Subcanopy and Inter-Canopy Supplemental Light Enhances and Standardizes Yields in Medicinal Cannabis (Cannabis sativa L.)
Abstract
1. Introduction
2. Results
2.1. Light Distribution Throughout the Canopy
2.2. Chlorophyll Content and Maximum Quantum Yield of Photosystem II (Fv/Fm)
2.3. Biomass and Secondary Metabolite Yield
2.3.1. Plant Growth and Biomass Yield
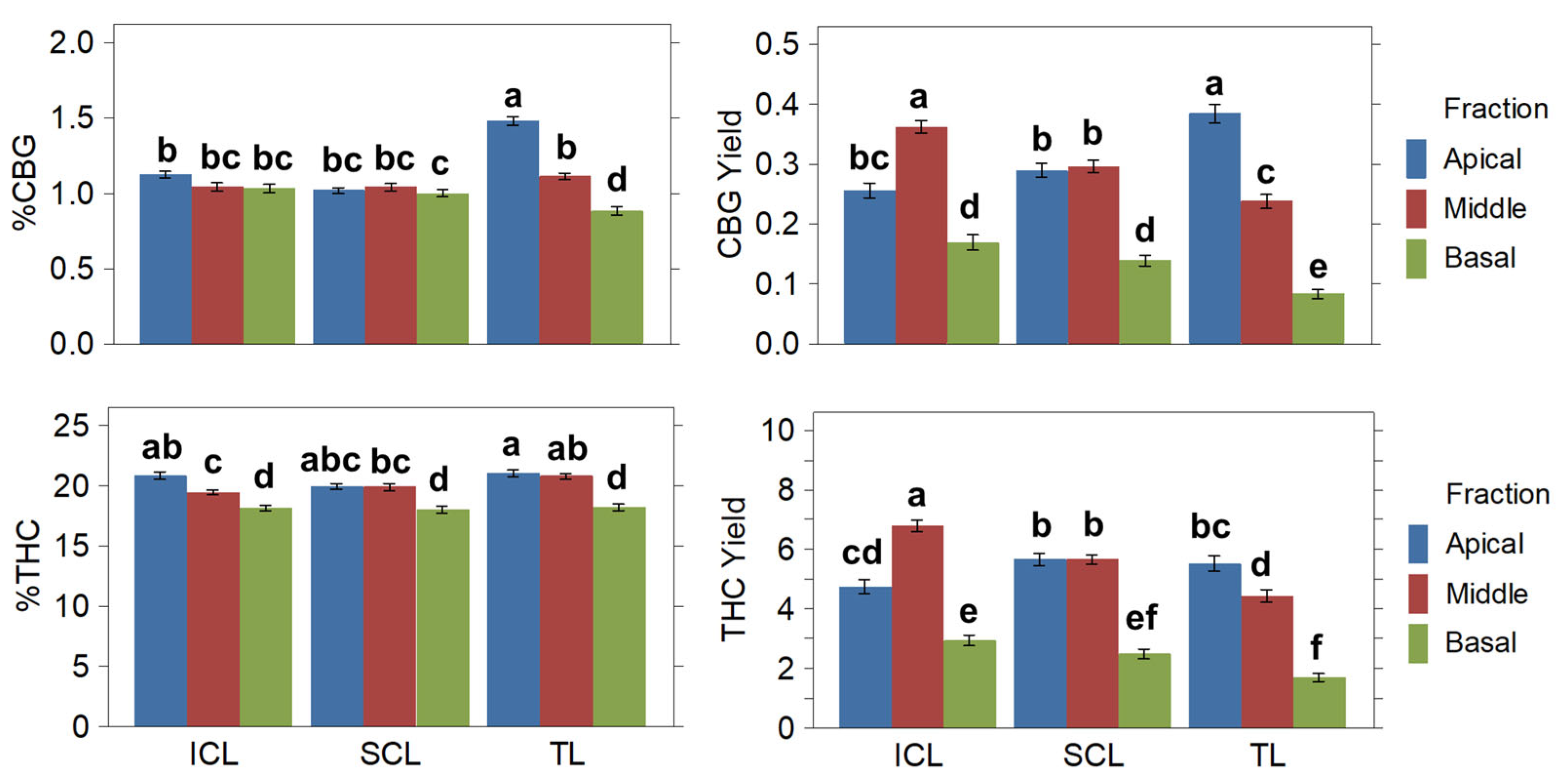
2.3.2. Cannabinoids
2.3.3. Terpenes
2.4. Yield Standardization
2.4.1. Biomass Standardization
2.4.2. Chemical Profile Standardization
2.5. Yield Enhancements and Energy Use Efficiency
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Light Treatments
4.3. Light Measurements
4.3.1. Photosynthetically Active Radiation (PAR)
4.3.2. Leaf Transmittance and Chlorophyll Fluorescence
4.4. Harvesting and Post-Harvest Analyses
4.4.1. Plant Processing
4.4.2. Secondary Metabolites
4.5. Energy Use Efficiency
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TL | Top lighting |
| SCL | Subcanopy lighting |
| ICL | Inter-canopy lighting |
| THC | Tetrahydrocannabinol |
| THCA | Tetrahydrocannabinolic acid |
| CBG | Cannabigerol |
| CBGA | Cannabigerolic acid |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic acid |
| CBC | Cannabichromene |
| CBCA | Cannabichromenic acid |
| RB | Red–blue light |
| RGB | Red–green–blue light |
| AEMPS | Agencia Española de Medicamentos y Productos Sanitarios |
| PPFD | Photosynthetic photon flux density |
| PAR | Photosynthetically active radiation |
| FR | Far-red light |
| SPAD | Single-photon avalanche diode |
| PSII | Photosystem II |
| Fv | Variable fluorescence |
| Fo | Minimum fluorescence |
| Fm | Maximum fluorescence |
| TDW | Total dry weight |
| FDW | Flowers (inflorescences) dry weight |
| LDW | Leaves dry weight |
| SDW | Stem dry weight |
| GC-MS | Gas chromatography–mass spectrometry |
| NIRS | Near infrared spectroscopy |
| HPLC-DAD | High-performance liquid chromatography with diode-array detection |
| REE | Raw electrical efficiency |
| EEE | Enhanced electrical efficiency |
| CV | Coefficient of variation |
| DLI | Daily light integral |
References
- Ren, G.; Zhang, X.; Li, Y.; Ridout, K.; Serrano-Serrano, M.L.; Yang, Y.; Liu, A.; Ravikanth, G.; Nawaz, M.A.; Mumtaz, A.S.; et al. Large-Scale Whole-Genome Resequencing Unravels the Domestication History of Cannabis sativa. Sci. Adv. 2021, 7, eabg2286. [Google Scholar] [CrossRef]
- Small, E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Balant, M.; Gras, A.; Gálvez, F.; Garnatje, T.; Vallès, J.; Vitales, D. CANNUSE, a Database of Traditional Cannabis Uses-an Opportunity for New Research. Database 2021, 2021, baab024. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Leinen, Z.J.; Mohan, R.; Premadasa, L.S.; Acharya, A.; Mohan, M.; Byrareddy, S.N. Therapeutic Potential of Cannabis: A Comprehensive Review of Current and Future Applications. Biomedicines 2023, 11, 2630. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Amin, H.I.M.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules 2022, 12, 1084. [Google Scholar] [CrossRef]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis Glandular Trichomes: A Cellular Metabolite Factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis Sativa—From Plant Genome to Humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Jin, D.; Jin, S.; Chen, J. Cannabis Indoor Growing Conditions, Management Practices, and Post-Harvest Treatment: A Review. Am. J. Plant Sci. 2019, 10, 925–946. [Google Scholar] [CrossRef]
- Fleming, H.; Chamberlain, Z.; Zager, J.J.; Lange, B.M. Controlled Environments for Cannabis Cultivation to Support “Omics” Research Studies and Production. In Biochemical Pathways and Environmental Responses in Plants: Part B; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Volume 680, pp. 353–380. ISBN 9780443185847. [Google Scholar]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis Sativa l: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Eichhorn Bilodeau, S.; Lyu, D.; Ahmed, M.B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the Yield Gap for Cannabis: A Meta-Analysis of Factors Determining Cannabis Yield. Front. Plant Sci. 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis Yield, Potency, and Leaf Photosynthesis Respond Differently to Increasing Light Levels in an Indoor Environment. Front. Plant Sci. 2021, 12, 646020. [Google Scholar] [CrossRef]
- Llewellyn, D.; Golem, S.; Foley, E.; Dinka, S.; Jones, A.M.P.; Zheng, Y. Indoor Grown Cannabis Yield Increased Proportionally with Light Intensity, but Ultraviolet Radiation Did Not Affect Yield or Cannabinoid Content. Front. Plant Sci. 2022, 13, 974018. [Google Scholar] [CrossRef]
- Sae-Tang, W.; Heuvelink, E.; Nicole, C.C.S.; Kaiser, E.; Sneeuw, K.; Holweg, M.M.S.F.; Carvalho, S.; Kappers, I.F.; Marcelis, L.F.M. High Light Intensity Improves Yield of Specialized Metabolites in Medicinal Cannabis (Cannabis sativa L.), Resulting from Both Higher Inflorescence Mass and Concentrations of Metabolites. J. Appl. Res. Med. Aromat. Plants 2024, 43, 100583. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light Matters: Effect of Light Spectra on Cannabinoid Profile and Plant Development of Medical Cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Holweg, M.M.S.F.; Kaiser, E.; Kappers, I.F.; Heuvelink, E.; Marcelis, L.F.M. The Role of Red and White Light in Optimizing Growth and Accumulation of Plant Specialized Metabolites at Two Light Intensities in Medical Cannabis (Cannabis sativa L.). Front. Plant Sci. 2024, 15, 1393803. [Google Scholar] [CrossRef]
- Gómez, C.; Morrow, R.C.; Bourget, C.M.; Massa, G.D.; Mitchell, C.A. Comparison of Intracanopy Light-Emitting Diode Towers and Overhead High-Pressure Sodium Lamps for Supplemental Lighting of Greenhouse-Grown Tomatoes. Horttechnology 2013, 23, 93–98. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.S.; Rufyikiri, A.S.; MacPherson, S.; Lefsrud, M. An Update on Plant Photobiology and Implications for Cannabis Production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- Reichel, P.; Munz, S.; Hartung, J.; Kotiranta, S.; Graeff-Hönninger, S. Impacts of Different Light Spectra on CBD, CBDA and Terpene Concentrations in Relation to the Flower Positions of Different Cannabis sativa L. Strains. Plants 2022, 11, 2695. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A. Using Light to Improve Commercial Value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Liu, K.; Heuvelink, E. LED Intercanopy Lighting in Blackberry During Spring Improves Yield as a Result of Increased Number of Fruiting Laterals and Has a Positive Carryover Effect on Autumn Yield. Front. Plant Sci. 2021, 12, 620642. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Photosynthesis, Plant Growth, and Fruit Production of Single-Truss Tomato Improves with Supplemental Lighting Provided from underneath or within the Inner Canopy. Sci. Hortic. 2017, 222, 221–229. [Google Scholar] [CrossRef]
- Stoochnoff, J.; Johnston, M.; Hoogenboom, J.; Graham, T.; Dixon, M. Intracanopy Lighting Strategies to Improve Green Bush Bean (Phaseolus vulgaris) Compatibility with Vertical Farming. Front. Agron. 2022, 4, 905286. [Google Scholar] [CrossRef]
- Hovi, T.; Näkkilä, J.; Tahvonen, R. Interlighting Improves Production of Year-Round Cucumber. Sci. Hortic. 2004, 102, 283–294. [Google Scholar] [CrossRef]
- Guo, X.; Hao, X.; Khosla, S.; Kumar, K.G.S.; Cao, R.; Bennett, N. Effect of LED Interlighting Combined with Overhead HPS Light on Fruit Yield and Quality of Year-Round Sweet Pepper in Commercial Greenhouse. Acta Hortic. 2016, 1134, 71–78. [Google Scholar] [CrossRef]
- Hawley, D.; Graham, T.; Stasiak, M.; Dixon, M. Improving Cannabis Bud Quality and Yield with Subcanopy Lighting. HortScience 2018, 53, 1593–1599. [Google Scholar] [CrossRef]
- Baratta, F.; Pignata, I.; Ravetto Enri, L.; Brusa, P. Cannabis for Medical Use: Analysis of Recent Clinical Trials in View of Current Legislation. Front. Pharmacol. 2022, 13, 888903. [Google Scholar] [CrossRef]
- Dor, M. Three Decades of Cannabis Research: What Are the Obstacles? Rambam Maimonides Med. J. 2022, 13, e0028. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Too Dense or Not Too Dense: Higher Planting Density Reduces Cannabinoid Uniformity but Increases Yield/Area in Drug-Type Medical Cannabis. Front. Plant Sci. 2022, 13, 713481. [Google Scholar] [CrossRef] [PubMed]
- Danziger, N.; Bernstein, N. Shape Matters: Plant Architecture Affects Chemical Uniformity in Large-Size Medical Cannabis Plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Plant Architecture Manipulation Increases Cannabinoid Standardization in ‘Drug-Type’ Medical Cannabis. Ind. Crop. Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Schipper, R.; van der Meer, M.; de Visser, P.H.B.; Heuvelink, E.; Marcelis, L.F.M. Consequences of Intra-Canopy and Top LED Lighting for Uniformity of Light Distribution in a Tomato Crop. Front. Plant Sci. 2023, 14, 1012529. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal Rate of Organic Fertilizer during the Flowering Stage for Cannabis Grown in Two Coir-Based Substrates. HortScience 2017, 52, 1796–1803. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. The Highs and Lows of P Supply in Medical Cannabis: Effects on Cannabinoids, the Ionome, and Morpho-Physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Dilena, E.; Close, D.C.; Hunt, I.; Garland, S.M. Investigating How Nitrogen Nutrition and Pruning Impacts on CBD and THC Concentration and Plant Biomass of Cannabis Sativa. Sci. Rep. 2023, 13, 19533. [Google Scholar] [CrossRef] [PubMed]
- Peterswald, T.J.; Mieog, J.C.; Azman Halimi, R.; Magner, N.J.; Trebilco, A.; Kretzschmar, T.; Purdy, S.J. Moving Away from 12:12; the Effect of Different Photoperiods on Biomass Yield and Cannabinoids in Medicinal Cannabis. Plants 2023, 12, 1061. [Google Scholar] [CrossRef]
- Sutton, D.B.; Punja, Z.K.; Hamarneh, G. Characterization of Trichome Phenotypes to Assess Maturation and Flower Development in Cannabis sativa L. (Cannabis) by Automatic Trichome Gland Analysis. Smart Agric. Technol. 2023, 3, 100111. [Google Scholar] [CrossRef]
- Holweg, M.M.S.F.; Curren, T.; Cravino, A.; Kaiser, E.; Kappers, I.F.; Heuvelink, E.; Marcelis, L.F.M. High Air Temperature Reduces Plant Specialized Metabolite Yield in Medical Cannabis, and Has Genotype-Specific Effects on Inflorescence Dry Matter Production. Environ. Exp. Bot. 2025, 230, 106085. [Google Scholar] [CrossRef]
- Massuela, D.C.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis Sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crop. Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. Light Quality Impacts Vertical Growth Rate, Phytochemical Yield and Cannabinoid Production Efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef] [PubMed]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Reichel, P.; Munz, S.; Hartung, J.; Graeff-Hönninger, S. Harvesting Light: The Interrelation of Spectrum, Plant Density, Secondary Metabolites, and Cannabis sativa L. Yield. Agronomy 2024, 14, 2565. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef]
- Moher, M.; Llewellyn, D.; Jones, M.; Zheng, Y. Light Intensity Can Be Used to Modify the Growth and Morphological Characteristics of Cannabis during the Vegetative Stage of Indoor Production. Ind. Crop. Prod. 2022, 183, 114909. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Lingvay, M.; Akhtar, P.; Sebők-Nagy, K.; Páli, T.; Lambrev, P.H. Photobleaching of Chlorophyll in Light-Harvesting Complex II Increases in Lipid Environment. Front. Plant Sci. 2020, 11, 849. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, J.M.; Bugbee, B. In Situ Measurement of Leaf Chlorophyll Concentration: Analysis of the Optical/Absolute Relationship. Plant. Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
- Khajuria, M.; Rahul, V.P.; Vyas, D. Photochemical Efficiency Is Negatively Correlated with the Δ9- Tetrahydrocannabinol Content in Cannabis sativa L. Plant Physiol. Biochem. 2020, 151, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Schouten, I.; Hawley, D.; Olschowski, S.; Ouzounis, T.; Kerstens, T.N.; Gianneas, T.; Ludovico, J.; Marcelis, L.F.M.; Heuvelink, E. Partially Substituting Top-Light with Intracanopy Light Increases Yield More at Higher LED Light Intensities. HortScience 2024, 59, 421–428. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between Chemistry and Morphology in Medical Cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Ahrens, A.; Llewellyn, D.; Zheng, Y. Is Twelve Hours Really the Optimum Photoperiod for Promoting Flowering in Indoor-Grown Cultivars of Cannabis sativa? Plants 2023, 12, 2605. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.; Llewellyn, D.; Zheng, Y. Longer Photoperiod Substantially Increases Indoor-Grown Cannabis’ Yield and Quality: A Study of Two High-THC Cultivars Grown under 12 h vs. 13 h Days. Plants 2024, 13, 433. [Google Scholar] [CrossRef]
- Anderson, S.L.; Pearson, B.; Kjelgren, R.; Brym, Z. Response of Essential Oil Hemp (Cannabis sativa L.) Growth, Biomass, and Cannabinoid Profiles to Varying Fertigation Rates. PLoS ONE 2021, 16, e0252985. [Google Scholar] [CrossRef]
- Garrido, J.; Rico, S.; Corral, C.; Sánchez, C.; Vidal, N.; Martínez-Quesada, J.J.; Ferreiro-Vera, C. Exogenous Application of Stress-Related Signaling Molecules Affect Growth and Cannabinoid Accumulation in Medical Cannabis (Cannabis sativa L.). Front. Plant Sci. 2022, 13, 1082554. [Google Scholar] [CrossRef]
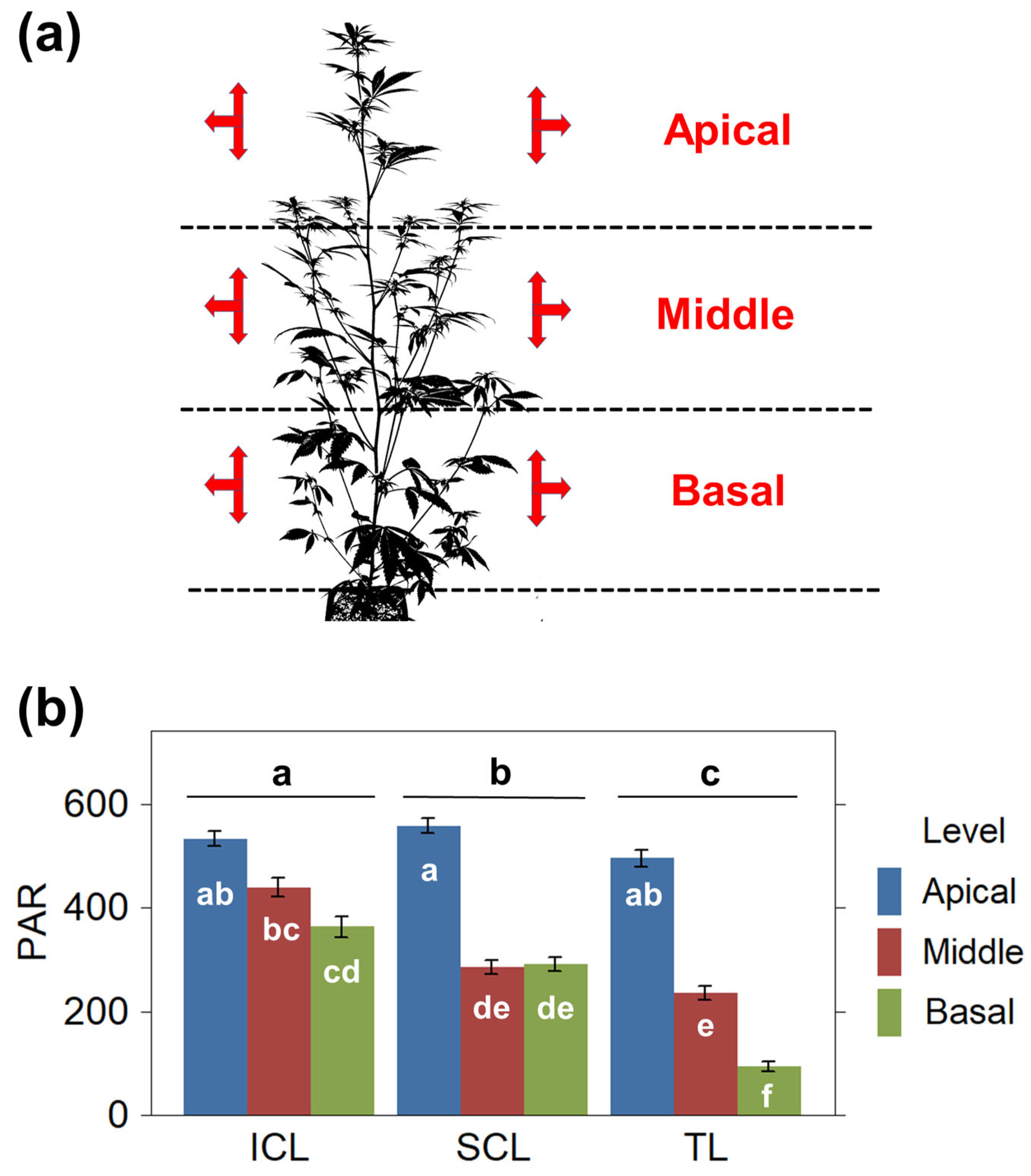



| Yields | Treatment | ||
|---|---|---|---|
| TL | SCL | ICL | |
| TDW (g·m−2) | 962.52 C | 1173.96 B | 1293.24 A |
| FDW (g·m−2) | 681.84 B | 849.48 A | 886.08 A |
| Total terpenes (%) | 2.95 B | 3.32 A | 3.31 A |
| CBG (%) | 1.25 A | 1.03 B | 1.07 B |
| THC (%) | 20.47 A | 19.55 B | 19.62 B |
| CBG (g·m−2) | 8.46 B | 8.69 B | 9.44 A |
| THC (g·m−2) | 139.74 B | 165.84 A | 173.88 A |
| Trait | Treatment | Mean (Plant) | Mean (g·m−2) |
|---|---|---|---|
| Height increase | ICL | 103.22 A | - |
| TL | 100.75 A | - | |
| SCL | 99.36 A | - | |
| FW | ICL | 396.94 A | 4763.28 A |
| SCL | 377.59 A | 4531.08 A | |
| TL | 323.19 B | 3878.28 B | |
| TDW | ICL | 107.77 A | 1293.24 A |
| SCL | 97.83 B | 1173.96 B | |
| TL | 80.21 C | 962.52 C | |
| FDW | ICL | 73.84 A | 886.08 A |
| SCL | 70.79 A | 849.48 A | |
| TL | 56.82 B | 681.84 B | |
| LDW | ICL | 16.04 A | 192.48 A |
| SCL | 14.68 A | 176.16 A | |
| TL | 12.75 B | 153 B | |
| SDW | ICL | 18.75 A | 225 A |
| TL | 16.77 AB | 201.24 AB | |
| SCL | 15.49 B | 185.88 B |
| Variables | %CBG | %THC | CBG (g·plant−1) | CBG (g·m−2) | THC (g·plant−1) | THC (g·m−2) | |
|---|---|---|---|---|---|---|---|
| Plant fraction | Apical | 1.21 A | 20.60 A | 0.309 A | 3.71 A | 5.31 A | 63.69 A |
| Middle | 1.07 B | 20.05 B | 0.299 A | 3.59 A | 5.63 A | 67.61 A | |
| Basal | 0.97 C | 18.13 C | 0.130 B | 1.56 B | 2.38 B | 28.56 B | |
| Treatment | TL | 1.25 A | 20.47 A | 0.705 B | 8.46 B | 11.65 B | 139.74 B |
| SCL | 1.03 B | 19.55 B | 0.724 B | 8.69 B | 13.82 A | 165.84 A | |
| ICL | 1.07 B | 19.62 B | 0.787 A | 9.44 A | 14.49 A | 173.88 A | |
| Yields | Treatment | ||
|---|---|---|---|
| TL | SCL | ICL | |
| TDW | 14.02 | 7.56 | 6.31 |
| FDW | 13.44 | 6.04 | 5.06 |
| Total terpenes (%) | 9.04 | 2.28 | 1.95 |
| CBG (%) | 5.18 | 5.32 | 6.54 |
| THC (%) | 4.05 | 4.42 | 3.62 |
| CBG | 11.7 | 4.87 | 6.82 |
| THC | 14.88 | 5.13 | 6.39 |
| Days after planting | Total power consumption (kWh·m−2) | % increase from TL | ||||
| 7 | 7 | 7 | 55 | |||
| Plant stage | Vegetative | Flowering | Flowering | Flowering | ||
| Daylength (hours) | 18 | 12 | 12 | 12 | ||
| TL power (W) | 530 | 530 | 1083 | 1083 | ||
| SCL power (W) | - | - | - | 210 | ||
| ICL power (W) | - | - | - | 420 | ||
| TL (kWh) | 66.78 | 44.52 | 91 | 715 | 458.65 | - |
| SCL (kWh) | 66.78 | 44.52 | 91 | 853.6 | 527.95 | 13.13 |
| Total ICL (kWh) | 66.78 | 44.52 | 91 | 992.2 | 597.25 | 23.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, J.; Corral, C.; García-Valverde, M.T.; Hidalgo-García, J.; Ferreiro-Vera, C.; Martínez-Quesada, J.J. Subcanopy and Inter-Canopy Supplemental Light Enhances and Standardizes Yields in Medicinal Cannabis (Cannabis sativa L.). Plants 2025, 14, 1469. https://doi.org/10.3390/plants14101469
Garrido J, Corral C, García-Valverde MT, Hidalgo-García J, Ferreiro-Vera C, Martínez-Quesada JJ. Subcanopy and Inter-Canopy Supplemental Light Enhances and Standardizes Yields in Medicinal Cannabis (Cannabis sativa L.). Plants. 2025; 14(10):1469. https://doi.org/10.3390/plants14101469
Chicago/Turabian StyleGarrido, José, Carolina Corral, María Teresa García-Valverde, Jesús Hidalgo-García, Carlos Ferreiro-Vera, and Juan José Martínez-Quesada. 2025. "Subcanopy and Inter-Canopy Supplemental Light Enhances and Standardizes Yields in Medicinal Cannabis (Cannabis sativa L.)" Plants 14, no. 10: 1469. https://doi.org/10.3390/plants14101469
APA StyleGarrido, J., Corral, C., García-Valverde, M. T., Hidalgo-García, J., Ferreiro-Vera, C., & Martínez-Quesada, J. J. (2025). Subcanopy and Inter-Canopy Supplemental Light Enhances and Standardizes Yields in Medicinal Cannabis (Cannabis sativa L.). Plants, 14(10), 1469. https://doi.org/10.3390/plants14101469







