Distinctive Traits of European Mistletoe (Viscum album spp. austriacum) and Its Impact on Host Tree Wood (Pinus sylvestris)
Abstract
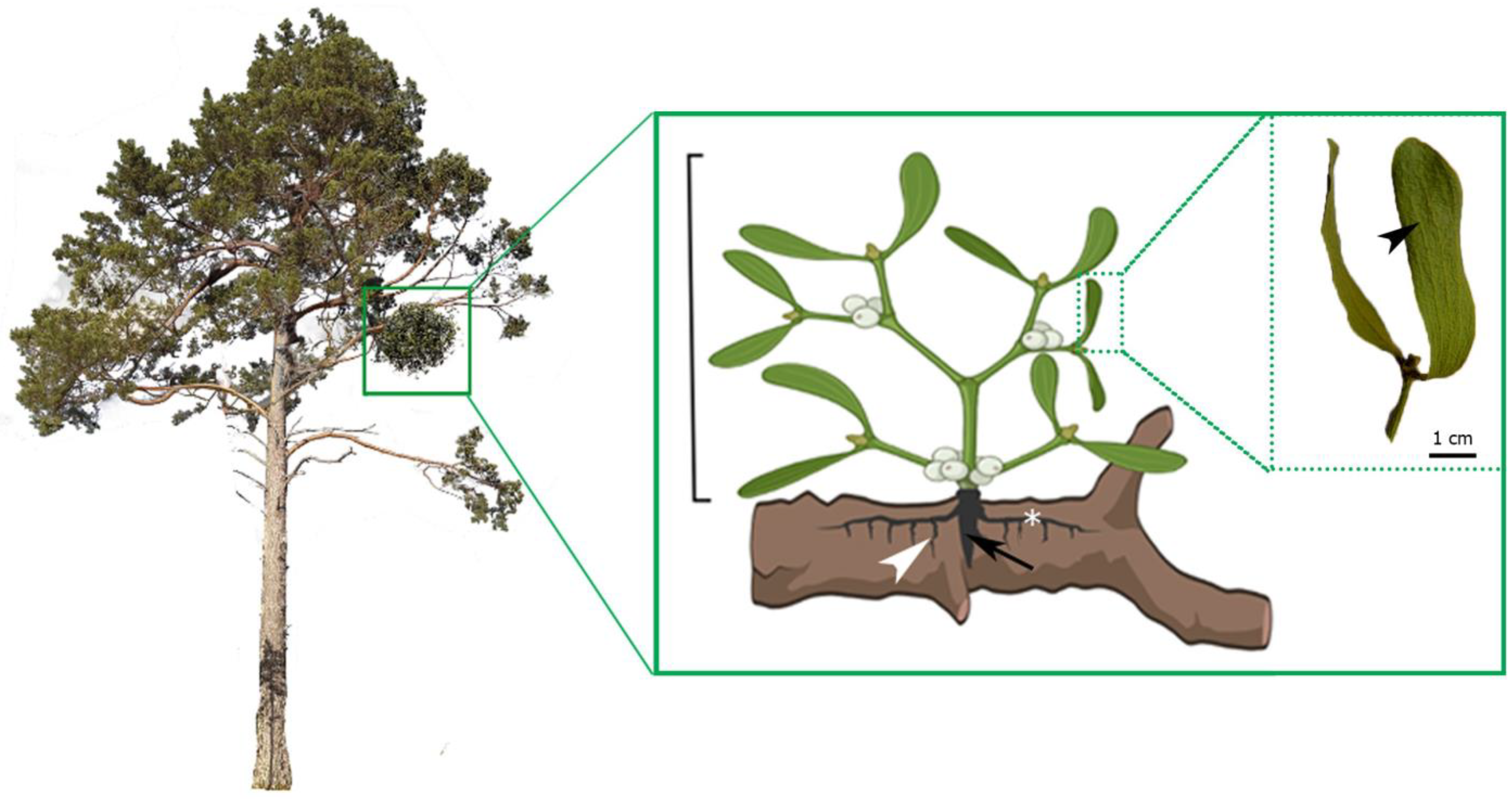
1. Introduction
- The pattern of growth and body organization of the outer system of V. album differs from those of the inner one.
- By adopting the structure–function concept in plants, we postulate that host trees develop structural and physiological adaptations to mistletoe infestation, which is visible at the level of host wood anatomy, especially concerning wood qualitative features.
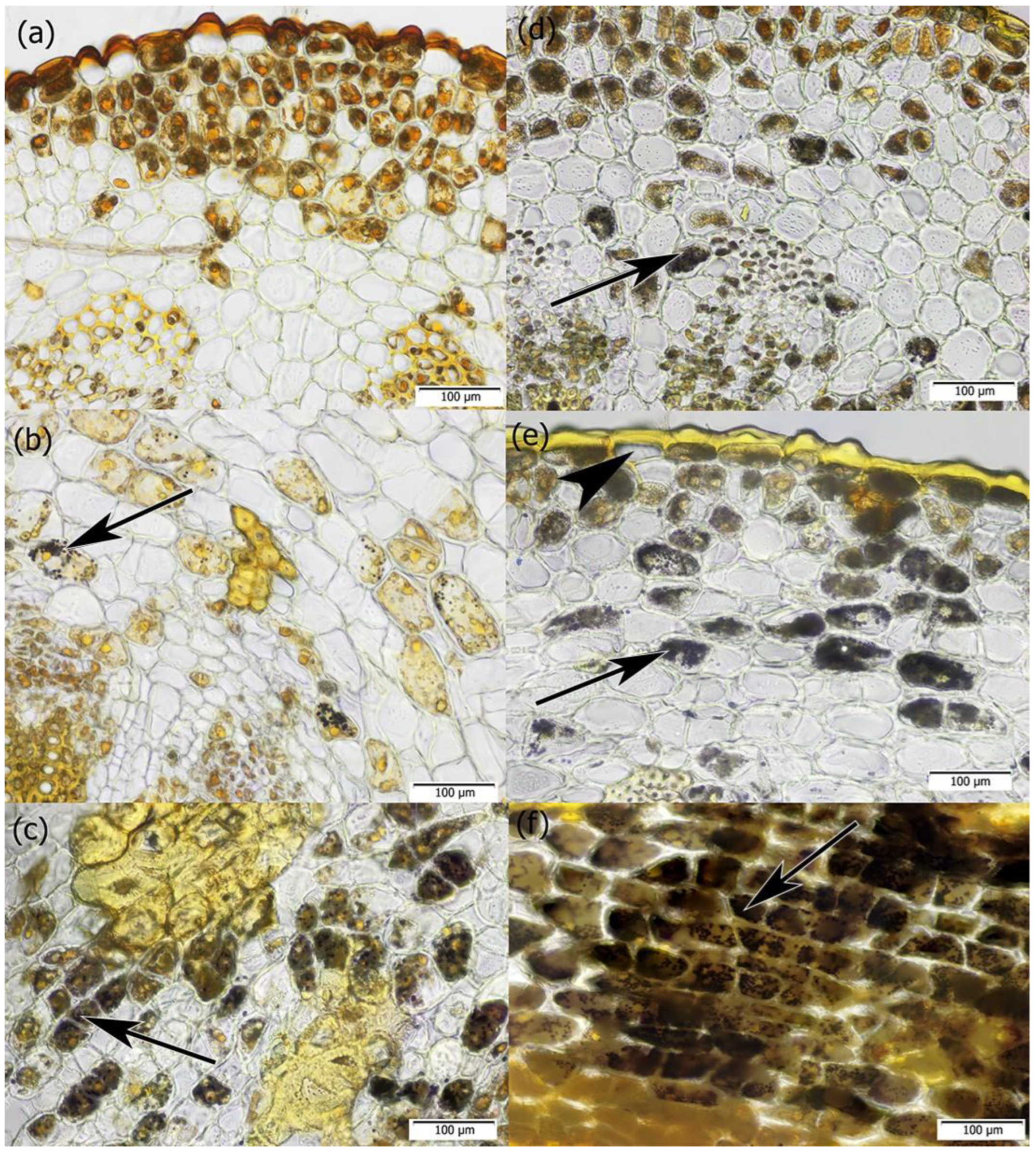
- Starch is stored in the cells of the internal system of mistletoe, and although it is osmotically inert, after hydrolysis it becomes a source of solutes (e.g., glucose) that affect the water potential of mistletoe cells. The reduced water potential of mistletoe cells favors the uptake of water from the host’s xylem sap.
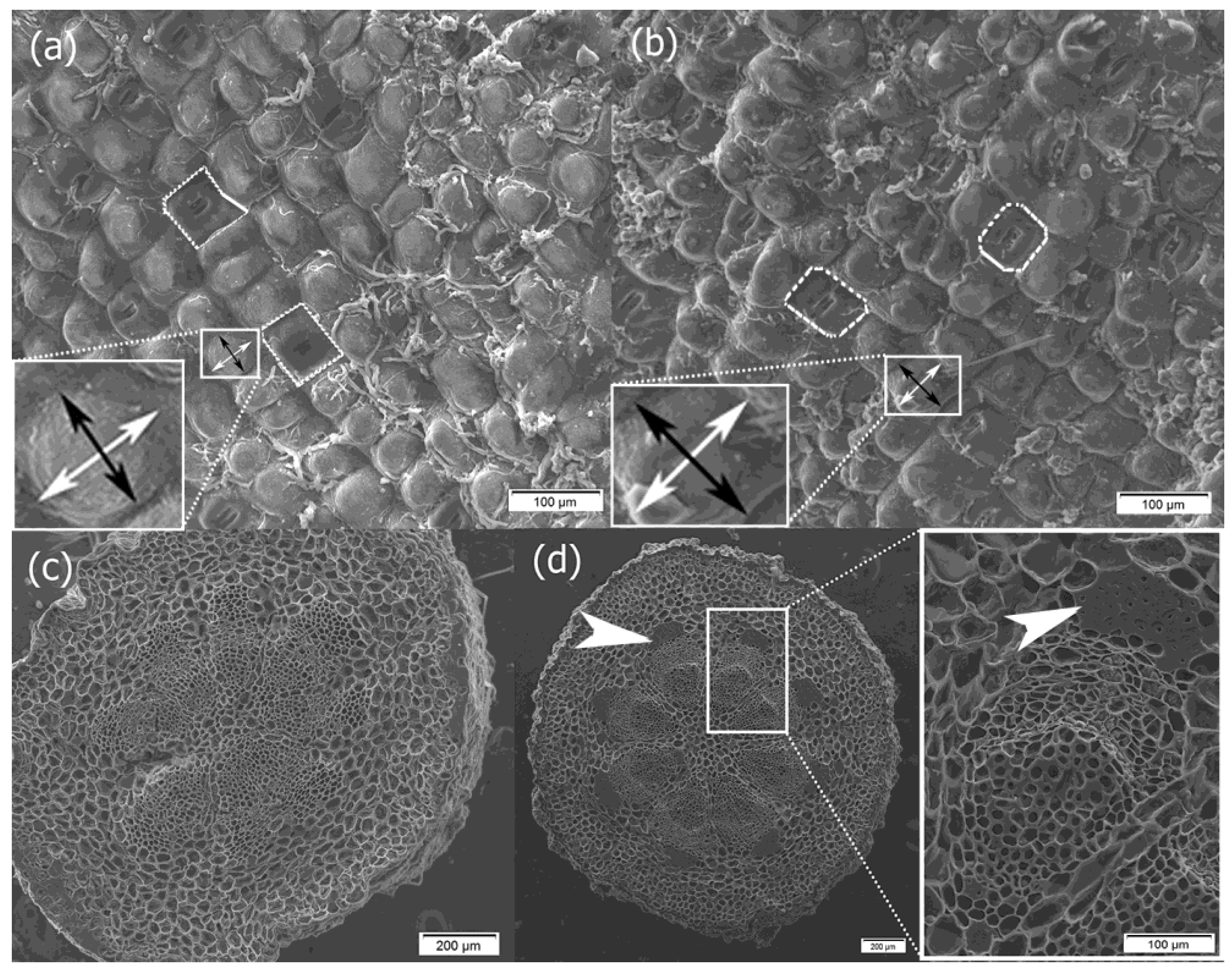
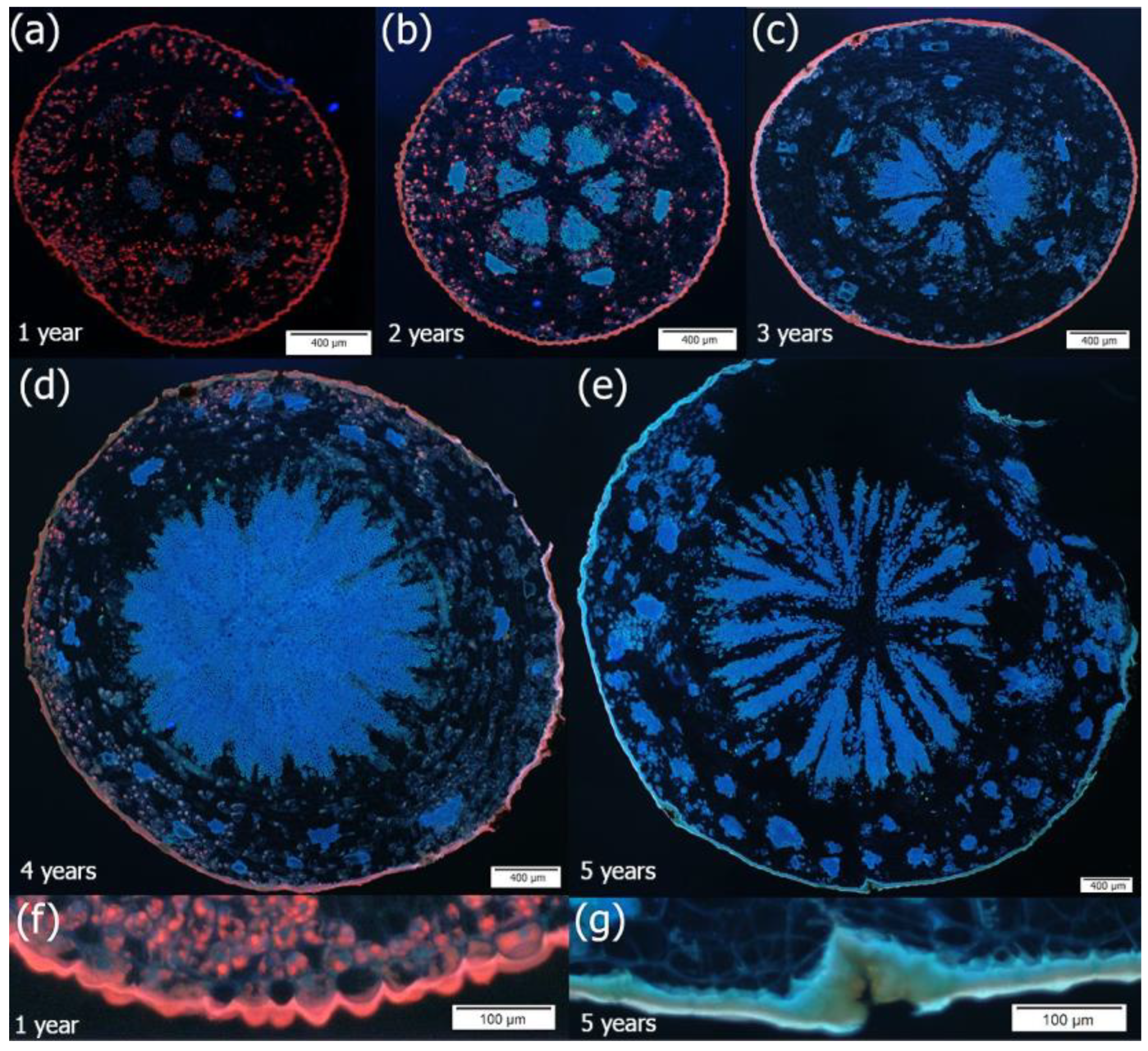
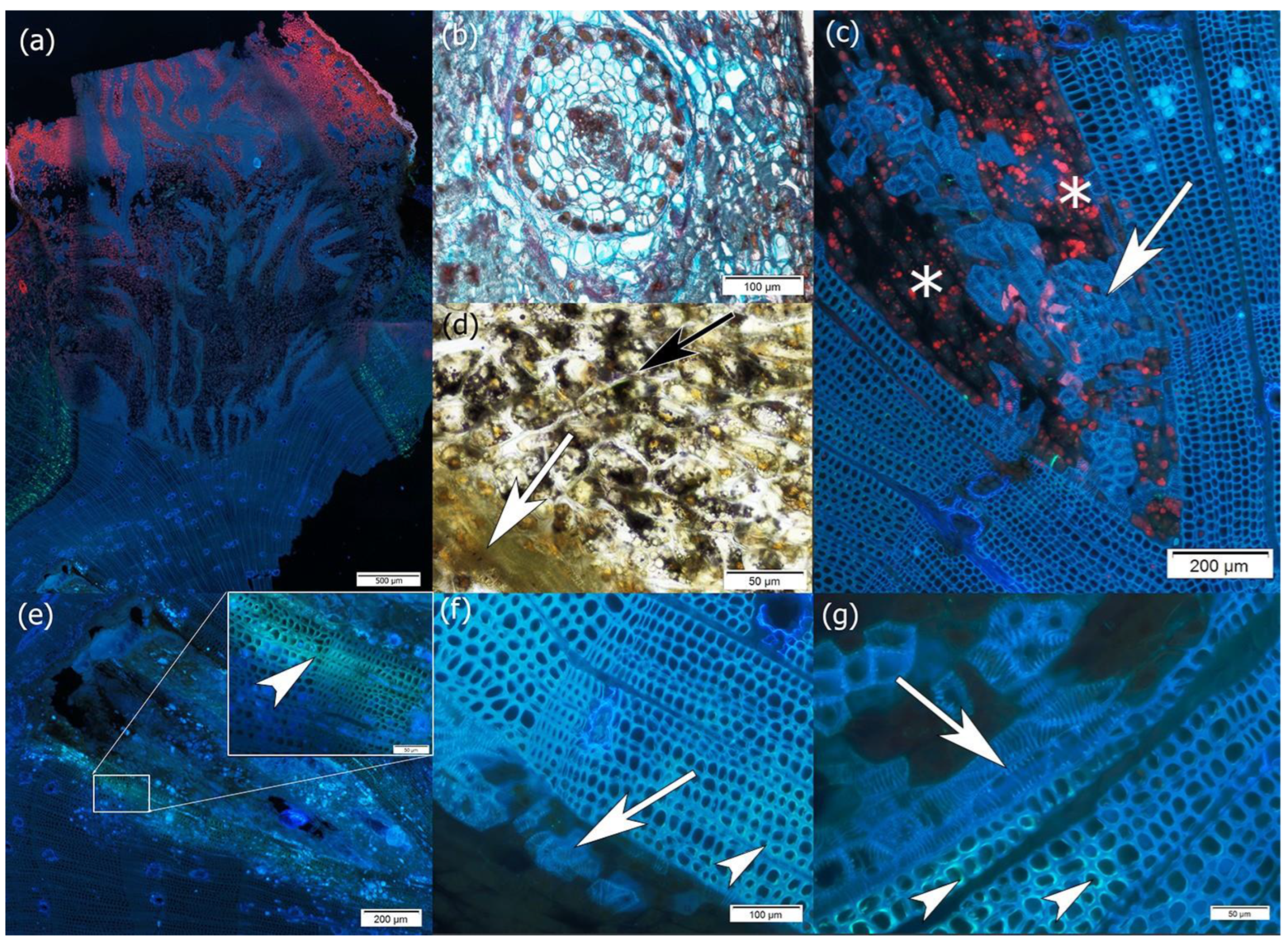
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulik, M. The Anatomical Traits of Trunk Wood and Their Relevance to Oak (Quercus robur L.) Vitality. Eur. J. Forest Res. 2014, 133, 845–855. [Google Scholar] [CrossRef]
- Venturas, M.; López, R.; Martín, J.A.; Gascó, A.; Gil, L. Heritability of Ulmus minor Resistance to Dutch Elm Disease and Its Relationship to Vessel Size, but Not to Xylem Vulnerability to Drought. Plant Pathol. 2014, 63, 500–509. [Google Scholar] [CrossRef]
- George, J.-P.; Sanders, T.G.M.; Timmermann, V.; Potočić, N.; Lang, M. European-Wide Forest Monitoring Substantiate the Neccessity for a Joint Conservation Strategy to Rescue European Ash Species (Fraxinus spp.). Sci. Rep. 2022, 12, 4764. [Google Scholar] [CrossRef] [PubMed]
- Bregant, C.; Batista, E.; Hilário, S.; Linaldeddu, B.T.; Alves, A. Phytophthora Species Involved in Alnus glutinosa Decline in Portugal. Pathogens 2023, 12, 276. [Google Scholar] [CrossRef]
- Tsopelas, P.; Angelopoulos, A.; Economou, A.; Soulioti, N. Mistletoe (Viscum album) in the Fir Forest of Mount Parnis, Greece. For. Ecol. Manag. 2004, 202, 59–65. [Google Scholar] [CrossRef]
- Rigling, A.; Eilmann, B.; Koechli, R.; Dobbertin, M. Mistletoe-Induced Crown Degradation in Scots Pine in a Xeric Environment. Tree Physiol. 2010, 30, 845–852. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Linares, J.C.; Julio Camarero, J. Drought and Mistletoe Reduce Growth and Water-Use Efficiency of Scots Pine. For. Ecol. Manag. 2013, 296, 64–73. [Google Scholar] [CrossRef]
- Mutlu, S.; Ilhan, V.; Turkoglu, H.I. Mistletoe (Viscum album) Infestation in the Scots Pine Stimulates Drought-Dependent Oxidative Damage in Summer. Tree Physiol. 2016, 36, 479–489. [Google Scholar] [CrossRef]
- Lüttge, U. Physiological Ecology of Tropical Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-71793-5. [Google Scholar]
- Ornelas, J.F.; Gándara, E.; Vásquez-Aguilar, A.A.; Ramírez-Barahona, S.; Ortiz-Rodriguez, A.E.; González, C.; Mejía Saules, M.T.; Ruiz-Sanchez, E. A Mistletoe Tale: Postglacial Invasion of Psittacanthus Schiedeanus (Loranthaceae) to Mesoamerican Cloud Forests Revealed by Molecular Data and Species Distribution Modeling. BMC Evol. Biol. 2016, 16, 78. [Google Scholar] [CrossRef]
- Reblin, J.S.; Logan, B.A.; Tissue, D.T. Impact of Eastern Dwarf Mistletoe (Arceuthobium pusillum) Infection on the Needles of Red Spruce (Picea rubens) and White Spruce (Picea glauca): Oxygen Exchange, Morphology and Composition. Tree Physiol. 2006, 26, 1325–1332. [Google Scholar] [CrossRef]
- Glatzel, G.; Geils, B.W. Mistletoe Ecophysiology: Host–Parasite interactions. This Review Is One of a Collection of Papers Based on a Presentation from the Stem and Shoot Fungal Pathogens and Parasitic Plants: The Values of Biological Diversity Session of the XXII International Union of Forestry Research Organization World Congress Meeting Held in Brisbane, Queensland, Australia, in 2005. Botany 2009, 87, 10–15. [Google Scholar] [CrossRef]
- Thomas, P.A.; Dering, M.; Giertych, M.J.; Iszkuło, G.; Tomaszewski, D.; Briggs, J. Biological Flora of Britain and Ireland: Viscum album. J. Ecol. 2022, 111, 701–739. [Google Scholar] [CrossRef]
- Marshall, J.D.; Ehleringer, J.R.; Schulze, E.-D.; Farquhar, G. Carbon Isotope Composition, Gas Exchange and Heterotrophy in Australian Mistletoes. Funct. Ecol. 1994, 8, 237–241. [Google Scholar] [CrossRef]
- Lüttge, U.; Haridasan, M.; Fernandes, G.W.; de Mattos, E.A.; Trimborn, P.; Franco, A.C.; Caldas, L.S.; Ziegler, H. Photosynthesis of Mistletoes in Relation to Their Hosts at Various Sites in Tropical Brazil. Trees 1998, 12, 167–174. [Google Scholar] [CrossRef]
- Zweifel, R.; Bangerter, S.; Rigling, A.; Sterck, F.J. Pine and Mistletoes: How to Live with a Leak in the Water Flow and Storage System? J. Exp. Bot. 2012, 63, 2565–2578. [Google Scholar] [CrossRef]
- Scalon, M.C.; Wright, I.J. Leaf Trait Adaptations of Xylem-Tapping Mistletoes and Their Hosts in Sites of Contrasting Aridity. Plant Soil. 2017, 415, 117–130. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Ehleringer, J.R. The Effect of Nitrogen Supply on Growth and Water-Use Efficiency of Xylem-Tapping Mistletoes. Planta 1984, 162, 268–275. [Google Scholar] [CrossRef]
- Escher, P.; Peuke, A.D.; Bannister, P.; Fink, S.; Hartung, W.; Jiang, F.; Rennenberg, H. Transpiration, CO2 Assimilation, WUE, and Stomatal Aperture in Leaves of Viscum album (L.): Effect of Abscisic Acid (ABA) in the Xylem Sap of Its Host (Populus x euamericana). Plant Physiol. Biochem. 2008, 46, 64–70. [Google Scholar] [CrossRef]
- Scalon, M.C.; Rossatto, D.R.; Domingos, F.M.C.B.; Franco, A.C. Leaf Morphophysiology of a Neotropical Mistletoe Is Shaped by Seasonal Patterns of Host Leaf Phenology. Oecologia 2016, 180, 1103–1112. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Woodruff, D.R.; Shaw, D.C. Integrated Responses of Hydraulic Architecture, Water and Carbon Relations of Western Hemlock to Dwarf Mistletoe Infection. Plant Cell Environ. 2004, 27, 937–946. [Google Scholar] [CrossRef]
- Bilgili, E.; Alperen Coşkuner, K.; Öztürk, M. Leaf Area—Sapwood Area Relationship in Scots Pine (Pinus sylvestris L.) under Mistletoe (Viscum slbum Ssp. austriacum) Infection. Dendrobiology 2020, 84, 1–11. [Google Scholar] [CrossRef]
- Yan, C.-F.; Gessler, A.; Rigling, A.; Dobbertin, M.; Han, X.-G.; Li, M.-H. Effects of Mistletoe Removal on Growth, N and C Reserves, and Carbon and Oxygen Isotope Composition in Scots Pine Hosts. Tree Physiol. 2016, 36, 562–575. [Google Scholar] [CrossRef]
- Dobbertin, M.; Hilker, N.; Rebetez, M.; Zimmermann, N.E.; Wohlgemuth, T.; Rigling, A. The Upward Shift in Altitude of Pine Mistletoe (Viscum album ssp. austriacum) in Switzerland—The Result of Climate Warming? Int. J. Biometeorol. 2005, 50, 40–47. [Google Scholar] [CrossRef]
- Hu, B.; Sakakibara, H.; Takebayashi, Y.; Peters, F.S.; Schumacher, J.; Eiblmeier, M.; Arab, L.; Kreuzwieser, J.; Polle, A.; Rennenberg, H. Mistletoe Infestation Mediates Alteration of the Phytohormone Profile and Anti-Oxidative Metabolism in Bark and Wood of Its Host Pinus Sylvestris. Tree Physiol. 2017, 37, 676–691. [Google Scholar] [CrossRef]
- Devkota, M. Biology of Mistletoes and Their Status in Nepal Himalaya. Himal. J. Sci. Vol. 2005, 3, 85–88. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Nickrent, D.L.; Shaw, D.C.; Watson, D.M. Mistletoes: Pathology, Systematics, Ecology, and Management. Plant Dis. 2008, 92, 988–1006. [Google Scholar] [CrossRef]
- Pilichowski, S.; Filip, R.; Koscielska, A.; Zaroffe, G.; Zyzniewska, A.; Iszkulo, G. Wpływ Viscum album ssp. austriacum (Wiesb.) Vollm. na przyrost radialny Pinus sylvestris L. Sylwan 2018, 162, 452–459. [Google Scholar]
- Srivastava, L.M.; Esau, K. Relation of Dwarfmistletoe (Arceuthobium) to the Xylem Tissue of Conifers. Ii. Effect of the Parasite on the Xylem Anatomy of the Host12. Am. J. Bot. 1961, 48, 209–215. [Google Scholar] [CrossRef]
- Ozturk, M.; Coskuner, K.A.; Usta, Y.; Serdar, B.; Bilgili, E. The Effect of Mistletoe (Viscum album) on Branch Wood and Needle Anatomy of Scots Pine (Pinus sylvestris). IAWA J. 2019, 40, 352–365. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of Parasitic Plants on Natural Communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef]
- Arruda, R.; Lunardelli, C.; Kitagawa, C.; Caires, C.S.; Teodoro, G.S.; Mourão, F.A. Two Mistletoes Are Too Many?: Interspecific Occurrence of Mistletoes on the Same Host Tree. Acta Bot. Bras. 2013, 27, 226–230. [Google Scholar] [CrossRef]
- Jasiczek, N.; Giertych, M.J.; Suszka, J. Wpływ Jemioły (Viscum album) Na Jakość Nasion Sosny Zwyczajnej (Pinus sylvestris). Sylwan 2017, 161, 558–564. [Google Scholar]
- Iszkuło, G.; Armatys, L.; Dering, M.; Ksepko, M.; Tomaszewski, D.; Ważna, A.; Giertych, M.J. Jemioła Jako Zagrożenie Dla Zdrowotności Drzewostanów Iglastych. Sylwan 2020, 164, 226–236. [Google Scholar]
- Pennings, S.C.; Callaway, R.M. Parasitic Plants: Parallels and Contrasts with Herbivores. Oecologia 2002, 131, 479–489. [Google Scholar] [CrossRef]
- Kuijt, J. Haustoria of Phanerogamic Parasites. Annu. Rev. Phytopathol. 1977, 17, 91–118. [Google Scholar] [CrossRef]
- Anselmo-Moreira, F.; Teixeira-Costa, L.; Ceccantini, G.; Furlan, C.M. Mistletoe Effects on the Host Tree Tapirira Guianensis: Insights from Primary and Secondary Metabolites. Chemoecology 2019, 29, 11–24. [Google Scholar] [CrossRef]
- Lázaro-González, A.; Hódar, J.A.; Zamora, R. Mistletoe Versus Host Pine: Does Increased Parasite Load Alter the Host Chemical Profile? J. Chem. Ecol. 2019, 45, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Noetzli, K.P.; Müller, B.; Sieber, T.N. Impact of Population Dynamics of White Mistletoe (Viscum album ssp. abietis) on European Silver Fir (Abies alba). Ann. For. Sci. 2003, 60, 773–779. [Google Scholar] [CrossRef]
- Szmidla, H.; Tkaczyk, M.; Plewa, R.; Tarwacki, G.; Sierota, Z. Impact of Common Mistletoe (Viscum album L.) on Scots Pine Forests—A Call for Action. Forests 2019, 10, 847. [Google Scholar] [CrossRef]
- Gibson, C.C.; Watkinson, A.R. Host Selectivity and the Mediation of Competition by the Root Hemiparasite Rhinanthus Minor. Oecologia 1991, 86, 81–87. [Google Scholar] [CrossRef]
- Fisher, J.P.; Phoenix, G.K.; Childs, D.Z.; Press, M.C.; Smith, S.W.; Pilkington, M.G.; Cameron, D.D. Parasitic Plant Litter Input: A Novel Indirect Mechanism Influencing Plant Community Structure. New Phytol. 2013, 198, 222–231. [Google Scholar] [CrossRef]
- Mellado, A.; Zamora, R. Parasites Structuring Ecological Communities: The Mistletoe Footprint in Mediterranean Pine Forests. Funct. Ecol. 2017, 31, 2167–2176. [Google Scholar] [CrossRef]
- Hawksworth, F.G. Dwarf Mistletoes: Biology, Pathology, and Systematics; U.S. Department of Agriculture, Forest Service: Washington, WA, USA, 1996.
- Zuber, D. Biological Flora of Central Europe: Viscum album L. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 181–203. [Google Scholar] [CrossRef]
- Watson, D.M. On Tropical Mistletoes: Tractable Models for Evolutionary Ecology, Ecosystem Function, and Phytochemistry. Botany 2017, 95, 211–217. [Google Scholar] [CrossRef]
- Lev, E.; Ephraim, M.; Ben-Arye, E. European and Oriental Mistletoe: From Mythology to Contemporary Integrative Cancer Care. Eur. J. Integr. Med. 2011, 3, e133–e137. [Google Scholar] [CrossRef]
- Bach, C.E.; Kelly, D.; Hazlett, B.A. Forest Edges Benefit Adults, but Not Seedlings, of the Mistletoe Alepis Flavida (Loranthaceae). J. Ecol. 2005, 93, 79–86. [Google Scholar] [CrossRef]
- Walas, Ł.; Kędziora, W.; Ksepko, M.; Rabska, M.; Tomaszewski, D.; Thomas, P.A.; Wójcik, R.; Iszkuło, G. The Future of Viscum album L. in Europe Will Be Shaped by Temperature and Host Availability. Sci. Rep. 2022, 12, 17072. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How Much Does Climate Change Threaten European Forest Tree Species Distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H.S. Parasitic Flowering Plants–Brill; Brill: Leiden-Boston, The Netherlands, 2008. [Google Scholar]
- Elkiran, O. Viscum album subsp. album L. ve Viscum album subsp. austriacum (Wiesb.) Vollman’ın karşılaştırmalı anatomi çalışması. Ejons Int. J. Math. Eng. Nat. Sci. 2022, 6, 12–17. [Google Scholar]
- de Almeida, V.P.; Monchak, I.T.; da Costa Batista, J.V.; Grazi, M.; Ramm, H.; Raman, V.; Baumgartner, S.; Holandino, C.; Manfron, J. Investigations on the Morpho-Anatomy and Histochemistry of the European Mistletoe: Viscum album L. subsp. album. Sci. Rep. 2023, 13, 4604. [Google Scholar] [CrossRef]
- Kollas, C.; Gutsch, M.; Hommel, R.; Lasch-Born, P.; Suckow, F. Mistletoe-Induced Growth Reductions at the Forest Stand Scale. Tree Physiol. 2018, 38, 735–744. [Google Scholar] [CrossRef]
- Ribeiro, C.; Stitt, M.; Hotta, C.T. How Stress Affects Your Budget—Stress Impacts on Starch Metabolism. Front. Plant Sci. 2022, 13, 774060. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Darwin, F. The Statolith-Theory of Geotropism. Nature 1903, 67, 571–572. [Google Scholar] [CrossRef]
- Iversen, T.-H.; Rommelhoff, A. The Starch Statolith Hypothesis and the Interaction of Amyloplasts and Endoplasmic Reticulum in Root Geotropism. J. Exp. Bot. 1978, 29, 1319–1328. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Guisinger, M.M.; Miller, A.J.; Stackhouse, K.S. Reduced Gravitropism in Hypocotyls of Starch-Deficient Mutants of Arabidopsis. Plant Cell Physiol. 1997, 38, 518–525. [Google Scholar] [CrossRef]
- Fujihira, K.; Kurata, T.; Watahiki, M.K.; Karahara, I.; Yamamoto, K.T. An Agravitropic Mutant of Arabidopsis, Endodermal-Amyloplast Less 1, That Lacks Amyloplasts in Hypocotyl Endodermal Cell Layer. Plant Cell Physiol. 2000, 41, 1193–1199. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z.; Herranz, R.; Medina, F.J. Light and Gravity Signals Synergize in Modulating Plant Development. Front. Plant Sci. 2014, 5, 563. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a Source, Starch as a Sink: The Bifunctional Role of Starch in Carbon Allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The Formation and Functions of a Fundamental Plant Tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K.C. The Formation and Function of Plant Cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Savaldi-Goldstein, S.; Peto, C.; Chory, J. The Epidermis Both Drives and Restricts Plant Shoot Growth. Nature 2007, 446, 199–202. [Google Scholar] [CrossRef]
- Butterfass, T. The Transverse Orientation of Stomata. Bot. Rev. 1987, 53, 415–441. [Google Scholar] [CrossRef]
- Khan, M.A.; Sharif, T.; Ahmad, M.; Zafar, M.; Tareen, R.B. Anatomical Characterization of Parasitic Plants of Pakistan. Pak. J. Bot. 2009, 41, 2661–2669. [Google Scholar]
- Caspar, T.; Pickard, B.G. Gravitropism in a Starchless Mutant of Arabidopsis. Planta 1989, 177, 185–197. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Kossmann, J. Transitory and Storage Starch Metabolism: Two Sides of the Same Coin? Curr. Opin. Biotechnol. 2015, 32, 143–148. [Google Scholar] [CrossRef]
- Kiss, J.Z. Statoliths Function in Gravity Perception in Plants: Yes, No, Yes! Planta 2025, 261, 45. [Google Scholar] [CrossRef]
- Wayne, R.; Staves, M.P. A Down-to-Earth Model of Gravisensing. Gravit. Space Biol. Bull. 1997, 10, 57–64. [Google Scholar]
- Krasensky, J.; Jonak, C. Drought, Salt, and Temperature Stress-Induced Metabolic Rearrangements and Regulatory Networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Pate, J.S.; True, K.C.; Rasins, E. Xylem Transport and Storage of Amino Acids by S.W. Australian Mistletoes and Their Hosts. J. Exp. Bot. 1991, 42, 441–451. [Google Scholar] [CrossRef]
- Wang, A.; Bose, A.K.; Lehmann, M.M.; Rigling, A.; Gessler, A.; Yu, L.; Li, M. Water Status and Macronutrient Concentrations, but Not Carbon Status, of Viscum album ssp. album Are Determined by Its Hosts: A Study across Nine Mistletoe–Host Pairs in Central Switzerland. Front. Plant Sci. 2023, 14, 1142760. [Google Scholar] [CrossRef] [PubMed]
- Pfanz, H.; Aschan, G.; Langenfeld-Heyser, R.; Wittmann, C.; Loose, M. Ecology and Ecophysiology of Tree Stems: Corticular and Wood Photosynthesis. Naturwissenschaften 2002, 89, 147–162. [Google Scholar] [CrossRef]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, Fate and Significance of CO2 in Tree Stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef]
- Ávila, E.; Herrera, A.; Tezara, W. Contribution of Stem CO2 Fixation to Whole-Plant Carbon Balance in Nonsucculent Species. Photosynthetica 2014, 52, 3–15. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H. More than Just CO2-Recycling: Corticular Photosynthesis as a Mechanism to Reduce the Risk of an Energy Crisis Induced by Low Oxygen. New Phytol. 2018, 219, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Wiedenhoeft, A.C. On the Possible Functions of Helical Thickenings in Conductive Cells in Wood. IAWA J. 2023, 44, 381–398. [Google Scholar] [CrossRef]
- Timell, T.E. Compression Wood in Gymnosperms; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Kurczyńska, E.U.; Dmuchowski, W.; Włoch, W.; Bytnerowicz, A. The Influence of Air Pollutants on Needles and Stems of Scots Pine (Pinus sylvestris L.) Trees. Environ. Pollut. 1997, 98, 325–334. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Kim, J.-Y. Callose Synthesis in Higher Plants. Plant Signal. Behav. 2009, 4, 489–492. [Google Scholar] [CrossRef]
- Ellinger, D.; Voigt, C.A. Callose Biosynthesis in Arabidopsis with a Focus on Pathogen Response: What We Have Learned within the Last Decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and Function of Defense-Related Callose Deposition in Plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, Z.; Yu, P.; Zeng, Y.; Du, S.; Huang, L.-J. The Multifarious Role of Callose and Callose Synthase in Plant Development and Environment Interactions. Front. Plant Sci. 2023, 14, 1183402. [Google Scholar] [CrossRef]
- Stass, A.; Horst, W.J. Chapter 4.4.4—Callose in Abiotic Stress. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 499–524. ISBN 978-0-12-373971-1. [Google Scholar]
- Jacobs, A.K.; Lipka, V.; Burton, R.A.; Panstruga, R.; Strizhov, N.; Schulze-Lefert, P.; Fincher, G.B. An Arabidopsis Callose Synthase, GSL5, Is Required for Wound and Papillary Callose Formation. Plant Cell 2003, 15, 2503–2513. [Google Scholar] [CrossRef]
- Nishimura, M.T.; Stein, M.; Hou, B.-H.; Vogel, J.P.; Edwards, H.; Somerville, S.C. Loss of a Callose Synthase Results in Salicylic Acid-Dependent Disease Resistance. Science 2003, 301, 969–972. [Google Scholar] [CrossRef]
- Pate, J.S. Haustoria in Action: Case Studies of Nitrogen Acquisition by Woody Xylem-Tapping Hemiparasites from Their Hosts. Protoplasma 2001, 215, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Muche, M.; Muasya, A.M.; Tsegay, B.A. Biology and Resource Acquisition of Mistletoes, and the Defense Responses of Host Plants. Ecol. Process 2022, 11, 24. [Google Scholar] [CrossRef]
- Klutsch, J.G.; Erbilgin, N. Dwarf Mistletoe Infection in Jack Pine Alters Growth–Defense Relationships. Tree Physiol. 2018, 38, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Ferrenberg, S. Dwarf Mistletoe Infection Interacts with Tree Growth Rate to Produce Opposing Direct and Indirect Effects on Resin Duct Defenses in Lodgepole Pine. Forests 2020, 11, 222. [Google Scholar] [CrossRef]
- Wojcik, R.; Wikalinski, M.; Kedziora, W. Ocena występowania jemioły pospolitej (Viscum album L.) na sośnie zwyczajnej (Pinus sylvestris L.) w Nadleśnictwie Kozienice. Sylwan 2021, 165, 3–8. [Google Scholar] [CrossRef]
- Etzold, H. Simultanfärbung von Pflanzenschnitten Mit Fuchsin, Chrysoidin Und Astrablau. Mikrokosmos 2002, 91, 316–318. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dołkin-Lewko, A.; Pulat, E.; Wójcik, R.; Yaman, B.; Zajączkowska, U.; Oszako, T.; Tulik, M. Distinctive Traits of European Mistletoe (Viscum album spp. austriacum) and Its Impact on Host Tree Wood (Pinus sylvestris). Plants 2025, 14, 1489. https://doi.org/10.3390/plants14101489
Dołkin-Lewko A, Pulat E, Wójcik R, Yaman B, Zajączkowska U, Oszako T, Tulik M. Distinctive Traits of European Mistletoe (Viscum album spp. austriacum) and Its Impact on Host Tree Wood (Pinus sylvestris). Plants. 2025; 14(10):1489. https://doi.org/10.3390/plants14101489
Chicago/Turabian StyleDołkin-Lewko, Alicja, Esra Pulat, Roman Wójcik, Barbaros Yaman, Urszula Zajączkowska, Tomasz Oszako, and Mirela Tulik. 2025. "Distinctive Traits of European Mistletoe (Viscum album spp. austriacum) and Its Impact on Host Tree Wood (Pinus sylvestris)" Plants 14, no. 10: 1489. https://doi.org/10.3390/plants14101489
APA StyleDołkin-Lewko, A., Pulat, E., Wójcik, R., Yaman, B., Zajączkowska, U., Oszako, T., & Tulik, M. (2025). Distinctive Traits of European Mistletoe (Viscum album spp. austriacum) and Its Impact on Host Tree Wood (Pinus sylvestris). Plants, 14(10), 1489. https://doi.org/10.3390/plants14101489






