Hydrogen-Sulfide-Mediated PpAOS3-JA Module Provides Insight into Salt Stress Resistance in Peach
Abstract
1. Introduction
2. Results
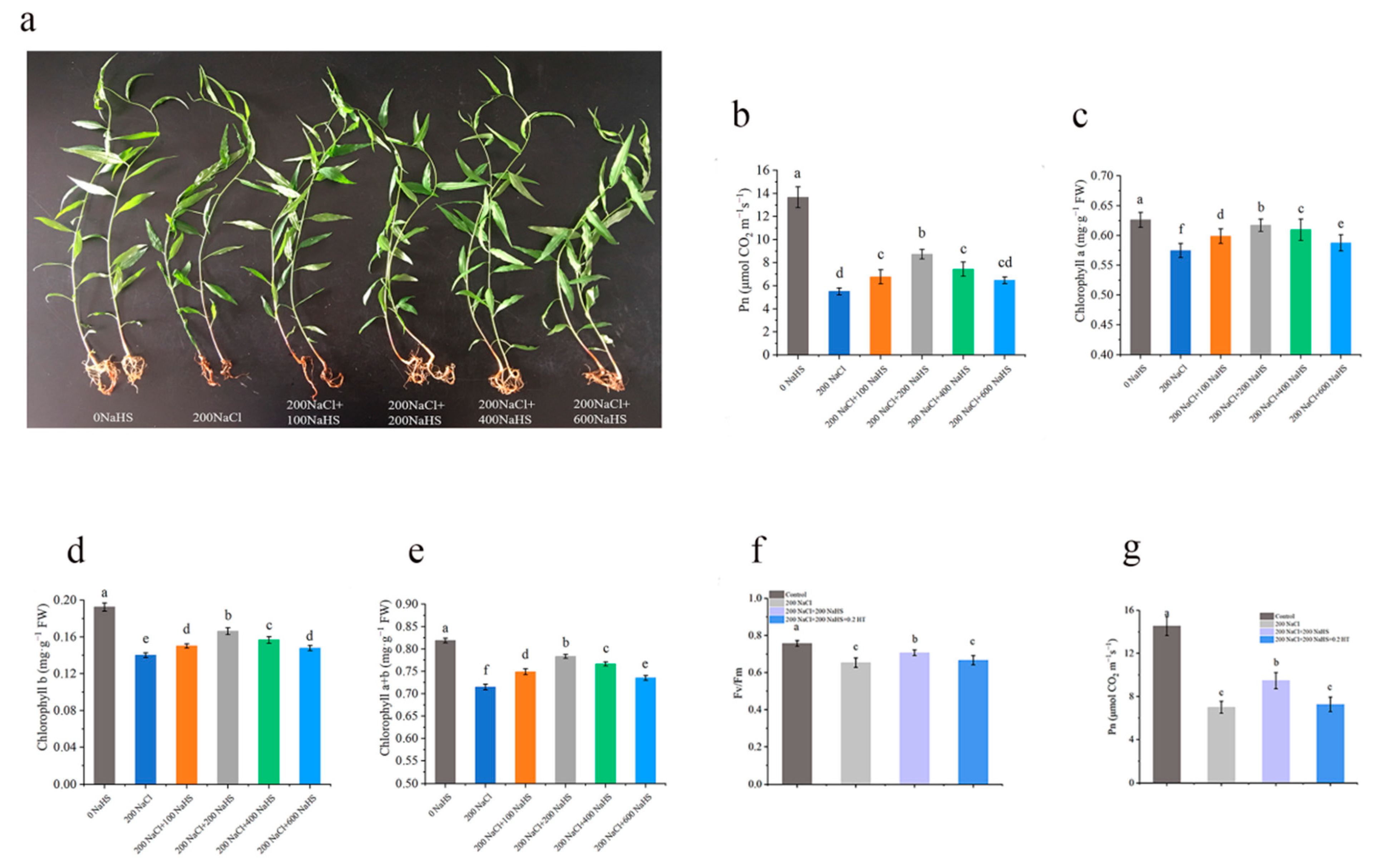
2.1. Salt Stress Impairs the Growth of Peach Seedlings
2.2. Exogenous Hydrogen Sulfide Alleviates the Decrease in Photosynthetic Rate of Seedlings Under Salt Stress
2.3. Exogenous Hydrogen Sulfide Alleviates Salt Stress Damage in Seedlings by Reducing ROS Production
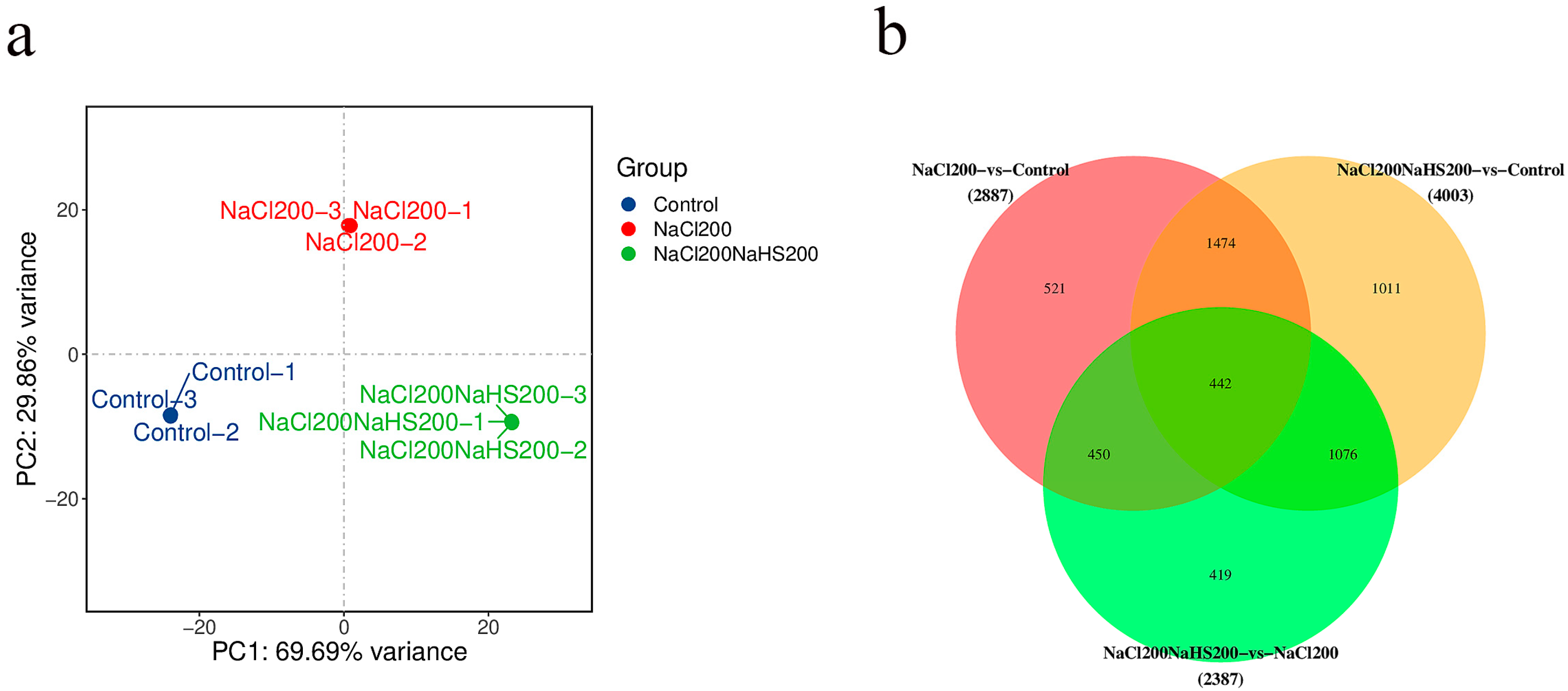
2.4. Transcriptome Analysis of Peach Seedlings Treated with Hydrogen Sulfide Under Salt Stress and Salt Stress Only
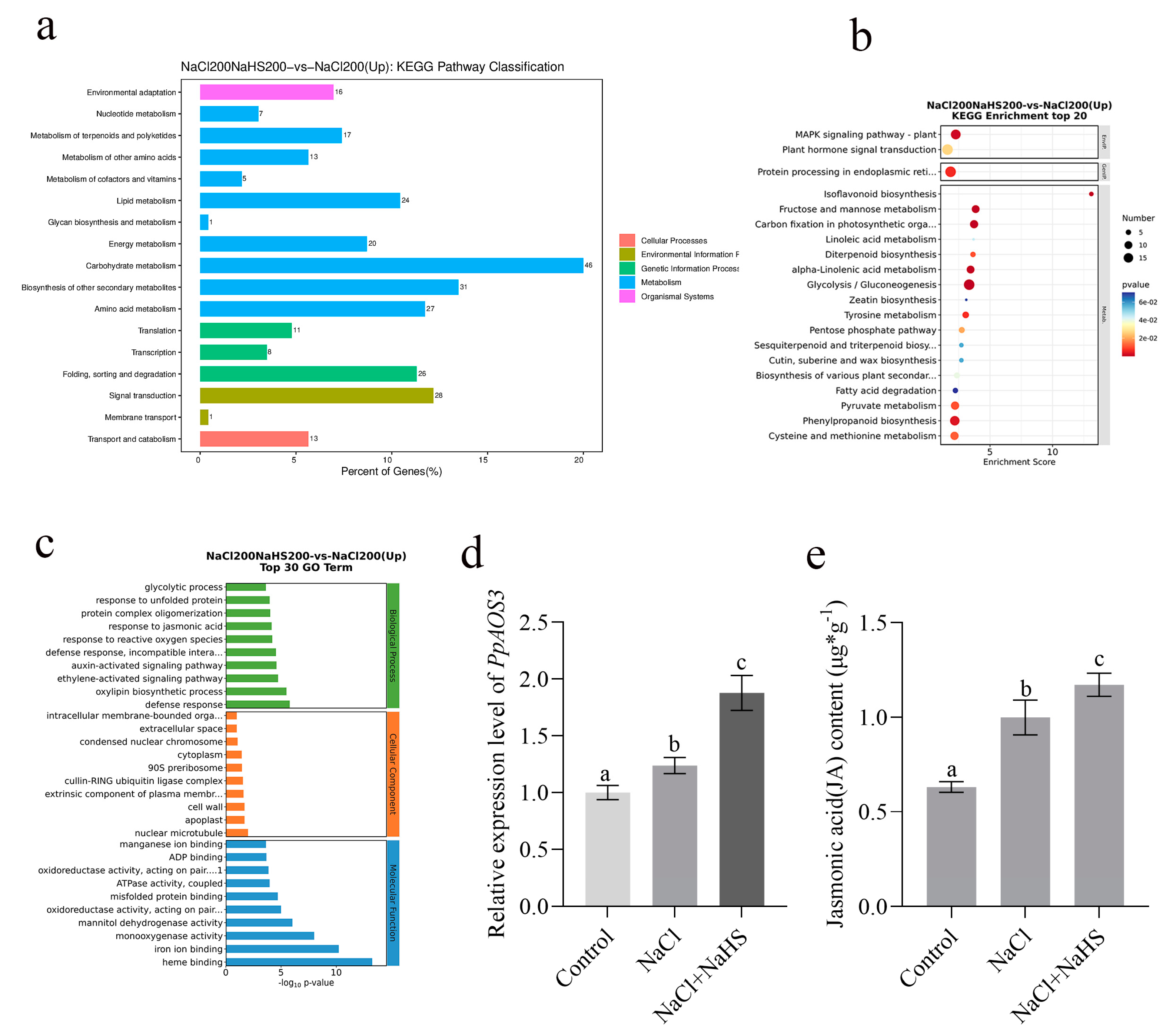
2.5. Differentially Expressed Genes Between Seedlings Treated with Hydrogen Sulfide Under Salt Stress and Salt Stress Only
2.6. PpAOS3 Is Highly Responsive to Exogenous Hydrogen Sulfide Under Salt Stress
2.7. Phenotypic Characterization of OE-PpAOS3 Arabidopsis
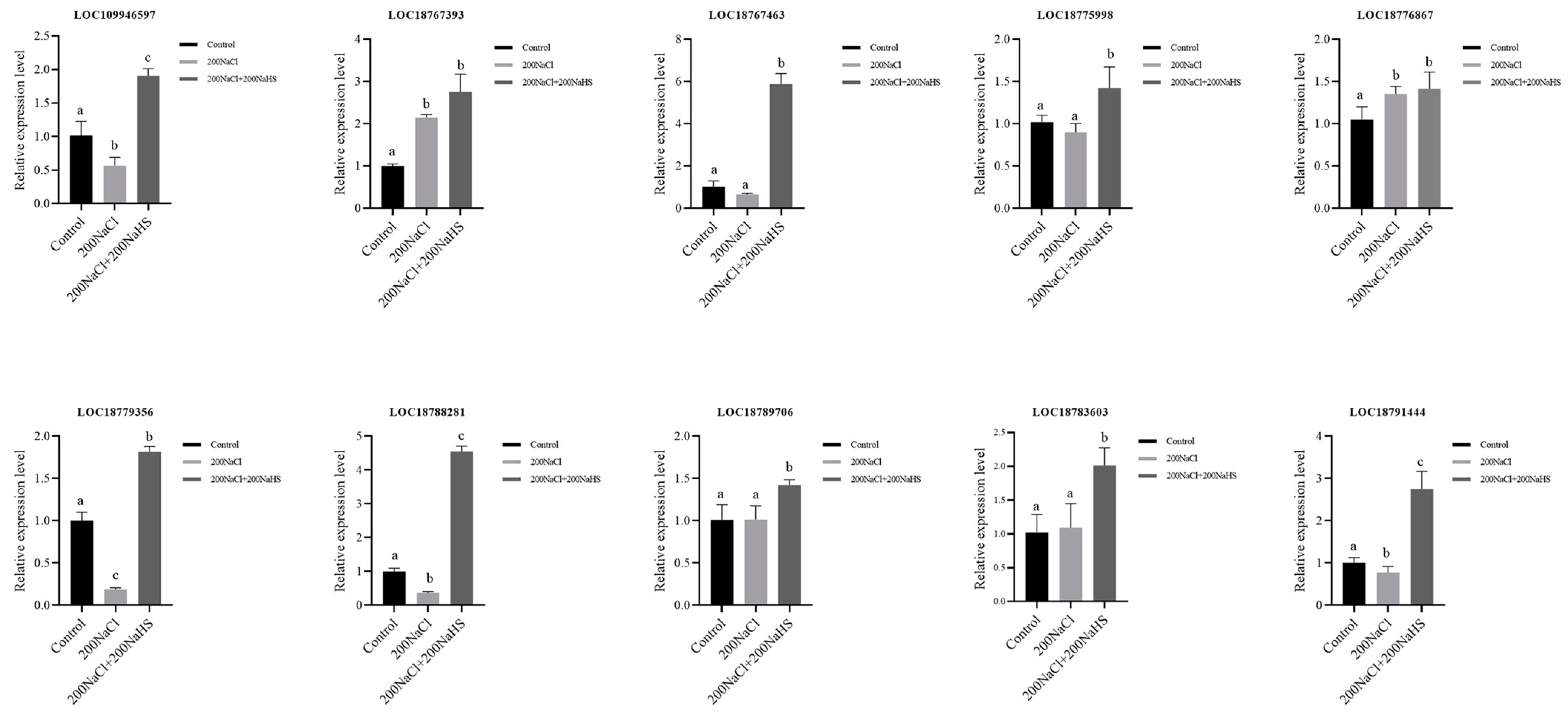
2.8. Encoding Oxidoreductase Genes’ Response to Hydrogen Sulfide and Its Expression Patterns
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, C.-X.; Schnabel, G.; Hu, M.; De Cal, A. Global distribution and management of peach diseases. Phytopathol. Res. 2022, 4, 30. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Aragüés, R.; Medina, E.; Martínez-Cob, A.; Faci, J. Effects of deficit irrigation strategies on soil salinization and sodification in a semiarid drip-irrigated peach orchard. Agric. Water Manag. 2014, 142, 1–9. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moreno, D.M.; Jiménez-Bremont, J.F.; Maruri-López, I.; Delgado-Sánchez, P. Effects of catalase on chloroplast arrangement in Opuntia streptacantha chlorenchyma cells under salt stress. Sci. Rep. 2017, 7, 8656. [Google Scholar] [CrossRef]
- Ghadakchi asl, A.; Mozafari Aa Ghaderi, N. Iron nanoparticles and potassium silicate interaction effect on salt-stressed grape cuttings under in vitro conditions: A morphophysiological and biochemical evaluation. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 510–518. [Google Scholar] [CrossRef]
- Lo’Ay, A.; EL-Ezz, S.F.A. Performance of ‘Flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Jia, X.-M.; Wang, H.; Svetla, S.; Zhu, Y.-F.; Hu, Y.; Cheng, L.; Zhao, T.; Wang, Y.-X. Comparative physiological responses and adaptive strategies of apple Malus halliana to salt, alkali and saline-alkali stress. Sci. Hortic. 2019, 245, 154–162. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Rodríguez-Ruiz, M.; Munoz-Vargas, M.A.; Palma Martínez, J.M. Nitric oxide and hydrogen sulfide share regulatory functions in higher plant events. Hydrog. Sulfide 2022, 46. [Google Scholar] [CrossRef]
- Wu, X.; Du, A.; Zhang, S.; Wang, W.; Liang, J.; Peng, F.; Xiao, Y. Regulation of growth in peach roots by exogenous hydrogen sulfide based on RNA-Seq. Plant Physiol. Biochem. 2021, 159, 179–192. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front. Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef]
- Montesinos-Pereira, D.; de la Torre-González, A.; Blasco, B.; Ruiz, J. Hydrogen sulphide increase the tolerance to alkalinity stress in cabbage plants (Brassica oleracea L.‘Bronco’). Sci. Hortic. 2018, 235, 349–356. [Google Scholar] [CrossRef]
- Min, Y.; Ping, W.; Mei-ling, L.; Lu, C.; Lei, C.; Ai, S.; Zhen, W.; Yan, Y. Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.). J. Integr. Agric. 2016, 15, 2745–2758. [Google Scholar]
- Shi, H.; Ye, T.; Chan, Z. Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2013, 71, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, S.; Chen, L.; Xiao, B. Effects of exogenous hydrogen sulfide on the antioxidant characteristics of tea plant (Camellia sinensis) under salt stress. Plant Physiol. J. 2017, 53, 497–504. [Google Scholar]
- Zhang, M.; Hu, K.; Ma, L.; Geng, M.; Zhang, C.; Yao, G.; Zhang, H. Persulfidation and phosphorylation of transcription factor SlWRKY6 differentially regulate tomato fruit ripening. Plant Physiol. 2024, 196, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Luo, S.; Li, J.; Zhang, J.; Li, L.; Xie, J. 5-Aminolevulinic acid and hydrogen sulphide alleviate chilling stress in pepper (Capsicum annuum L.) seedlings by enhancing chlorophyll synthesis pathway. Plant Physiol. Biochem. 2021, 167, 567–576. [Google Scholar] [CrossRef]
- Chen, P.; Yang, W.; Jin, S.; Liu, Y. Hydrogen sulfide alleviates salinity stress in Cyclocarya paliurus by maintaining chlorophyll fluorescence and regulating nitric oxide level and antioxidant capacity. Plant Physiol. Biochem. 2021, 167, 738–747. [Google Scholar] [CrossRef]
- Wei, M.-Y.; Liu, J.-Y.; Li, H.; Hu, W.-J.; Shen, Z.-J.; Qiao, F.; Zhu, C.-Q.; Chen, J.; Liu, X.; Zheng, H.-L. Proteomic analysis reveals the protective role of exogenous hydrogen sulfide against salt stress in rice seedlings. Nitric Oxide 2021, 111, 14–30. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.-G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Farmer, E.E.; Goossens, A. Jasmonates: What ALLENE OXIDE SYNTHASE does for plants. J. Exp. Bot. 2019, 70, 3373–3378. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.; Shin, D.; Park, S.; Jang, S.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Zheng, Z.; Lin, H.; Cui, H. Effect of exogenous hydrogen sulfide on photosynthesis parameters and chlorophyll fluorescence characteristics of processing tomato (Lycopersicon esculentum Mill ssp. subspontaneum Brezh) seedlings under NaCl stress. J. Nucl. Agric. Sci. 2017, 31, 1426–1435. [Google Scholar]
- Khan, M.I.R.; Syeed, S.; Nazar, R.; Anjum, N.A. An insight into the role of salicylic acid and jasmonic acid in salt stress tolerance. Phytohorm. Abiotic Stress Toler. Plants 2012, 277–300. [Google Scholar] [CrossRef]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.-T.; Figueroa, C.R. Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules—Current perspectives and future directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Tajima, H.; Liang, Y.-C.; Ohto, M.-a.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. Correction: The Arabidopsis Na⁺/H⁺ Antiporters NHX1 and NHX2 Control Vacuolar pH and K⁺ Homeostasis to Regulate Growth, Flower Development, and Reproduction. Plant Cell 2011, 23, 4526. [Google Scholar] [CrossRef]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-Um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Li, T.; Marcelis, L.F. NaCl affects photosynthetic and stomatal dynamics by osmotic effects and reduces photosynthetic capacity by ionic effects in tomato. J. Exp. Bot. 2022, 73, 3637–3650. [Google Scholar] [CrossRef]
- Yadav, S.P.; Bharadwaj, R.; Nayak, H.; Mahto, R.; Singh, R.K.; Prasad, S.K. Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ors, S.; Dursun, A. Physiological, morphological and biochemical responses of exogenous hydrogen sulfide in salt-stressed tomato seedlings. Sustainability 2023, 15, 1098. [Google Scholar] [CrossRef]
- Jiang, J.-L.; Tian, Y.; Li, L.; Yu, M.; Hou, R.-P.; Ren, X.-M. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front. Plant Sci. 2019, 10, 678. [Google Scholar] [CrossRef]
- Baral, A. Strawberries under salt stress: ALA and ROS to the rescue. Physiol. Plant. 2019, 167, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Luan, X.T.; Wang, L.Y.; Zhang, Z.W.; Hui, Z.M. Effects of EBR pretreatment on antioxidant substances and enzyme activities of grapevine seedling leaves under salt stress. Acta Bot. Boreal.-Occident. Sin. 2018, 38, 291–297. [Google Scholar] [CrossRef]
- Baniasadi, F.; Saffari, V.R.; Moud, A.A.M. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Hortic. 2018, 234, 312–317. [Google Scholar] [CrossRef]
- Luo, X.; Tian, T.; Bonnave, M.; Tan, X.; Huang, X.; Li, Z.; Ren, M. The molecular mechanisms of Phytophthora infestans in response to reactive oxygen species stress. Phytopathology 2021, 111, 2067–2079. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.; Alhammad, B.A.; Hassan, M.M.; Shami, A. Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Laudert, D.; Weiler, E.W. Allene oxide synthase: A major control point in Arabidopsis thaliana octadecanoid signalling. Plant J. 1998, 15, 675–684. [Google Scholar] [CrossRef]
- Li, L.-H.; Yi, H.-L.; Liu, X.-P.; Qi, H.-X. Sulfur dioxide enhance drought tolerance of wheat seedlings through H2S signaling. Ecotoxicol. Environ. Saf. 2021, 207, 111248. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.G.; Ma, Q.J.; Sun, C.H.; Sun, M.H.; You, C.X.; Hao, Y.J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plant. 2016, 156, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Patterson, B. The effect of chilling temperature on the level of superoxide dismutase, catalase and hydrogen peroxide in some plant leaves. CABI J. 1988, 323–328. [Google Scholar]
- Omran, R.G. Peroxide levels and the activities of catalase, peroxidase, and indoleacetic acid oxidase during and after chilling cucumber seedlings. Plant Physiol. 1980, 65, 407–408. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Steffens, B.; Sauter, M. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 2009, 21, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Schmelz, E.A.; Alborn, H.T.; Cardoza, Y.J.; Huang, J.; Tumlinson, J.H. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal. Biochem. 2003, 312, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, 1–14. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Li, M.; Gong, Q.; Li, G.; Yu, H.; Dong, X.; Wang, X.; Gong, Z.; Wang, Z.; Xiao, Y.; et al. Hydrogen-Sulfide-Mediated PpAOS3-JA Module Provides Insight into Salt Stress Resistance in Peach. Plants 2025, 14, 1477. https://doi.org/10.3390/plants14101477
Gao X, Li M, Gong Q, Li G, Yu H, Dong X, Wang X, Gong Z, Wang Z, Xiao Y, et al. Hydrogen-Sulfide-Mediated PpAOS3-JA Module Provides Insight into Salt Stress Resistance in Peach. Plants. 2025; 14(10):1477. https://doi.org/10.3390/plants14101477
Chicago/Turabian StyleGao, Xiaolan, Miao Li, Qingtao Gong, Guixiang Li, Haixiang Yu, Xiaomin Dong, Xiaoyou Wang, Zheng Gong, Zhongtang Wang, Yuansong Xiao, and et al. 2025. "Hydrogen-Sulfide-Mediated PpAOS3-JA Module Provides Insight into Salt Stress Resistance in Peach" Plants 14, no. 10: 1477. https://doi.org/10.3390/plants14101477
APA StyleGao, X., Li, M., Gong, Q., Li, G., Yu, H., Dong, X., Wang, X., Gong, Z., Wang, Z., Xiao, Y., & Zhang, A. (2025). Hydrogen-Sulfide-Mediated PpAOS3-JA Module Provides Insight into Salt Stress Resistance in Peach. Plants, 14(10), 1477. https://doi.org/10.3390/plants14101477



