Identification and Functional Analysis of Endophytic Bacteria Bacillus cereus in Sphagnum palustre
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Test Materials
2.2. Culture Medium Preparation
2.3. Isolation and Purification of Strains
2.4. Preparation of Bacterial Liquid Medium for Strain J11
2.5. Morphological Characterization of Strain J11
- 1.
- Morphological identification
- 2.
- Gram staining
- 3.
- Spore staining
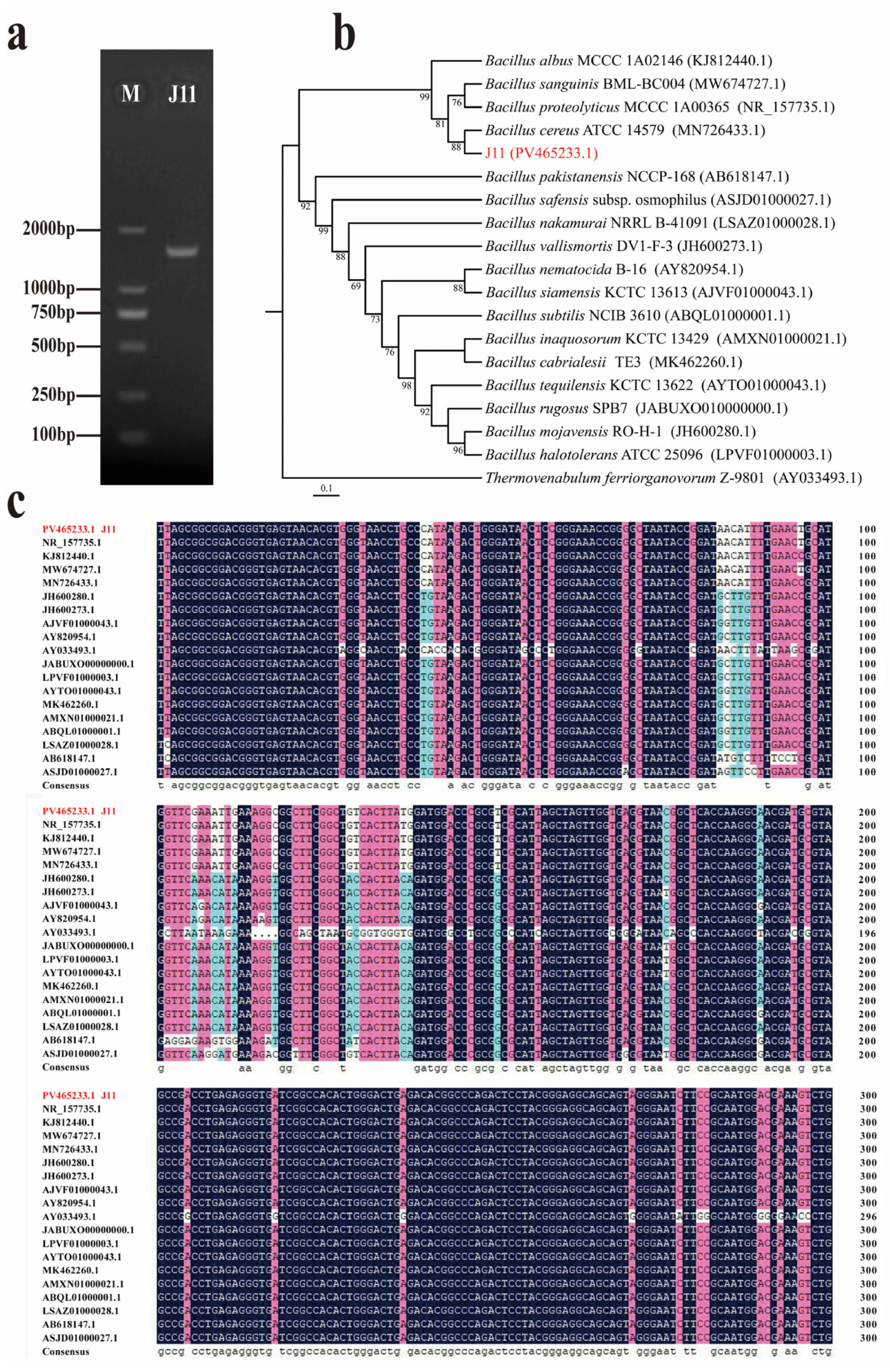
2.6. Molecular Biology Identification of Strain J11
2.7. Characterization of Physiological and Biochemical Indices for Strain J11
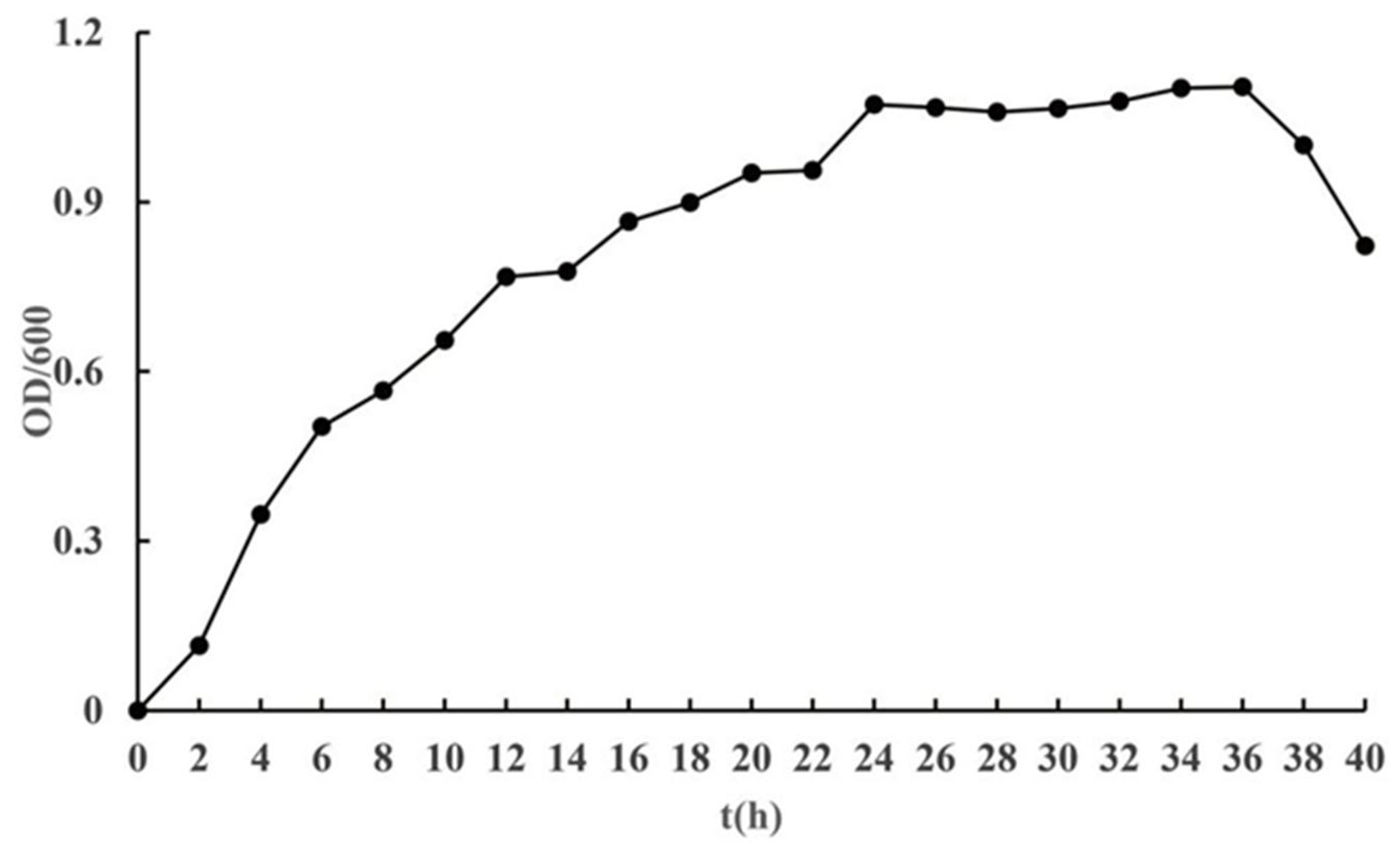
2.8. Growth Curve of Strain J11
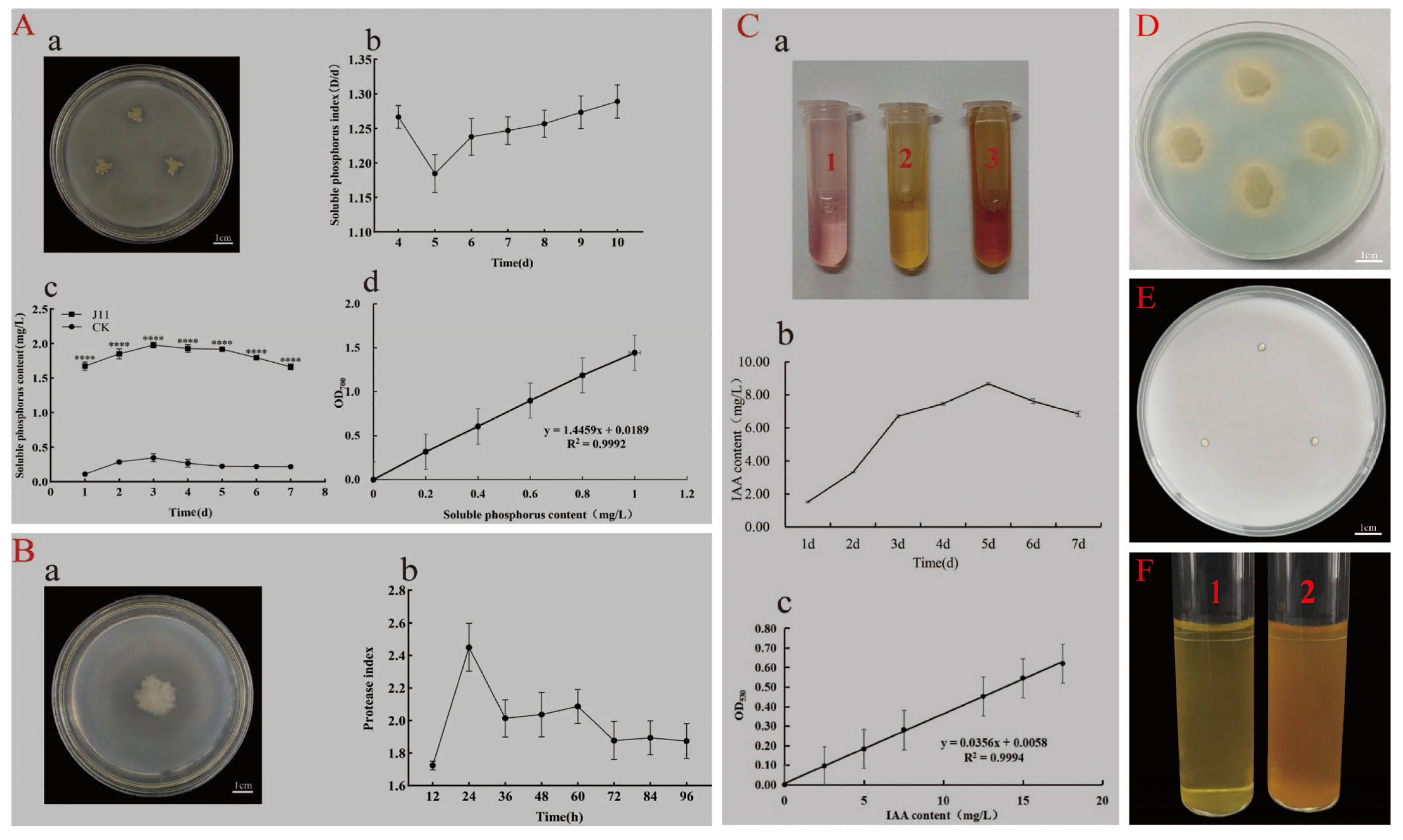
2.9. Functional Study of Strain J11
2.10. Strain J11’s Promoting Test on Maize
2.10.1. Germination Test
2.10.2. Houseplant Experiment
2.11. Data Analysis
3. Results and Analysis
3.1. Identification of Endophytic Bacteria J11 Within S. palustre
3.1.1. Morphological Identification
3.1.2. Molecular Biology Identification
3.2. Physiological and Biochemical Characteristics of Endophytic Bacteria J11 Within S. palustre
3.3. Growth Curve Analysis of Strain J11
3.4. Functional Analysis of Strain J11
3.5. The Effect of Strain J11 on the Growth of Maize Seeds and Seedlings
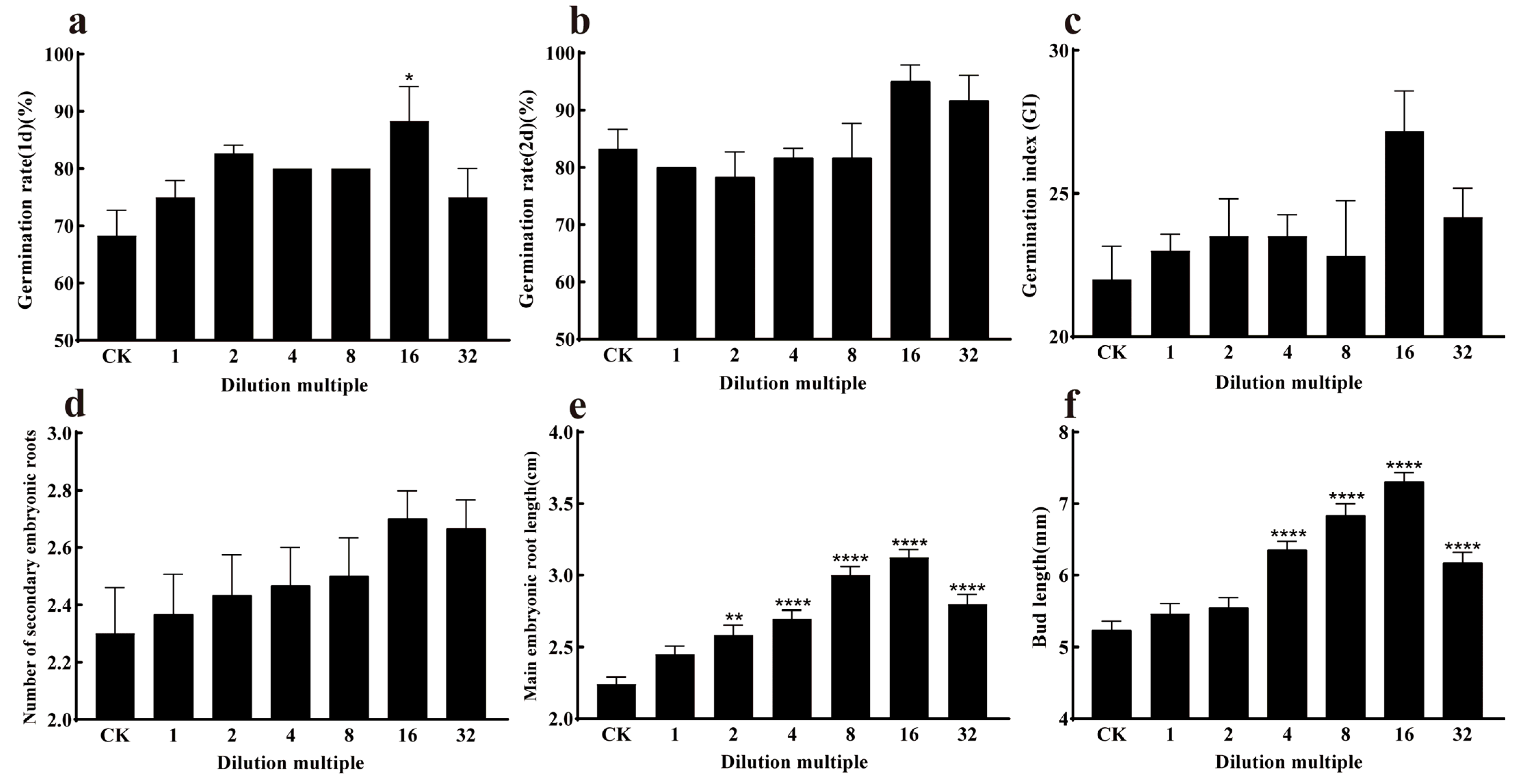
3.5.1. Effect of Strain J11 on Maize Seed Germination
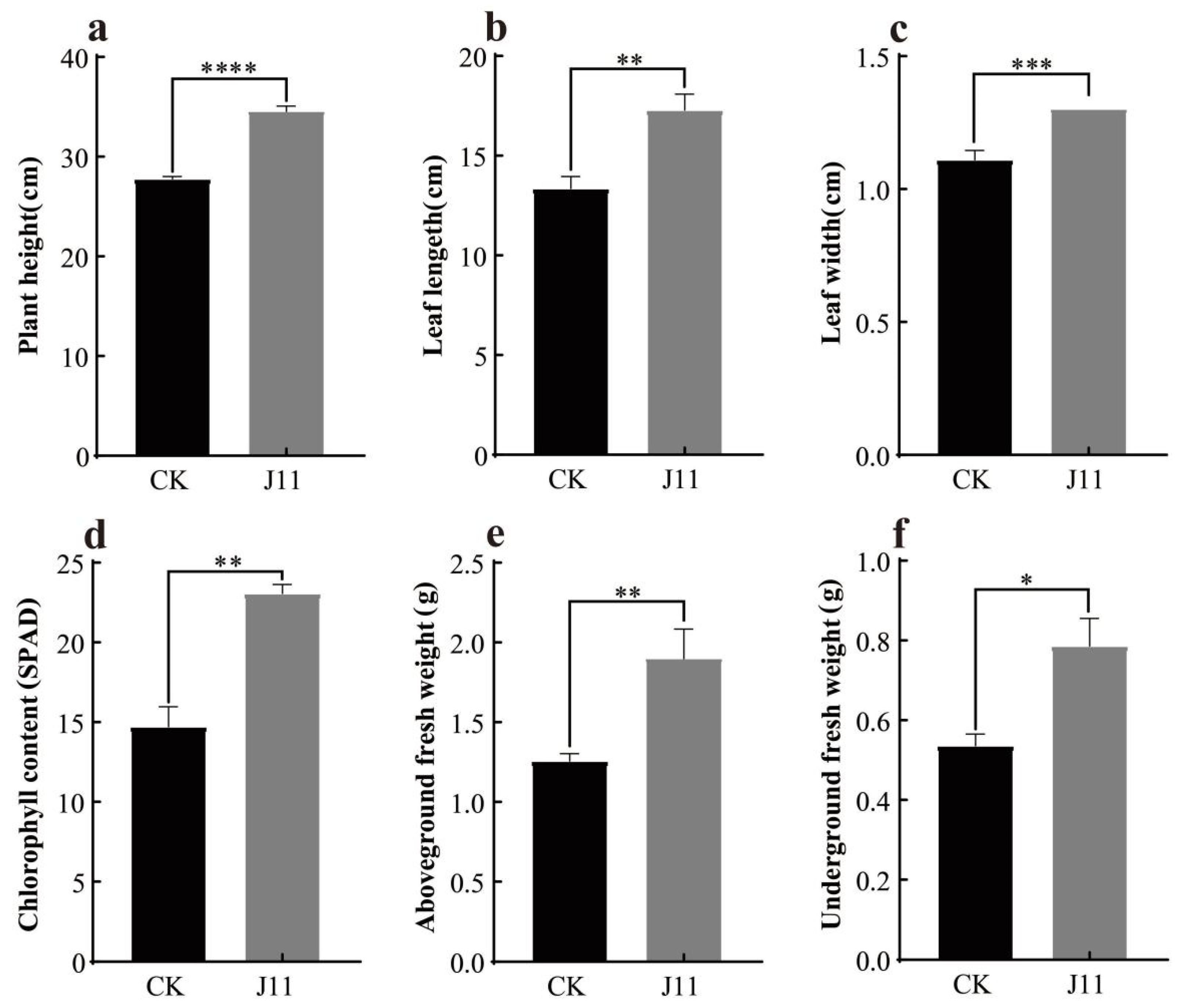
3.5.2. Effect of Strain J11 on the Growth of Maize Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, Q. Bryophyta of China (Volume I); Science Press: Beijing, China, 1994. [Google Scholar]
- Zhu, R.L. Sphagnum mosses: A group of ecologically, economically and scientifically important plants for carbon sequestration. Chin. Bull. Bot. 2022, 57, 559–578. [Google Scholar]
- Xiong, Y.X. Journal of Guizhou Bryophytes (Volume I); Science and Technology Press: Guizhou, China, 2014. [Google Scholar]
- Huang, Z.F.; Xin, J.K.; Jiang, S. Optimization of culture medium for Sphagnum palustre by Response Surface. Mol. Plant Breed. 2024, 1, 1–13. Available online: https://link.cnki.net/urlid/46.1068.S.20240426.1143.002 (accessed on 6 March 2025).
- Huang, Z.F. Research on Rapid Propagation and Floating Cultivation of Sphagnum. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2024. [Google Scholar]
- Zhang, W.; Zhang, Z.Z.; Liu, F.J.; Liang, T.X. Spatio-temporal variation of Vegetation Index of Sphagnum swamp in Dajiu Lake. Geospat. Inf. 2024, 22, 106–110. [Google Scholar]
- Healey, A.L.; Piatkowski, B.; Lovell, J.T.; Sreedasyam, A.; Carey, S.B.; Mamidi, S.; Shu, S.; Plott, C.; Jenkins, J.; Lawrence, T.; et al. Newly identified sex chromosomes in the Sphagnum (peat moss) genome alter carbon sequestration and ecosystem dynamics. Nat. Plants 2023, 9, 238–254. [Google Scholar] [CrossRef]
- Kox, M.A.R.; Aalto, S.L.; Penttilä, T.; Ettwig, K.F.; Jetten, M.S.M.; Kessel, M.A.H.J.V. The influence of oxygen and methane on nitrogen fixation in subarctic Sphagnum mosses. AMB Express 2018, 8, 76. [Google Scholar] [CrossRef]
- Piatkowski, B.T.; Yavitt, J.B.; Turetsky, M.R.; Jonathan, S.A. Natural selection on a carbon cycling trait drives ecosystem engineering by Sphagnum (peat moss). Proc. R. Soc. B. 2021, 288, 20210609. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Liu, C.Z.; Li, X.Q.; Ma, L.X.; Zhai, G.Q.; Feng, X.J. Sphagnum increases soil’s sequestration capacity of mineral-associated organic carbon via activating metal oxides. Nat. Commun. 2023, 14, 5052. [Google Scholar] [CrossRef]
- Kostka, J.E.; Weston, D.J.; Glass, J.B.; Lilleskov, E.A.; Shaw, A.J.; Turetsky, M.R. The Sphagnum microbiome: New insights from an ancient plant lineage. New Phytol. 2016, 211, 57–64. [Google Scholar] [CrossRef]
- Pacheco-Cancino, P.A.; Carrillo-López, R.F.; Sepulveda-Jauregui, A.; Somos-Valenzuela, M.A. Sphagnum mosses, the impact of disturbances and anthropogenic management actions on their ecological role in CO2 fluxes generated in peatland ecosystems. Glob. Change Biol. 2024, 30, e16972. [Google Scholar] [CrossRef]
- Hu, H.X.; Zhang, Y.Y.; He, W.; Tian, R.; Zhong, X.; Han, S.S.; Li, S.S.; Wang, J.J.; Chen, W.F.; Yang, Y.; et al. Simulation of Cd(II), Cu(II), Pb(II) and Zn(II) depuration by peat moss swamp wetland in Dajiu Lake, Shennongjia Lake, China. Resour. Environ. Yangtze Basin 2009, 18, 1050–1057. [Google Scholar]
- Liu, H.H. Bacteriostasis and Heavy Metals Enrichment Ability of Three Sphagnums Tissue Culture Seedlings Study. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2020. [Google Scholar]
- Hou, M.D. Identification and the Function Analysis of Key Genes of Sphagnum cuspidatum to Chromium Based on Transcriptome Sequencing. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Zhu, D. Effects of Heavy Metal Pollution and Soil Physicochemical Properties on the Soil Microbial Community from Sphagnum Farmland. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Gao, Y.F.; Zhao, Y.B.; Xu, L.; Sun, J.Y.; Xia, Y.X.; Xue, D.; Wu, H.W.; Ning, H.; Wu, A.; Wu, L. Long-term Sphagnum cultivation in cold waterlogged paddy fields increases organic carbon content and decreases soil extracellular enzyme activities. Chin. Agric. Sci. 2024, 57, 1533–1546. [Google Scholar]
- Gao, Y.F. Effect of Conversion of Cold Leached Fields to Sphagnum on Soil Organic Carbon and Its Fractions. Master’s Thesis, Hubei Minzu University, Wuhan, China, 2024. [Google Scholar]
- Stelmasiewicz, M.; Świątek, Ł.; Gibbons, S.; Ludwiczuk, A. Bioactive compounds produced by endophytic microorganisms associated with Bryophytes—The “Bryendophytes”. Molecules 2023, 28, 3246. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.J.; Zhou, H.; Yao, T.; Dong, W.Q.; Zhang, J.G.; Han, D.R. Community structure and function of cultivable endophytic bacteria isolated from four moss species in Qilian Mountain. Symbiosis 2020, 80, 257–267. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, D.; Chen, X.H.; Qiu, Q.; Chen, H. Structure and functions of endophytic bacterial communities associated with Sphagnum mosses and their drivers in two different nutrient types of peatlands. Microb. Ecol. 2024, 87, 47. [Google Scholar] [CrossRef]
- Holland-Moritz, H.; Stuart, J.E.M.; Lewis, L.R.; Miller, S.N.; Mack, M.C.; Ponciano, J.M.; McDaniel, S.F.; Fierer, N. The bacterial communities of Alaskan mosses and their contributions to N2-fixation. Microbiome 2021, 9, 53. [Google Scholar] [CrossRef]
- Opelt, K.; Chobot, V.; Hadacek, F.; Schönmann, S.; Eberl, L.; Berg, G. Investigations of the structure and function of bacterial communities associated with Sphagnum mosses. Environ. Microbiol. 2007, 9, 2795–2809. [Google Scholar] [CrossRef]
- Shcherbakov, A.V.; Bragina, A.V.; Kuzmina, E.Y.; Berg, C.; Muntyan, A.N.; Makarova, N.M.; Malfanova, N.V.; Cardinale, M.; Berg, G.; Chebotar, V.K.; et al. Endophytic bacteria of Sphagnum mosses as promising objects of agricultural microbiology. Microbiology 2013, 82, 306–315. [Google Scholar] [CrossRef]
- Vile, M.A.; Kelman Wieder, R.; Živković, T.; Scott, K.D.; Vitt, D.H.; Hartsock, J.A.; Iosue, C.L.; Quinn, J.C.; Petix, M.; Fillingim, H.M.; et al. N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands. Biogeochemistry 2014, 121, 317–328. [Google Scholar] [CrossRef]
- Raghoebarsing, A.A.; Smolders, A.J.P.; Schmid, M.C.; Rijpstra, W.I.C.; Wolters-Arts, M.; Derksen, J.; Jetten, M.S.M.; Schouten, S.; Damsté, J.S.S.; Lamers, L.P.M.; et al. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 2005, 436, 1153–1156. [Google Scholar] [CrossRef]
- Stępniewska, Z.; Kuźniar, A.; Pytlak, A.; Szymczycha, J. Detection of methanotrophic endosymbionts in Sphagnum sp. originating from Moszne peat bog (East Poland). Afr. J. Microbiol. Res. 2013, 7, 1319–1325. [Google Scholar]
- Stępniewska, Z.; Goraj, W.; Kuźniar, A.; Szafranek-Nakonieczna, A.; Banach, A.; Górski, A.; Pytlak, A.; Urban, D. Methane Oxidation by Endophytic Bacteria inhabiting Sphagnum sp. and some Vascular Plants. Wetlands 2018, 38, 411–422. [Google Scholar] [CrossRef]
- Lotte, R.; Chevalier, A.; Boyer, L.; Ruimy, R. Bacillus cereus invasive infections in preterm neonates: An up-to-date review of the literature. Clin. Microbiol. Rev. 2022, 35, e0008821. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.C.; Lu, Y.F.; Liu, C.Y.; Ma, D.H.; Xue, J.D. Isolation, identification and virulence gene distribution and pathogenicity of three Bacillus cereus strains. Chin. J. Biol. 2023, 36, 935–940. [Google Scholar]
- Song, L.L.; Zhang, L.Y.; Jia, W.J.; Bai, Z.H.; Liu, Y.; Chen, Y.J.; Wang, X.L. Isolation, identification and virulence gene detection of Bacillus cereus in cattle. Acta Agric. Boreali-Occident. Sin. 2019, 28, 1735–1741. [Google Scholar]
- Jiang, D.K.; Xiang, M.Y.; Wang, Y.; Yao, X.P.; Yang, Z.X.; Zhang, P.F.; Liao, C.Y.; Zhu, G.H.; Jiang, R.J. Identification and biological characteristics analysis of a Bacillus cereus strain from swine. Chin. J. Prev. Vet. Med. 2019, 41, 1063–1066. [Google Scholar]
- Dou, P.P.; Wang, L.; Fang, Q.; Li, J. Isolation, identification and drug resistance analysis of Bacillus cereus isolated from Fish. Chin. Anim. Husb. Vet. Med. 2019, 46, 2745–2752. [Google Scholar]
- Shang, Y.L.; Chen, H.F.; Wang, L.M.; Wang, Y.J.; Wang, Q.; Zhang, B.X.; Mei, R.H. Molecular cloning of two Mn superoxide dismutase genes from Bacillus cereus M22 and their expression in Escherichia coli. Acta Phytopathol. Sin. 2004, 34, 487–494. [Google Scholar]
- Huang, C.J.; Wang, T.K.; Chung, S.C.; Chen, C.Y. Identification of an antifungal Chitinase from a potential biocontrol agent, Bacillus cereus 28-9. J. Biochem. Mol. Biol. 2005, 38, 82–88. [Google Scholar] [CrossRef]
- Xiao, L.; Wan, J.W.; Yao, J.H.; Feng, H.; Wei, L.L. Effects of Bacillus cereus strain Jdm1 on Meloidogyne incognita and the bacterial community in tomato rhizosphere soil. 3 Biotech 2018, 8, 319. [Google Scholar] [CrossRef]
- Huang, W.X.; Liu, X.Y.; Zhou, X.S.; Wang, X.L.; Liu, X.Y.; Liu, H.X. Calcium signaling is suppressed in Magnaporthe oryzae conidia by Bacillus cereus HS24. Phytopathology 2020, 110, 309–316. [Google Scholar] [CrossRef]
- Gao, X. The Diversity and Functional Study of the Endophytic Bacteria in Four Species of Moss. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2018. [Google Scholar]
- Ding, Y.Z.; Yao, X.X.; Sun, Z.; Yang, L.M.; Han, Z.M.; Wang, Y.H. Isolation, screening and the optimization of fermentation conditions of biocontrol bacteria against Eleutherococcus senticosus Black Spot. Biotechnol. Bull. 2024, 40, 218–226. [Google Scholar]
- Wang, J.; Li, B.; Li, L.P.; Wei, J.; Jiang, M.G.; Zhou, Y. Screening and identification of plant growth-promoting rhizobacteria and its growth promoting effects in subtropical region in Guangxi. J. Nanjing Agric. Univ. 2024, 47, 468–476. [Google Scholar]
- Li, W.P.; Li, S.J.; Li, J.C.; Ye, G.C.; Zhu, L.F.; Wang, X.X.; An, W.X.; Yang, L.; Liu, Y. Isolation and identification of the protease producing endophyte bacteria from Nepenthes mirabilis. Acta Bot. Boreal. Occident. Sin. 2012, 32, 2551–2555. [Google Scholar]
- Bai, J.; Yao, T.; Wang, Z.J.; Lei, Y.; Yang, X.L.; Zhang, W. Isolation and identification as well as growth enhancement and saline-alkali tolerance properties of Cerasus humilis plant growth-promoting endophytes. Agric. Res. Arid. Areas 2022, 40, 132–138. [Google Scholar]
- Chen, W.; Shu, J.H.; Chen, Y.; Zeng, Q.F.; Wang, X.L.; Lu, R.X.; Fu, W. Screening, identification and fermentation condition optimun of a siderophore-producing bacteria WN-H3 from rhizosphere of ryegrass. Biotechnol. Bull. 2016, 32, 219–226. [Google Scholar]
- Chang, X.Y.; Zhang, W.M.; Yin, S.Q.; Chen, K.S.; Yang, Y.X.; Xing, Y.H. Screening and utilization of thermophilic nitrogen-fixing bacteria during high temperature aerobic composting. J. Gansu Agric. Univ. 2022, 57, 198–207. [Google Scholar]
- Huo, T.Q.; Han, X.; Bu, X.Y.; Yang, R.J.; Yang, W.L. Community structure analysis, isolation and identification of endophytic bacteria in Nauclea officinalis. Mol. Plant Breed. 2023, 1, 1–15. Available online: https://link.cnki.net/urlid/46.1068.S.20231114.1503.012 (accessed on 28 April 2025).
- Dong, X.Z.; Cai, M.Y. Handbook of Systematic Identification of Common Bacteria; Science Press: Beijing, China, 2001. [Google Scholar]
- Shen, P.; Chen, X.D. Experimental Microbiology, 4th ed.; Higher Education Press: Beijing, China, 2007. [Google Scholar]
- GB4789.14-2014; Food Safety National Standard for Microbiological Examination of Food—Bacillus cereus Examination. Standards Press of China: Beijing, China, 2014.
- Huang, M.Q.; Tan, P.T.; Chen, Y.H.; Zhao, C.X.; Liu, L.P.; Peng, G.X.; Tan, Z.Y. Endophytic bacteria from Populus tomentosa: Isolation, identification, and screening of strains promoting rice growth. Microbiol. China 2024, 52, 1572–1586. [Google Scholar]
- Yang, G.M.; Liu, C.; Gu, L.D.; Chen, Q.F.; Zhang, X.N. Studies on the phosphorus-solubilizing ability of Isaria cateinannulata and its influence on the growth of Fagopyrum tataricum plants. Plants 2024, 13, 1694. [Google Scholar] [CrossRef]
- Tang, J.C.; Liang, Y.M.; Ma, J.S.; Peng, G.X.; Tan, Z.Y. Diversity and Growth Promotion of Endophytic Bacteria Isolated from Passiflora edulia Sims. Biotechnol. Bull. 2022, 38, 86–97. [Google Scholar]
- Li, Z.L.; Zhou, X.L.; Wang, W.X.; Niu, L.Y.; Wan, J.X.; Wang, W.W. Isolation and characterization of a strain of bacterial hydroxide with pro-biotic effect. Jiangsu Agric. Sci. 2016, 44, 500–503. [Google Scholar]
- Gong, W.F.; Sun, Y.; Wang, R.Q.; Wei, L.P. Isolation of endophytes from rapeseeds in Tibet and screening of antagonistic bacteria with multiple plant growth promoting traits. J. Plant Prot. 2022, 49, 1053–1062. [Google Scholar]
- Peng, X.; Wu, Y.; Zhang, X.N.; Chen, W.H.; Zhu, L.W.; Chen, Q.F.; Deng, J. Screening of high germination rate strains of Entomopathogenic Fungi against Buckwheat seeds. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2022, 53, 393–400. [Google Scholar]
- Peng, X. Effects of Colonization by Isaria cateinannulata on Enzyme Activity and Metabolites of Buckwheat Seeds During Germination. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Luo, Y.J.; Xie, L.Y.; Liu, H.; Liu, L.F.; He, L.L.; Li, F.S. Functional characterization of two strains of sugarcane endophytes. Jiangsu Agric. Sci. 2024, 52, 268–273. [Google Scholar]
- Zhao, B.R. Experiment on water absorption law of maize seeds and the best soaking time for germination. Heilongjiang Agric. Sci. 1999, 1, 61–63. [Google Scholar]
- Xiong, C.M.; Liu, Y.K.; Yuan, Y.; You, L. Effect of different methods of seed soaking on the germination of maize seeds. J. Green Sci. Technol. 2022, 24, 191–193. [Google Scholar]
- Du, F.; Sun, Y.H.; Li, J.H.; Jian, Y.; Zhang, L.; Yang, M.F. Effects of melatonin soaking on seed germination, seedling emergence and physiological metabolism of spring maize under low temperature stress. Jiangsu Agric. Sci. 2024, 52, 65–71. [Google Scholar]
- Zhang, H.N. Soilless Culture of Licorice and Scutellaria baicalensis Seedlings and Their Response to Water and Nitrogen Interaction. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2022. [Google Scholar]
- Lv, N.; Li, Y.; Li, W.; Hu, Q.X.; Chen, X.M.; Zhan, Y.J.; Ma, Y.H.; Wang, Y.L.; Wang, X.L. Isolation and characterization of Bacillus cereus of forest musk origin and pathogenicity test. Prog. Vet. Med. 2024, 45, 141–144. [Google Scholar]
- Miu, Y.X. Handbook of Bacteriology for Berger’s Identification and Handbook of Bacteriology for Berger’s Classification. Microbiol. China 1992, 1, 249. [Google Scholar]
- Chen, Y.Q. Comparison of methods for the determination of growth in microbial growth curves. Light. Ind. Sci. Technol. 2020, 36, 7–812. [Google Scholar]
- Peng, X.; Chen, W.H.; Zhang, X.N.; Meng, Z.Y.; Zhu, L.W.; Deng, J.; Chen, Q.F. Determination of the growth curve of Isaria cateinannulata. J. Guizhou Norm. Univ. (Nat. Sci.) 2023, 41, 102–106. [Google Scholar]
- Yan, H.X.; Li, L.; Liu, L.; Liang, W.H.; Zhang, P.P.; Zhao, H.T. Optimization of fermentation conditions of Bacillus cereus B-21. Shandong Agric. Sci. 2016, 48, 62–65. [Google Scholar]
- Zhou, Y.H.; Li, Q.Y.; Zeng, Y.; Wu, S.L.; Miu, D. Optimization of fermentation conditions and culture medium of probiotic Bacillus cereus. Feed Res. 2007, 1, 56–58. [Google Scholar]
- Ling, Y.H.; Wu, S.F.; Ke, C.H.; Zhao, J. Optimization of culture medium and fermentation conditions of probiotics Bacillus stratosphericus A3440 from farmed abalone. J. Xiamen Univ. (Nat. Sci.) 2016, 55, 336–342. [Google Scholar]
- Feng, Z.Y.; Chen, S.S.; Wang, W.C.; Yang, H.; Deng, Z.L.; Li, Z.; Wang, S.M.; Xu, Y.C. Screening and identification of several phosphate-solubilizing bacteria and effect of their p-solubility. J. Nanjing Agric. Univ. 2017, 40, 842–849. [Google Scholar]
- Wang, D.M. Studies on the Polymorphism and Fermentation Conditions of Bacillus cereus 6371. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2004. [Google Scholar]
- Son, H.J.; Park, G.T.; Cha, M.S.; Heo, M.S. Solubilization of insoluble inorganic phosphates by a novel salt-and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210. [Google Scholar] [CrossRef]
- Hu, Y.L.; Li, H.D.; Wang, H.F.; Jia, C.H.; Chang, L.X.; Tang, H.M. Enzyme screening and isolation of Suaeda Salsa endophytes. J. Tangshan Norm. Univ. 2018, 40, 70–72+76. [Google Scholar]
- Ma, Y.; Hu, S.J.; Wang, X.; Wang, L.H.; Zhao, D.P. Isolation of cultivable endophytic bacteria from Didymodon tectorum and effects of extracts from strain fermentation broths on host plant growth. Chin. J. Appl. Environ. Biol. 2017, 23, 1028–1034. [Google Scholar]
- Ha, F. Preliminary Studies of the Rile of Auxin Gradient During Organogenesis in Arabidopsis thaliana. Master’s Thesis, Lanzhou University, Lanzhou, China, 2010. [Google Scholar]
- Yang, C.L. Analysis on the Factors of Coiling of Bermudagrass Sod and the Effect of Auxin on the Growth of Bermudagrass. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar]
- Pang, Y.P. Studies on the Effects of Plant Growth Regulators on the Changes of Rooting in Shoot Cuttings of Keteleeria fortune var. cyclolepis. Master’s Thesis, Guangxi University, Nanning, China, 2024. [Google Scholar]
- Wang, X.Y.; Zhang, M.; Tian, X.L.; Yuan, P.; Li, H.X.; Luo, Z.B.; Yue, Q.Q.; Zhao, L. Isolation and screening of IAA-producing bacteria from glutinous sorghum leaves and its plant growth-promoting function. Guihaia 2024, 44, 1807–1816. [Google Scholar]
- Li, X.; Bian, Z.J.; Ning, Z.S.; Liu, H.Y.; Zeng, B.; Dong, W. Studies on the growth-promoting effect of Bacillus strain from rhizosphere in Ionic Rare Earth Ores. Biotechnol. Bull. 2024, 40, 259–268. [Google Scholar]
- Zhao, X.; Deng, Y.; Chen, S.F.; Zhang, B.L. Effects of nitrogen combined with phosphate applying on yield and water and fertilizer use efficiency of Tatary Buckwheat in dryland. Acta Agric. Boreali-Sin. 2016, 31, 350–355. [Google Scholar]
- Cruz-Ramírez, A.; Oropeza-Aburto, A.; Razo-Hernández, F.; Ramirez-chavez, E.; Herrera-Estrella, L. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2006, 103, 6765–6770. [Google Scholar] [CrossRef] [PubMed]
- Carrell, A.A.; Lawrence, T.J.; Cabugao, K.G.M.; Car, D.L.; Pelletier, D.A.; Lee, J.H.; Jawdy, S.S.; Grimwod, J.; Schmutz, J.; Hanson, P.J.; et al. Habitat-adapted microbial communities habitat-adapted microbial communities mediate Sphagnum peatmoss resilience to warming. New Phytol. 2022, 234, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J. Study on the Retention Mechanism of C and N Organic Matter by Montmorillonite Mediated by Bacillus cereus. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2024. [Google Scholar]
- Jabeen, Z.; Irshad, F.; Habib, A.; Hussain, N.; Sajjad, M.; Mumtaz, S.; Rehman, S.; Haider, W.; Hassan, M.N. Alleviation of cadmium stress in rice by inoculation of Bacillus cereus. PeerJ 2002, 10, e13131. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.Y.; Zhang, Q.F.; Yang, P.; Liu, Q.W.; Li, Y.G.; Zhang, L.H.; Dong, J.G. Isolation, identification, and herbicidal activity of Bacillus cereus XG1. J. Hebei Agric. Univ. 2016, 39, 81–86. [Google Scholar]
- Yang, G.J.; Liu, H.H.; Ma, Y.; Shi, Y.Q.; Xia, X.D.; Zhang, C.L. Isolation, identification of Bacillus cereus from brown rice and its virulence genes detection and antibiotic susceptibility. Mod. Food Sci. Technol. 2020, 36, 290–299. [Google Scholar]
- Rasool, U.; Ahmad, A.; Badroo, G.A.; Mudasir, M.; Fayaz, S.; Mustafa, R. Isolation and identification of Bacillus cereus from fish and their handlers from Jammu, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 441–447. [Google Scholar] [CrossRef]
- Mairizal; Marlida, Y.; Mirzah; Manin, F. Isolation and characterization of mannanase-producing Bacillus cereus isolated from the hindgut of termites. Pak. J. Nutr. 2018, 17, 116–123. [Google Scholar]
- Li, M.H.; Gao, X.Y.; Li, C.; Yang, C.L.; Fu, C.A.; Liu, J.; Wang, R.; Chen, L.X.; Lin, J.Q.; Liu, X.M.; et al. Isolation and identification of Chromium reducing Bacillus cereus species from chromium-contaminated soil for the biological detoxification of Chromium. Int. J. Environ. Res. Public Health 2020, 17, 2118. [Google Scholar] [CrossRef]
- Afzal, I.; Shah, A.A.; Hameed, Z.; Hameed, A.; Hasan, F. Isolation and characterization of cellulase producing Bacillus cereus MRLB1 from soil. Minerva Biotecnol. 2012, 24, 101–109. [Google Scholar]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef] [PubMed]
- Bizani, D.; Brandelli, A. Influence of media and temperature on bacteriocin production by Bacillus cereus 8A during batch cultivation. Appl. Microbiol. Biotechnol. 2004, 65, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wu, B.T.; Yang, L.Z.; He, M.; Hu, C.Y.; Liu, H.; Yi, M.H. Optimization of fermentation conditions for high production of a novel neutral protease from Bacillus cereus. J. Chin. Inst. Food Sci. Technol. 2020, 20, 109–117. [Google Scholar]
- Sherpa, M.T.; Bag, N.; Das, S.; Haokip, P.; Sharma, L. Isolation and characterization of plant growth promoting rhizobacteria isolated from organically grown high yielding pole type native pea (Pisum sativum L.) variety Dentami of Sikkim, India. Curr. Res. Microb. Sci. 2021, 2, 100068. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Swiecicka, I. Natural occurrence of Bacillus Thuringiensis and Bacillus cereus in eukaryotic organisms: A case for symbiosis. Biocontrol Sci. Technol. 2008, 18, 221–239. [Google Scholar] [CrossRef]
- Wu, J.W.; Tan, J.J.; Li, L.L.; Hao, D.J. Effect of Bacillus cereus NJSZ-13 on seed germination and seeding growth of Pinus massoniana Lamb. J. Northeast For. Univ. 2019, 47, 28–31. [Google Scholar]
- Ali, A.M.; Awad, M.Y.M.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Ku, Y.; Xu, G.; Tian, X.; Xie, H.; Yang, X.; Cao, C.; Chen, Y.L. Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS ONE 2018, 13, e0210035. [Google Scholar]
- Baliyan, N.; Dhiman, S.; Dheeman, S.; Kumar, S.; Arora, N.K.; Maheshwari, D.K.; Dheeman, S.; Arora, N.K. Optimization of Gibberellic Acid production in endophytic Bacillus cereus using Response Surface Methodology and its use as plant growth regulator in chickpea. J. Plant Growth Regul. 2022, 41, 3019–3029. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ikhajiagbe, B. The growth response of rice (Oryza sativa L. var. FARO 44) in vitro after inoculation with bacterial isolates from a typical ferruginous ultisol. Bull. Natl. Res. Cent. 2021, 45, 70. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of endophytic Bacillus cereus T4S and its plant growth-promoting traits. Plants 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pahal, V.; Gupta, A.; Vadhan, R.; Chandra, H.; Dubey, R.C. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea Mays. Sci. Rep. 2020, 10, 20409. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-Deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]

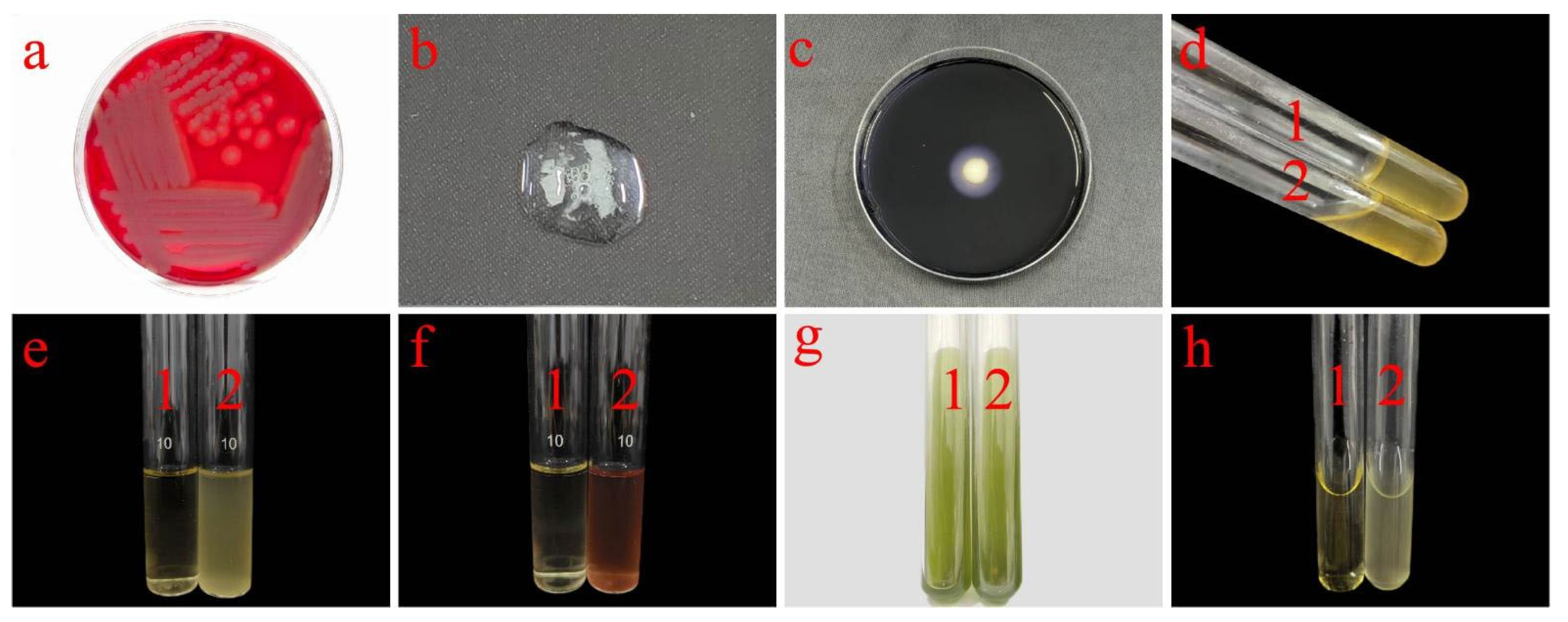


| Test Names | Experimental Phenomenon | Results |
|---|---|---|
| Hemolysis test | Colonies surrounded by a completely transparent hemolytic ring (beta hemolysis) | + |
| Catalase test | Bubble | + |
| Starch hydrolysis test | With colorless transparent ring | + |
| Gelatin liquefaction test | The medium is liquid | + |
| Lysozyme resistance test | The medium becomes cloudy | + |
| V-P test | The culture medium is red | + |
| Citrate utilization test | The medium is green in color | − |
| Methylred test | The reaction solution is yellow | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xin, J.; Zhang, X.; Jiang, S. Identification and Functional Analysis of Endophytic Bacteria Bacillus cereus in Sphagnum palustre. Plants 2025, 14, 1476. https://doi.org/10.3390/plants14101476
Wang H, Xin J, Zhang X, Jiang S. Identification and Functional Analysis of Endophytic Bacteria Bacillus cereus in Sphagnum palustre. Plants. 2025; 14(10):1476. https://doi.org/10.3390/plants14101476
Chicago/Turabian StyleWang, Hongying, Jiankang Xin, Xiaona Zhang, and Shan Jiang. 2025. "Identification and Functional Analysis of Endophytic Bacteria Bacillus cereus in Sphagnum palustre" Plants 14, no. 10: 1476. https://doi.org/10.3390/plants14101476
APA StyleWang, H., Xin, J., Zhang, X., & Jiang, S. (2025). Identification and Functional Analysis of Endophytic Bacteria Bacillus cereus in Sphagnum palustre. Plants, 14(10), 1476. https://doi.org/10.3390/plants14101476






