhas de videira. Semin. Agrar. 2013, 34, 3333. [Google Scholar] [CrossRef]
- Carmona, M.A.; Sautua, F.J.; E Grijalba, P.; Cassina, M.; Pérez-Hernández, O. Effect of potassium and manganese phosphites in the control of Pythium damping-off in soybean: A feasible alternative to fungicide seed treatments. Pest Manag. Sci. 2017, 74, 366–374. [Google Scholar] [CrossRef]
- Yáñez-Juárez, M.G.; López-Orona, C.A.; Ayala-Tafoya, F.; Partida-Ruvalcaba, L.; Velázquez-Alcaraz, T.d.J.; Medina-López, R. Los fosfitos como alternativa para el manejo de problemas fitopatológicos. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2018, 36, 79–94. [Google Scholar] [CrossRef]
- Monteiro, A.C.A.; de Resende, M.L.V.; Valente, T.C.T.; Junior, P.M.R.; Pereira, V.F.; da Costa, J.R.; da Silva, J.A.G. Manganese phosphite in coffee defence against Hemileia vastatrix, the coffee rust fungus: Biochemical and molecular analyses. J. Phytopathol. 2016, 164, 1043–1053. [Google Scholar] [CrossRef]
- da Silva, A.C.; Resende, M.L.V.; de Souza, P.E.; Silva, N.C.N.; Silva, M.B.; Vitorino, L.R.R. Coffee-leaf extract and phosphites on the curative control of powdery mildew in eucalyptus mini-stumps. For. Pathol. 2013, 43, 297–305. [Google Scholar] [CrossRef]
- Yogev, E.; Sadowsky, A.; Solel, Z.; Oren, Y.; Orbach, Y. The performance of potassium phosphite for controlling Alternaria brown spot of citrus fruit. J. Plant Dis. Prot. 2006, 113, 207–213. [Google Scholar] [CrossRef]
- Silva, O.; Santos, H.; Pria, M.D.; Mio, L.M.-D. Potassium phosphite for control of downy mildew of soybean. Crop. Prot. 2011, 30, 598–604. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Lazarovits, G. Seed Treatment with phosphonate (AG3) suppresses pythium damping-off of cucumber seedlings. Plant Dis. 2006, 90, 459–464. [Google Scholar] [CrossRef]
- Mayton, H.; Amirkhani, M.; Loos, M.; Johnson, B.; Fike, J.; Johnson, C.; Myers, K.; Starr, J.; Bergstrom, G.C.; Taylor, A. Evaluation of Industrial Hemp Seed Treatments for Management of Damping-Off for Enhanced Stand Establishment. Agriculture 2022, 12, 591. [Google Scholar] [CrossRef]
- Lobato, M.C.; Olivieri, F.P.; Altamiranda, E.A.G.; Wolski, E.A.; Daleo, G.R.; Caldiz, D.O.; Andreu, A.B. Phosphite compounds reduce disease severity in potato seed tubers and foliage. Eur. J. Plant Pathol. 2008, 122, 349–358. [Google Scholar] [CrossRef]
- Simonetti, E.; Viso, N.P.; Montecchia, M.; Zilli, C.; Balestrasse, K.; Carmona, M. Evaluation of native bacteria and manganese phosphite for alternative control of charcoal root rot of soybean. Microbiol. Res. 2015, 180, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Thao, H.T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- McDonald, A.E.; Grant, B.R.; Plaxton, W.C. Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. J. Plant Nutr. 2001, 24, 1505–1519. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Kubiak, K.A.; Sawicki, J.; Nowakowska, J.A.; Oszako, T. The use of phosphates in forestry. For. Res. Pap. 2016, 77, 76–81. [Google Scholar] [CrossRef]
- Whiley, A.; Hargreaves, P.; Pegg, K.; Doogan, V.; Ruddle, L.; Saranah, J.; Langdon, P. Changing sink strengths influence translocation of phosphonate in avocado (Persea americana Mill.) trees. Aust. J. Agric. Res. 1995, 46, 1079–1090. [Google Scholar] [CrossRef]
- Magalhães, L.P.P.; Sales, N.d.L.P.; Barroso, P.D.; da Silva, R.A.F.; Pinho, D.B.; Zanuncio, J.C.; da Silva, A.C. Pseudoplagiostoma humilis sp. nov., a New Fungal Species Causing Shoot Blight and Dieback in Anacardium humile in Brazil. Curr. Microbiol. 2024, 81, 378. [Google Scholar] [CrossRef]
- Brasil Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2009; 399p. [Google Scholar]
- Machado, J.; Oliveira, J.; Vieira, M.; Alves, M. Inoculação artificial de sementes de soja por fungos, utilizando solução de manitol. Rev. Bras. Sement. 2001, 23, 95–101. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination—Aid in selection and evaluation for seedling emergence and vigor. Crop. Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Araújo, L.; Valdebenito-Sanhueza, R.M.; Stadnik, M.J. Avaliação de formulações de fosfito de potássio sobre Colletotrichum gloeosporioides in vitro e no controle pós-infeccional da mancha foliar de Glomerella em macieira. Trop. Plant Pathol. 2010, 35, 54–59. [Google Scholar] [CrossRef]
- Torres-Calzada, C.; Tapia-Tussell, R.; Higuera-Ciapara, I.; Martin-Mex, R.; Nexticapan-Garcez, A.; Perez-Brito, D. Sensitivity of Colletotrichum truncatum to four fungicides and characterization of thiabendazole-resistant isolates. Plant Dis. 2015, 99, 1590–1595. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Urbanek, H.; Kuzniak-Gebarowska, E.; Herka, H. Elicitation of defence responses in common bean leaves by Botrytis cinerea polygalacturonase. Acta Phisiologiae Plant. 1991, 13, 43–50. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Giannopolotis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Shaner, G.; Finney, R.F. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathol. 1977, 67, 1051–1056. [Google Scholar] [CrossRef]
- Doster, M.A.; Bostock, R.M. Quantification of lignin formation in almond bark in response to wounding and infection by Phytophthora species. Phytopathology 1988, 78, 473–477. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson Seedless grape juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Förster, H.; Adaskaveg, J.E.; Kim, D.H.; Stanghellini, M.E. Effect of Phosphite on Tomato and Pepper Plants and on Susceptibility of Pepper to Phytophthora Root and Crown Rot in Hydroponic Culture. Plant Dis. 1998, 82, 1165–1170. [Google Scholar] [CrossRef]
- Guest, D.; Grant, B. The complex action of phosphonates as antifungal agents. Biol. Rev. 1991, 66, 159–187. [Google Scholar] [CrossRef]
- Malusà, E.; Tosi, L. Phosphorous acid residues in apples after foliar fertilization: Results of field trials. Food Addit. Contam. 2005, 22, 541–548. [Google Scholar] [CrossRef]
- Marks, G.; Smith, I. Metalaxyl and phosphonate as prophylactic and curative agents against stem infection of Leucadendron caused by Phytophthora cinnamomi. Aust. J. Exp. Agric. 1992, 32, 255–259. [Google Scholar] [CrossRef]
- Howard, K.; Dell, B.; Hardy, G.E. Phosphite and mycorrhizal formation in seedlings of three Australian Myrtaceae. Aust. J. Bot. 2000, 48, 725–729. [Google Scholar] [CrossRef]
- Tambascio, C.; Covacevich, F.; Lobato, M.C.; de Lasa, C.; Caldiz, D.; Dosio, G.; Andreu, A. The application of K phosphites to seed tubers enhanced emergence, early growth and mycorrhizal colonization in potato (Solanum tuberosum). Am. J. Plant Sci. 2014, 05, 132–137. [Google Scholar] [CrossRef]
- Liljeroth, E.; Lankinen, Å.; Wiik, L.; Burra, D.D.; Alexandersson, E.; Andreasson, E. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop. Prot. 2016, 86, 42–55. [Google Scholar] [CrossRef]
- Puerari, H.H.; Dias-Arieira, C.; Tavares Silva, C.A.; Arieira, J.O.; Biela, F.; Poletine, J.P. Ecolife nd manganese phosphite in the control of Meloidogyne javanica and in the development of soybean cultivars susceptible and resistant to the nematode. Nematropica 2013, 43, 105–112. [Google Scholar]
- da Silva, A.C.; de Resende, M.L.V.; de Souza, P.E.; Pôssa, K.F.; Júnior, M.B.d.S. Plant extract, zinc phosphite and zinc sulphate in the control of powdery mildew in the eucalyptus. Rev. Cienc. Agron. 2016, 47, 93–100. [Google Scholar] [CrossRef]
- King, M.; Reeve, W.; Van der Hoek, M.B.; Williams, N.; McComb, J.; O’brien, P.A.; Hardy, G.E.S.J. Defining the phosphite-regulated transcriptome of the plant pathogen Phytophthora cinnamomi. Mol. Genet. Genomics 2010, 284, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lobato, M.C.; Olivieri, F.P.; Daleo, G.R.; Andreu, A.B. Antimicrobial activity of phosphites against different potato pathogens. J. Plant Dis. Prot. 2010, 117, 102–109. [Google Scholar] [CrossRef]
- Dobrowolski, M.P.; Shearer, B.L.; Colquhoun, I.J.; O’brien, P.A.; Hardy, G.E.S. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathol. 2008, 57, 928–936. [Google Scholar] [CrossRef]
- Brown, S.; Koike, S.T.; Ochoa, O.E.; Laemmlen, F.; Michelmore, R.W.; Brown, S.; Koike, S.T.; Ochoa, O.E.; Steingrímsdóttir, M.M.; Petersen, A.; et al. Insensitivity to the fungicide fosetyl-aluminum in California isolates of the lettuce downy mildew pathogen, Bremia lactucae. Plant Dis. 2004, 88, 502–508. [Google Scholar] [CrossRef]
- Duvenhage, J.A. Monitoring the Resistance of Phytophthora cinnamomito Fosetyl-Al and H3PO3. In Duivelskloof: Yearbook; South African Avocado Growers’ Association: Tzaneen, South Africa, 1994; Volume 17, pp. 35–37. [Google Scholar]
- Perez, V.M.; Huet, J.-C.; Pernollet, J.-C. Enhanced secretion of elicitins by Phytophthora fungi exposed to phosphonate. Cryptogam. Mycol. 1995, 16, 191–194. [Google Scholar] [CrossRef]
- Machinandiarena, M.F.; Lobato, M.C.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B. Potassium phosphite primes defense responses in potato against Phytophthora infestans. J. Plant Physiol. 2012, 169, 1417–1424. [Google Scholar] [CrossRef]
- Pilbeam, R.A.; Howard, K.; Shearer, B.L.; Hardy, G.E.S.J. Phosphite stimulated histological responses of Eucalyptus marginata to infection by Phytophthora cinnamomi. Trees 2011, 25, 1121–1131. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Chai, T.; Zhang, Y.; Tan, J.; Ma, S. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Demirevska-Kepova, K.; Simova-Stoilova, L.; Stoyanova, Z.; Hölzer, R.; Feller, U. Biochemical changes in barley plants after excessive supply of copper and manganese. Environ. Exp. Bot. 2004, 52, 253–266. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D.; Pedler, J.F. Manganese nutrition and accumulation of phenolics and lignin as related to differential resistance of wheat genotypes to the take-all fungus. Plant Soil 1993, 151, 255–263. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture: A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Stangarlin, J.R.; Kuhn, O.J.; Toledo, M.V.; Portz, R.L.; Schan-Estrada, K.R.F.; Pascholati, S.F. A defesa vegetal contra fitopatógenos. Sci. Agrar. Parana 2011, 10, 18–46. [Google Scholar]
- Griffith, J.M.; Coffey, M.D.; Grant, B.R. Phosphonate inhibition as a function of phosphate concentration in isolates of Phytophthora palmivora. J. Gen. Microbiol. 1993, 139, 2109–2116. [Google Scholar] [CrossRef]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Zhang, Z.; Xi, Y.; Han, H.; Lan, F.; Zhang, B.; Wang-Pruski, G. Effects of potassium phosphite on biochemical contents and enzymatic activities of Chinese potatoes inoculated by Phytophthora infestans. Appl. Ecol. Environ. Res. 2019, 17, 4499–4514. [Google Scholar] [CrossRef]

| Treatments | Germination (%) | Incidence of C. truncatum in the Seeds (%) | Reduction of C. truncatum Incidence in the Seeds (%) |
|---|---|---|---|
| Inoculated control | 25 ± 1.0 d* | 96 ± 3.7 e* | 0 |
| Uninoculated control | 85 ± 3.4 a | 4 ± 4.4 a | 95 |
| Protreat® fungicide | 83 ± 10.8 a | 27 ± 6.9 b | 72 |
| Copper phosphite | 69 ± 3.4 b | 70 ± 7.2 d | 27 |
| Manganese phosphite | 81 ± 3.4 a | 29 ± 5.7 b | 69 |
| Potassium phosphite | 59 ± 10.8 c | 49 ± 4.8 c | 49 |
| Zinc phosphite | 85 ± 5.2 a | 33 ± 4.1 b | 65 |
| Treatments | ESI * | IS * (%) | FS * (%) | H * (cm) | R * (cm) | BA * (g) | BR * (g) |
|---|---|---|---|---|---|---|---|
| Inoculated control | 21.5 ± 1.2 d* | 32.2 ± 1.5 e* | 34.0 ± 4.1 e* | 24.3 ± 0.7 c* | 20.4 ± 2.5 c* | 2.9 ± 0.4 c* | 1.6 ± 0.1 d* |
| Uninoculated control | 65.7 ± 2.8 a | 93.0 ± 2.9 a | 95.0 ± 2.1 a | 28.9 ± 1.7 b | 32.8 ± 1.1 a | 7.5 ± 0.7 a | 5.1 ± 0.6 a |
| Protreat® fungicide | 45.9 ± 3.2 b | 71.0 ± 3.5 b | 76.7 ± 5.6 b | 30.3 ± 0.7 a | 30.2 ± 3.0 a | 6.8 ± 0.7 a | 3.8 ± 0.7 b |
| Copper phosphite | 33.3 ± 5.4 c | 51.5 ± 7.0 d | 55.0 ± 9.6 d | 28.6 ± 1.0 b | 25.0 ± 2.1 b | 4.6 ± 0.6 b | 2.6 ± 0.8 c |
| Manganese phosphite | 44.1 ± 2.7 b | 62.5 ± 7.5 c | 80.2 ± 1.2 b | 30.5 ± 1.6 a | 30.2 ± 0.9 a | 7.0 ± 0.3 a | 4.2 ± 0.2 b |
| Potassium phosphite | 39.0 ± 5.8 c | 60.0 ± 8.2 c | 64.0 ± 6.9 c | 30.2 ± 2.5 a | 24.4 ± 4.0 b | 5.8 ± 0.97 a | 2.4 ± 0.3 c |
| Zinc phosphite | 47.7 ± 2.9 b | 75.7 ± 5.3 b | 80.0 ± 3.3 b | 31.9 ± 1.6 a | 30.5 ± 0.1 a | 6.5 ± 0.2 a | 3.7 ± 0.4 b |
| Treatments | EC50 mL.L−1 (g.L−1 a.i.) | MCI mL.L−1 (g.L−1 a.i.) |
|---|---|---|
| Manganese phosphite | 2.48 (0.248) | 8.64 (0.864) |
| Zinc phosphite | 2.35 (0.235) | 8.44 (0.844) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Júnior, M.B.; de Resende, M.L.V.; Pozza, E.A.; de Resende, A.R.M.; Silveira, G.C.D.; da Veiga, J.D.; Oliveira, J.M.; da Silva, A.C. Phosphite Compounds Suppress Anthracnose in Soybean Seeds Infected by Colletotrichum truncatum and Stimulate Growth and Defense Mechanisms. Plants 2025, 14, 1494. https://doi.org/10.3390/plants14101494
da Silva Júnior MB, de Resende MLV, Pozza EA, de Resende ARM, Silveira GCD, da Veiga JD, Oliveira JM, da Silva AC. Phosphite Compounds Suppress Anthracnose in Soybean Seeds Infected by Colletotrichum truncatum and Stimulate Growth and Defense Mechanisms. Plants. 2025; 14(10):1494. https://doi.org/10.3390/plants14101494
Chicago/Turabian Styleda Silva Júnior, Manoel Batista, Mário Lúcio Vilela de Resende, Edson Ampélio Pozza, Alexandre Ribeiro Maia de Resende, Gustavo César Dias Silveira, Jayne Deboni da Veiga, Júlia Marques Oliveira, and André Costa da Silva. 2025. "Phosphite Compounds Suppress Anthracnose in Soybean Seeds Infected by Colletotrichum truncatum and Stimulate Growth and Defense Mechanisms" Plants 14, no. 10: 1494. https://doi.org/10.3390/plants14101494
APA Styleda Silva Júnior, M. B., de Resende, M. L. V., Pozza, E. A., de Resende, A. R. M., Silveira, G. C. D., da Veiga, J. D., Oliveira, J. M., & da Silva, A. C. (2025). Phosphite Compounds Suppress Anthracnose in Soybean Seeds Infected by Colletotrichum truncatum and Stimulate Growth and Defense Mechanisms. Plants, 14(10), 1494. https://doi.org/10.3390/plants14101494







