Drought-Driven Divergence in Photosynthetic Performance Between Two Cunninghamia lanceolata Provenances: Insights from Gas Exchange and Chlorophyll Fluorescence Dynamics
Abstract
:1. Introduction
2. Results
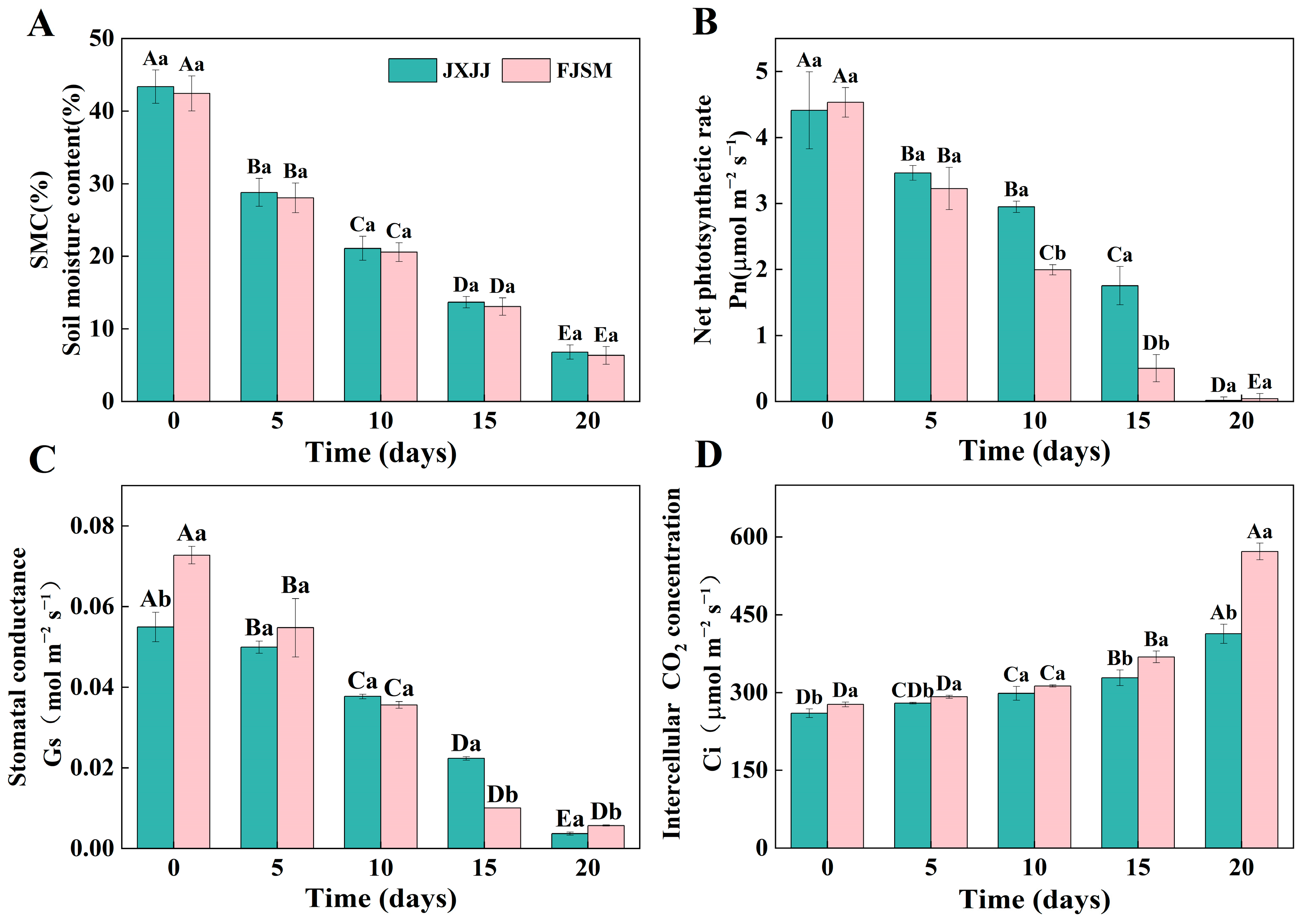
2.1. Soil Moisture Content and Gas Exchange Parameters
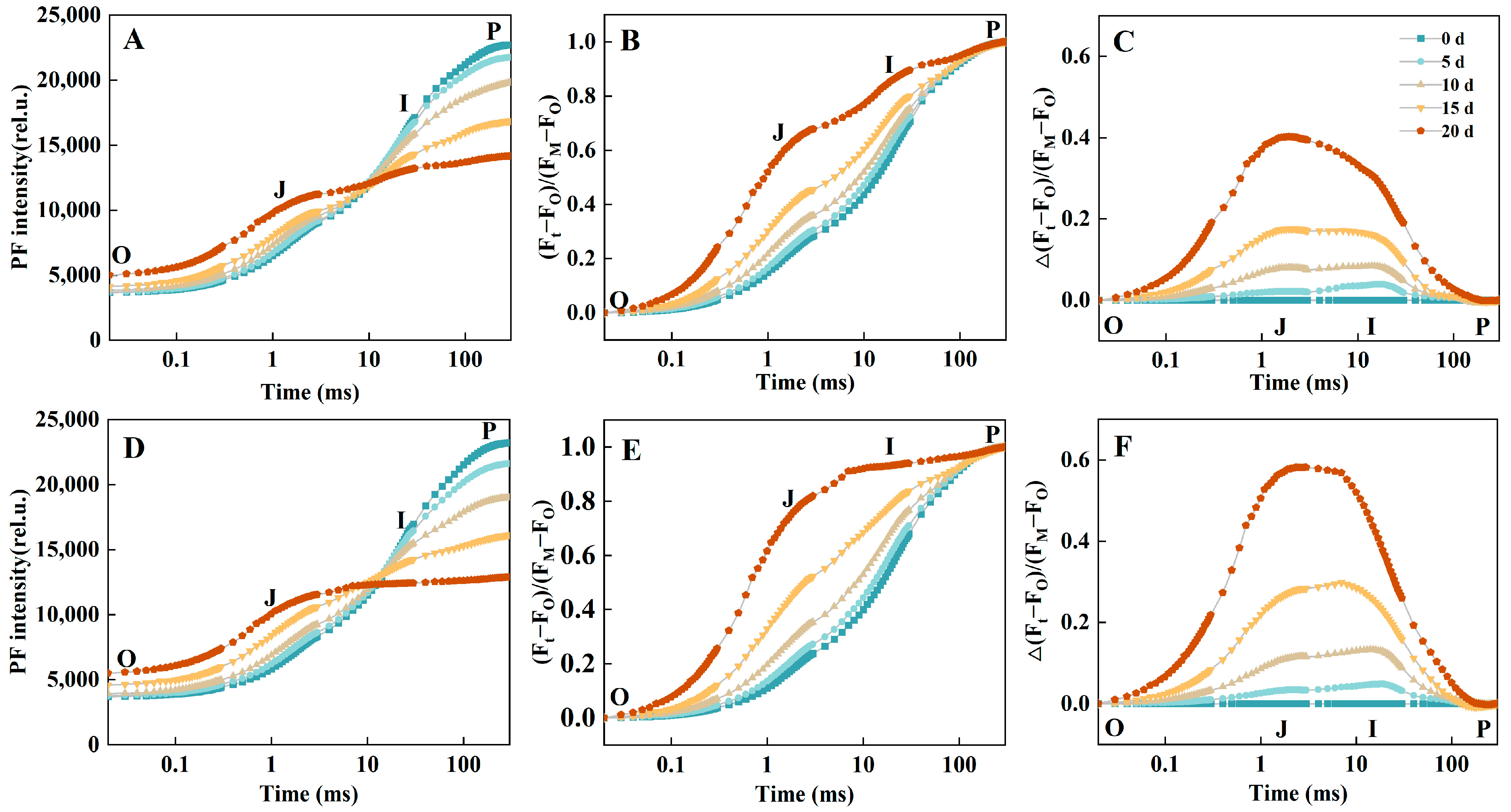
2.2. Prompt Fluorescence OJIP Transient Analysis
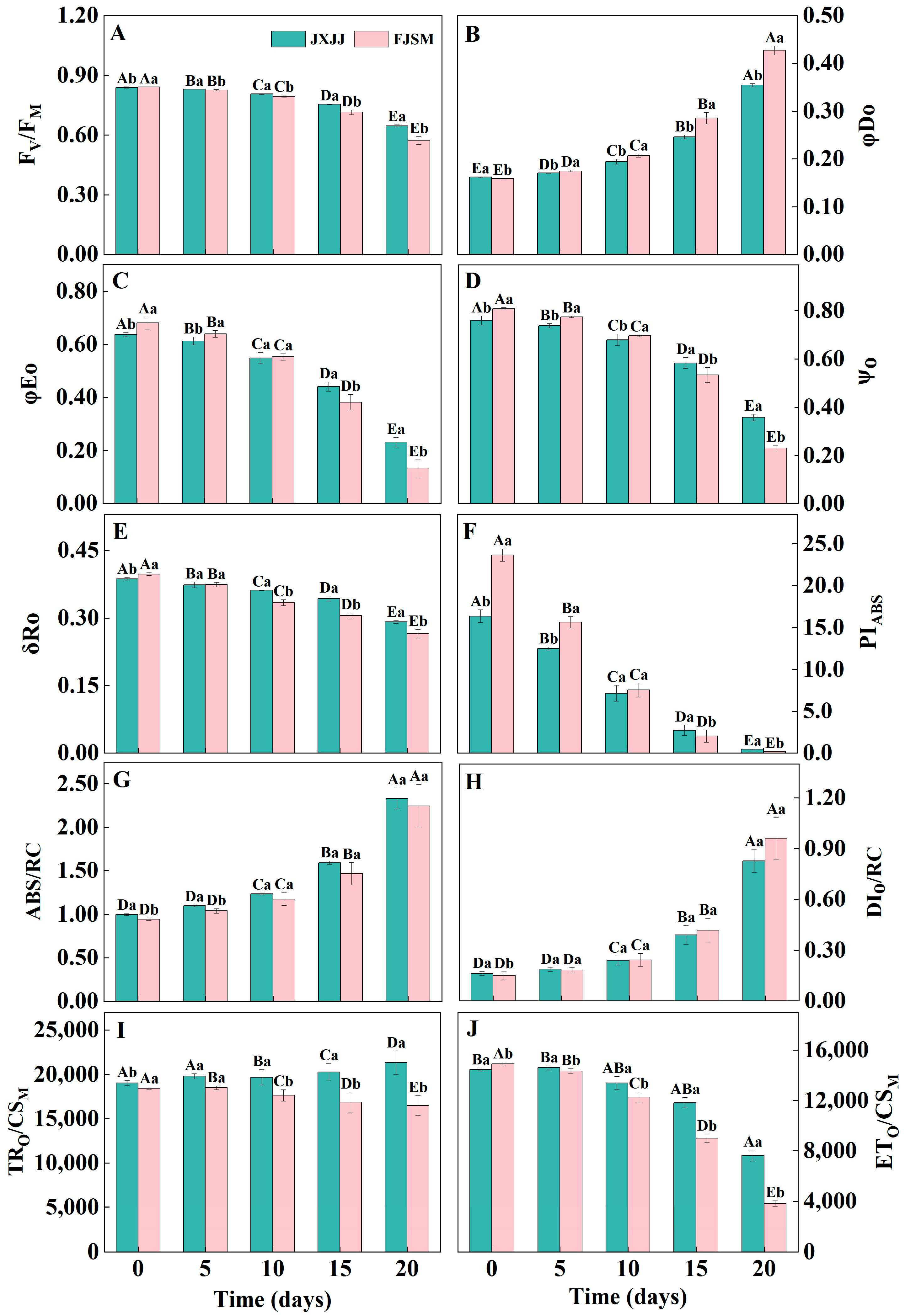
2.3. JIP-Test
2.4. MR/MR0 Transient Analysis
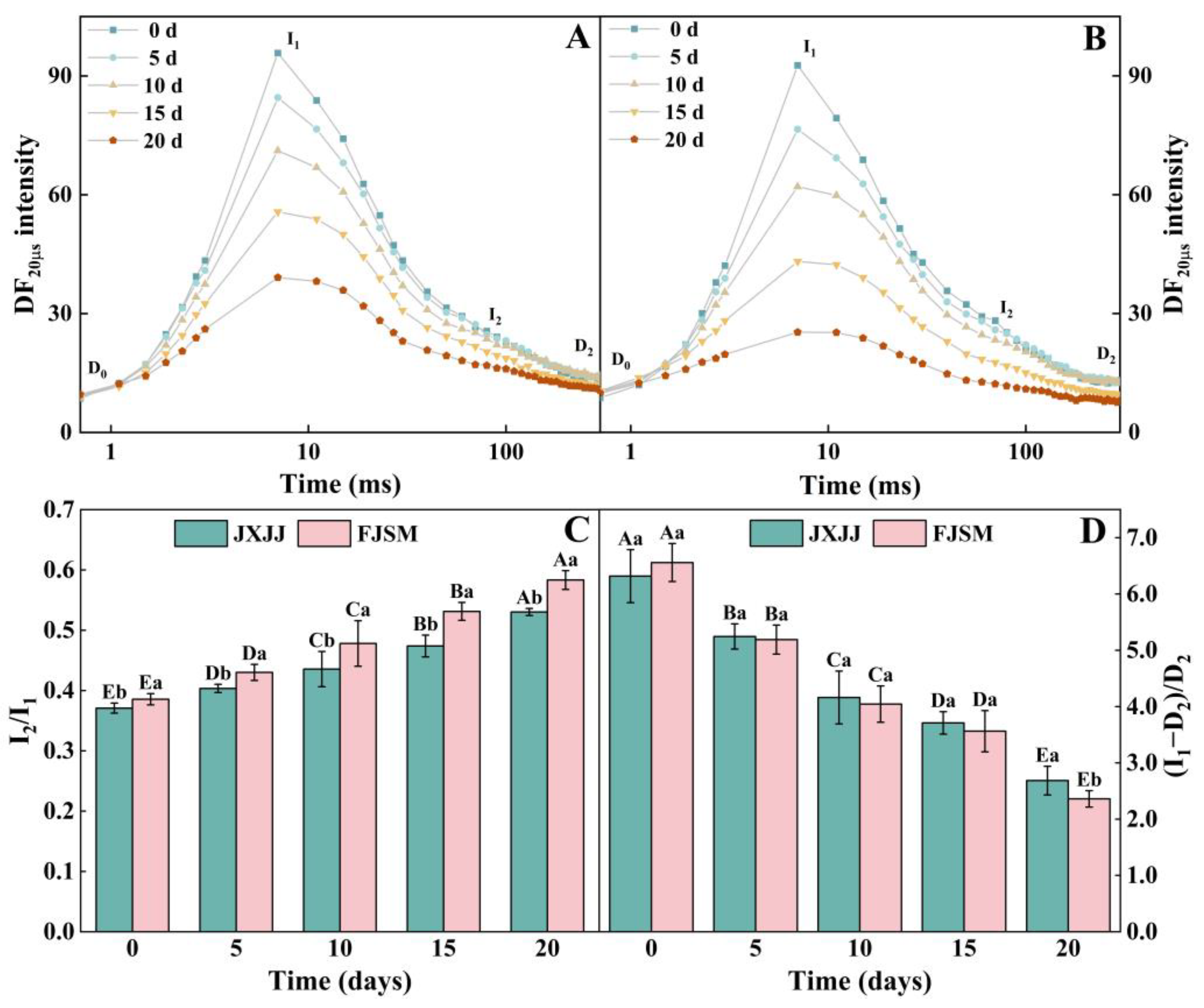
2.5. DF Induction and Decay Transient Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment
4.2. Soil Moisture Content and Gas Exchange Measurement
4.3. Simultaneous Measurements of the Kinetics of PF, DF, and MR
4.4. MR and DF Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohsin, N.; Jianfan, S.; Samina, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar]
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Jiang, Y.F. Physiological and Structural Changes in the Root System of Seeds and Seedlings of Amorpha fruticosa Under PEG-6000 Stress. Master’s Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Rabara, R.C.; Tripathi, P.; Reese, R.N.; Rushton, D.L.; Alexander, D.; Timko, M.P.; Shen, Q.J.; Rushton, P.J. Tobacco drought stress responses reveal new targets for Solanaceae crop improvement. BMC Genom. 2015, 16, 1–23. [Google Scholar] [CrossRef]
- Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.A.; Alhejeli, A.M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 339–348. [Google Scholar] [CrossRef]
- State Forestry Administration. China Forest Resources Report; State Forestry Administration: Beijing, China, 2019. [Google Scholar]
- Wang, C.; Liu, S.; Zhu, Y.; Smith, A.R.; Ning, Y.; Deng, D. Aboveground carbon sequestration of Cunninghamia lanceolata forests: Magnitude and drivers. For. Ecosyst. 2024, 11, 100165. [Google Scholar] [CrossRef]
- Zhang, J.M.; Liu, Y.F.; Wu, H.; Zhang, J.; Zhao, H. Characteristics of atmospheric circulation anomalies and drought in summer and autumn in Hunan Province. J. Arid Meteorol. 2018, 36, 353. [Google Scholar]
- Yuan, W.; Cai, W.; Chen, Y.; Liu, S.; Dong, W.; Zhang, H.; Yu, G.; Chen, Z.; He, H.; Guo, W.; et al. Severe summer heatwave and drought strongly reduced carbon uptake in southern China. Sci. Rep. 2016, 6, 18813. [Google Scholar] [CrossRef]
- Aiqin, L.; Xiangqing, M.; Lizhen, F. A study on physiological responses of different Chinese fir clones under water stress. J. Fujian Coll. For. 1998, 18, 28–31. [Google Scholar]
- Liu, X.; Zhong, H. Resistance response of Cunninghamia lanceolata seedlings from different provenances to drought stress. Shanxi Froest Sci. Technol. 2023, 51, 15–19. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Cui, Y.; Pan, C.; Ma, L.; Zou, C.; Niu, F.; Zhang, G. The differential responses of gas exchange and evapotranspiration to experimental drought for seedlings of two typical tree species on the Loess Plateau. Ecol. Indic. 2024, 167, 112721. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Kalaji, M.H.; Baczewska, A.H.; Pawluśkiewicz, B.; Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Paunov, M.; Goltsev, V. Delayed chlorophyll a fluorescence, MR820, and gas exchange changes in perennial ryegrass under salt stress. J. Lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, M.; Yang, T.; Chen, W.; Yang, T. Global atmospheric sulfur deposition and associated impaction on nitrogen cycling in ecosystems. J. Clean. Prod. 2018, 195, 1–9. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, M.; Gao, J.; Li, P.; Goltsev, V.; Ma, F. Thermotolerance of apple tree leaves probed by chlorophyll a fluorescence and modulated 820 nm reflection during seasonal shift. J. Photochem. Photobiol. B Biol. 2015, 152, 347–356. [Google Scholar] [CrossRef]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Shao, X.; Song, Y.; Wang, X. Influence of tree species composition on leaf and soil properties and soil enzyme activity in mixed and pure Oak (Quercus variabilis) stands. Forests 2025, 16, 471. [Google Scholar] [CrossRef]
- Tang, J.M.; Chai, S.F.; Zou, R.; Chen, Z.; Shi, Y.; Jiang, Y.; Wei, X. Effests of water stress on photosynthetic characteristics of Camellia tunghinensis seedlings. Guahaia 2020, 40, 1764–1772. [Google Scholar]
- Mahmoud, E.S.A.; Hassanin, M.A.; Borham, T.I.; Emara, E.I. Tolerance of some sugar beet varieties to water stress. Agric. Water Manag. 2018, 201, 144–151. [Google Scholar] [CrossRef]
- Omae, H.; Kumar, A.; Shono, M. Adaptation to high temperature and water deficit in the common bean (Phaseolus vulgaris L.) during the reproductive period. J. Bot. 2012, 2012, 803413. [Google Scholar]
- Wang, J.; Liu, Y.; Xu, Y.; Chen, W.; Han, Y.; Wang, G.G.; Jin, S. Sexual differences in gas exchange and chlorophyll fluorescence of Torreya grandis under drought stress. Trees 2022, 36, 283–294. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhou, G.S.; Ma, X.Y. Responses of summer maize leaf water content and photosynthetic characteristics to consecutive drought with different intensities. Chin. J. Ecol. 2015, 34, 3111–3117. [Google Scholar]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Oukarroum, A.; Goltsev, V.; Strasser, R.J. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. PLoS ONE 2013, 8, e59433. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Wang, X.; Yang, L.; Han, S.; Chen, S.; Strasser, R.J.; Valverde, B.E.; Qiang, S. Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 128, 1–12. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B Biol. 2016, 157, 22–31. [Google Scholar] [CrossRef]
- Zong, Y.Z.; Wang, W.F.; Xue, Q.W.; Shangguan, Z.P. Interactive effects of elevated CO2 and drought on photosynthetic capacity and PSII performance in maize. Photosynthetica 2014, 52, 63–70. [Google Scholar] [CrossRef]
- Gao, J.; Li, P.; Ma, F.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol. B Biol. 2014, 137, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Stirbet, A.D. Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O–J–I–P). Math. Comput. Simul. 1998, 48, 3–9. [Google Scholar] [CrossRef]
- Mlinaric, S.; Dunic, J.A.; Babojelic, M.S.; Cesar, V.; Lepeduš, H. Differential accumulation of photosynthetic proteins regulates diurnal photochemical adjustments of PSII in common fig (Ficus carica L.) leaves. J. Plant Physiol. 2017, 209, 1–10. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise OJIP. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Salvatori, E.; Fusaro, L.; Gottardini, E.; Pollastrini, M.; Goltsev, V.; Strasser, R.J.; Bussotti, F. Plant stress analysis: Application of prompt, delayed chlorophyll fluorescence and 820 nm modulated reflectance. Insights from independent experiments. Plant Physiol. Biochem. 2014, 85, 105–113. [Google Scholar] [CrossRef]
- Cheng, H.; Wan, Z.; Xu, Y.; Shen, J.; Li, X.; Jin, S. Transcriptome and photosynthetic analyses provide new insight into the molecular mechanisms underlying heat stress tolerance in Rhododendron× pulchrum Sweet. Tree Physiol. 2024, 44, tpad133. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Furutani, R.; Ifuku, K.; Suzuki, Y.; Noguchi, K.; Shimakawa, G.; Wada, S.; Makino, A.; Sohtome, T.; Miyake, C. P700 oxidation suppresses the production of reactive oxygen species in photosystem I. Adv. Bot. Res. 2020, 96, 151–176. [Google Scholar]
- Miyake, C. Molecular mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef]
- Tsuyama, M.; Kobayashi, Y. Reduction of the primary donor P700 of photosystem I during steady-state photosynthesis under low light in Arabidopsis. Photosynth. Res. 2009, 99, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Pace, R.J. Photoreduction of the secondary Photosystem I electron acceptor vitamin K1 in intact spinach chloroplasts and Cyanobacteria in vivo. Biochim. Biophys. Acta (BBA)-Bioenerg. 1997, 1318, 133–144. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R.J. Delayed fluorescence in photosynthesis. Photosynth. Res. 2009, 101, 217–232. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fluorescence rise OJIP and the MR820 signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, J. A kinetic model structure for delayed fluorescence from plants. Biosystems 2009, 95, 98–103. [Google Scholar] [CrossRef]
- Todorenko, D.; Volgusheva, A.; Timofeev, N.; Kovalenko, I.; Matorin, D.; Antal, T. Multiple in vivo effects of cadmium on photosynthetic electron transport in pea plants. Photochem. Photobiol. 2021, 97, 1516–1526. [Google Scholar] [CrossRef]
- Mehta, P.; Kraslavsky, V.; Bharti, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of salt stress induced changes in Photosystem II heterogeneity by prompt fluorescence and delayed fluorescence in wheat (Triticum aestivum) leaves. J. Photochem. Photobiol. B Biol. 2011, 104, 308–313. [Google Scholar] [CrossRef]
- Lazár, D. Modelling of light-induced chlorophyll a fluorescence rise (OJIP transient) and changes in 820 nm-transmittance signal of photosynthesis. Photosynthetica 2009, 47, 483–498. [Google Scholar] [CrossRef]
- Sun, W.; Qi, L.; Chen, H.; Song, Y.; Jiang, J.; Zhang, P.; Wang, B.; Wang, Q.; Meng, G.; Ji, T.; et al. Mycorrhization of Quercus dentata seedlings with Laccaria bicolor enhances salt tolerance of plants only under relatively moderate soil salinity level. Forests 2025, 16, 413. [Google Scholar] [CrossRef]
- Kan, X.; Ren, J.; Chen, T.; Cui, M.; Li, C.; Zhou, R.; Zhang, Y.; Liu, H.; Deng, D.; Yin, Z. Effects of salinity on photosynthesis in maize probed by prompt fluorescence, delayed fluorescence and P700 signals. Environ. Exp. Bot. 2017, 140, 56–64. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Q.S.; Yang, X.Q.; Sheng, Z.T.; Nan, G.N. The alternation between PSII and PSI in ivy (Hedera nepalensis) demonstrated by in vivo chlorophyll a fluorescence and modulated 820 nm reflection. Plant Physiol. Biochem. 2016, 108, 499–506. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Wan, Z.; Jin, P.; Jin, S.; Li, X. Drought-Driven Divergence in Photosynthetic Performance Between Two Cunninghamia lanceolata Provenances: Insights from Gas Exchange and Chlorophyll Fluorescence Dynamics. Plants 2025, 14, 1487. https://doi.org/10.3390/plants14101487
Gong X, Wan Z, Jin P, Jin S, Li X. Drought-Driven Divergence in Photosynthetic Performance Between Two Cunninghamia lanceolata Provenances: Insights from Gas Exchange and Chlorophyll Fluorescence Dynamics. Plants. 2025; 14(10):1487. https://doi.org/10.3390/plants14101487
Chicago/Turabian StyleGong, Xiaofei, Ziyun Wan, Peng Jin, Songheng Jin, and Xueqin Li. 2025. "Drought-Driven Divergence in Photosynthetic Performance Between Two Cunninghamia lanceolata Provenances: Insights from Gas Exchange and Chlorophyll Fluorescence Dynamics" Plants 14, no. 10: 1487. https://doi.org/10.3390/plants14101487
APA StyleGong, X., Wan, Z., Jin, P., Jin, S., & Li, X. (2025). Drought-Driven Divergence in Photosynthetic Performance Between Two Cunninghamia lanceolata Provenances: Insights from Gas Exchange and Chlorophyll Fluorescence Dynamics. Plants, 14(10), 1487. https://doi.org/10.3390/plants14101487







