Exogenous GA3 Promotes Germination by Reducing Endogenous Inhibitors in Sainfoin (Onobrychis viciifolia) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
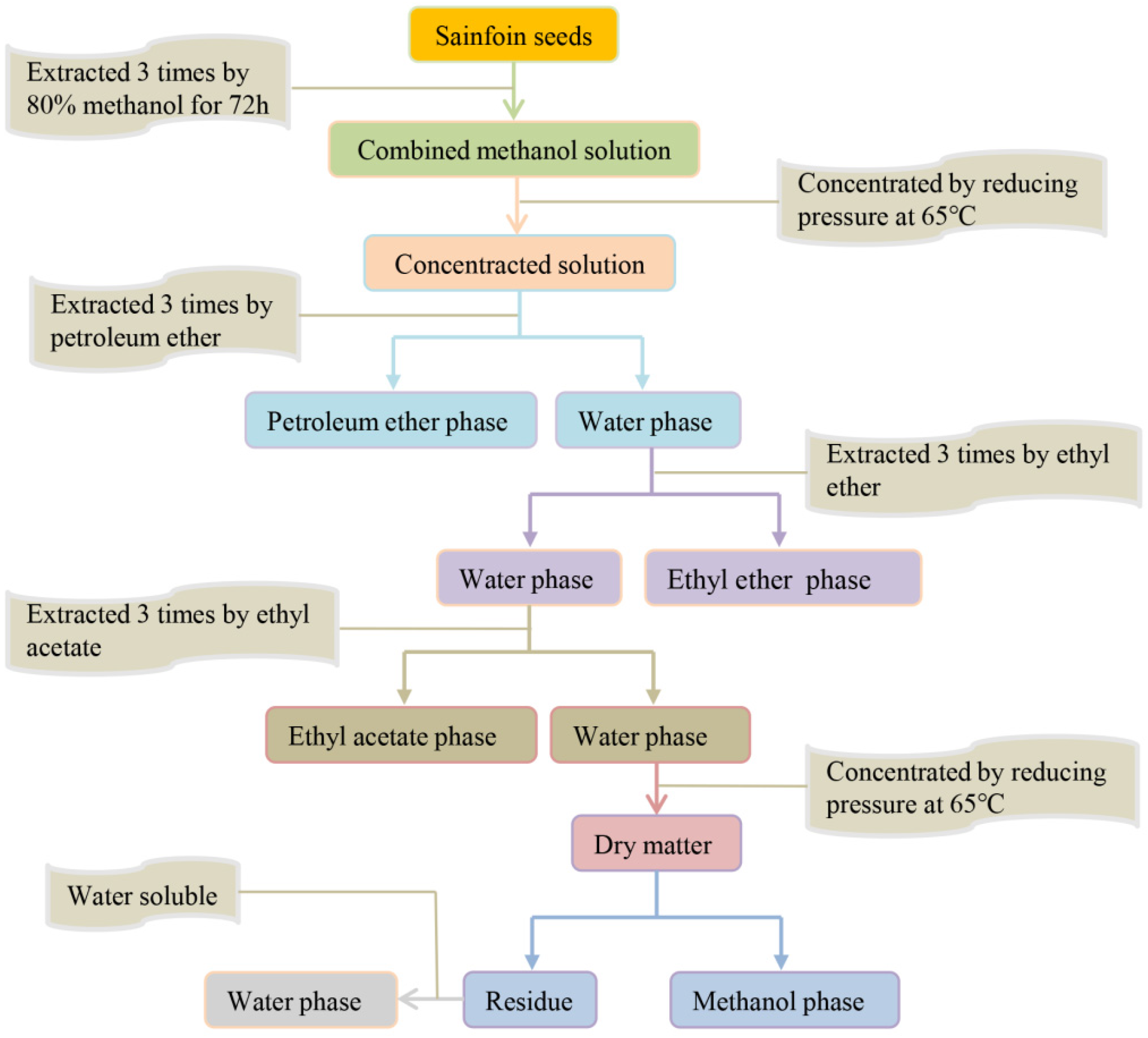
2.2. Extraction of Endogenous Inhibitors from Different Parts of Sainfoin Seeds
2.3. Preliminary Isolation of Endogenous Inhibitors from Sainfoin Seeds
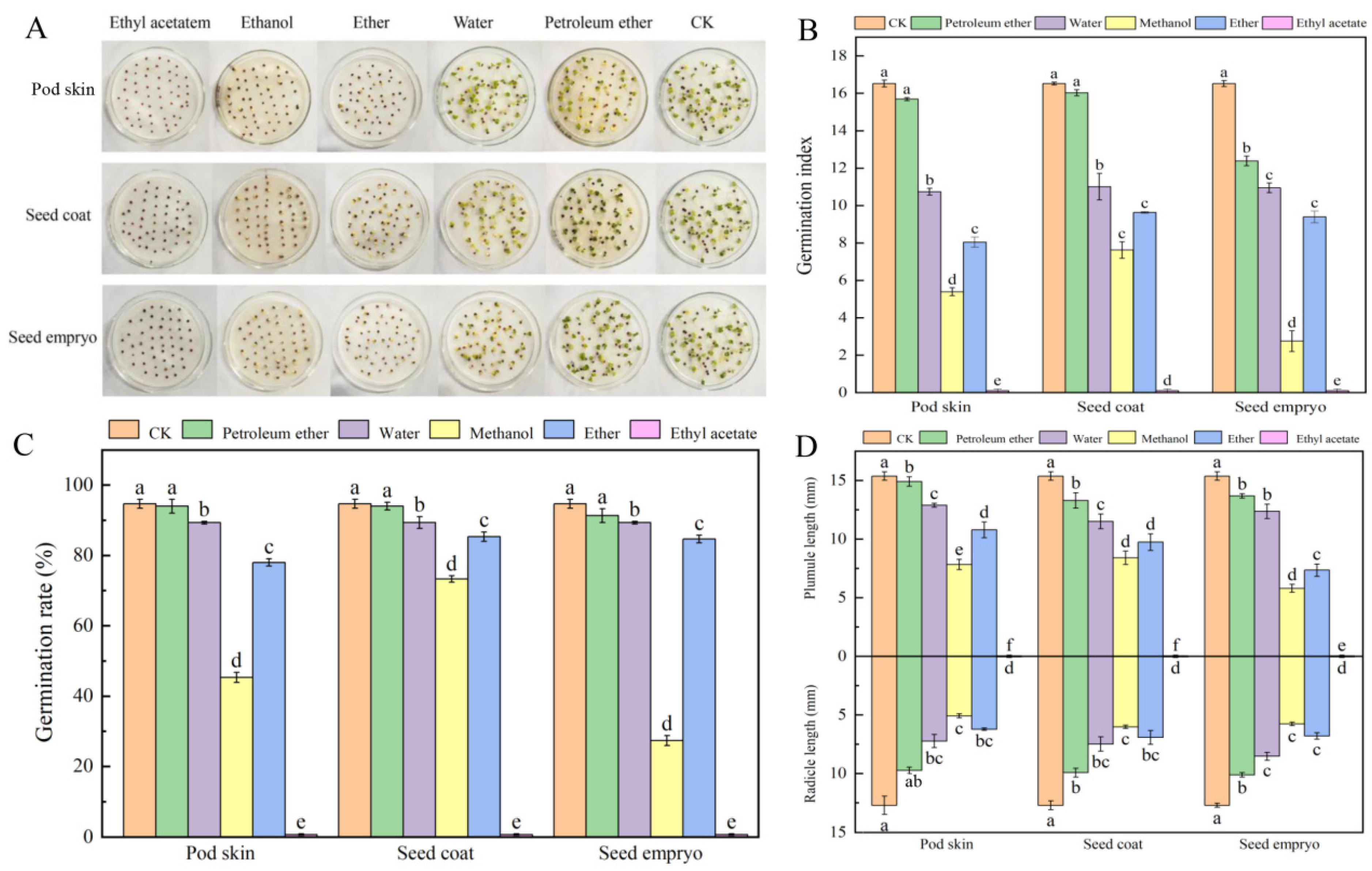
2.4. Determination of the Activity of Each Isolated Phase Using Cabbage
2.5. Determination of the Chemical Composition of Each Isolated Phase in the Sainfoin Seeds
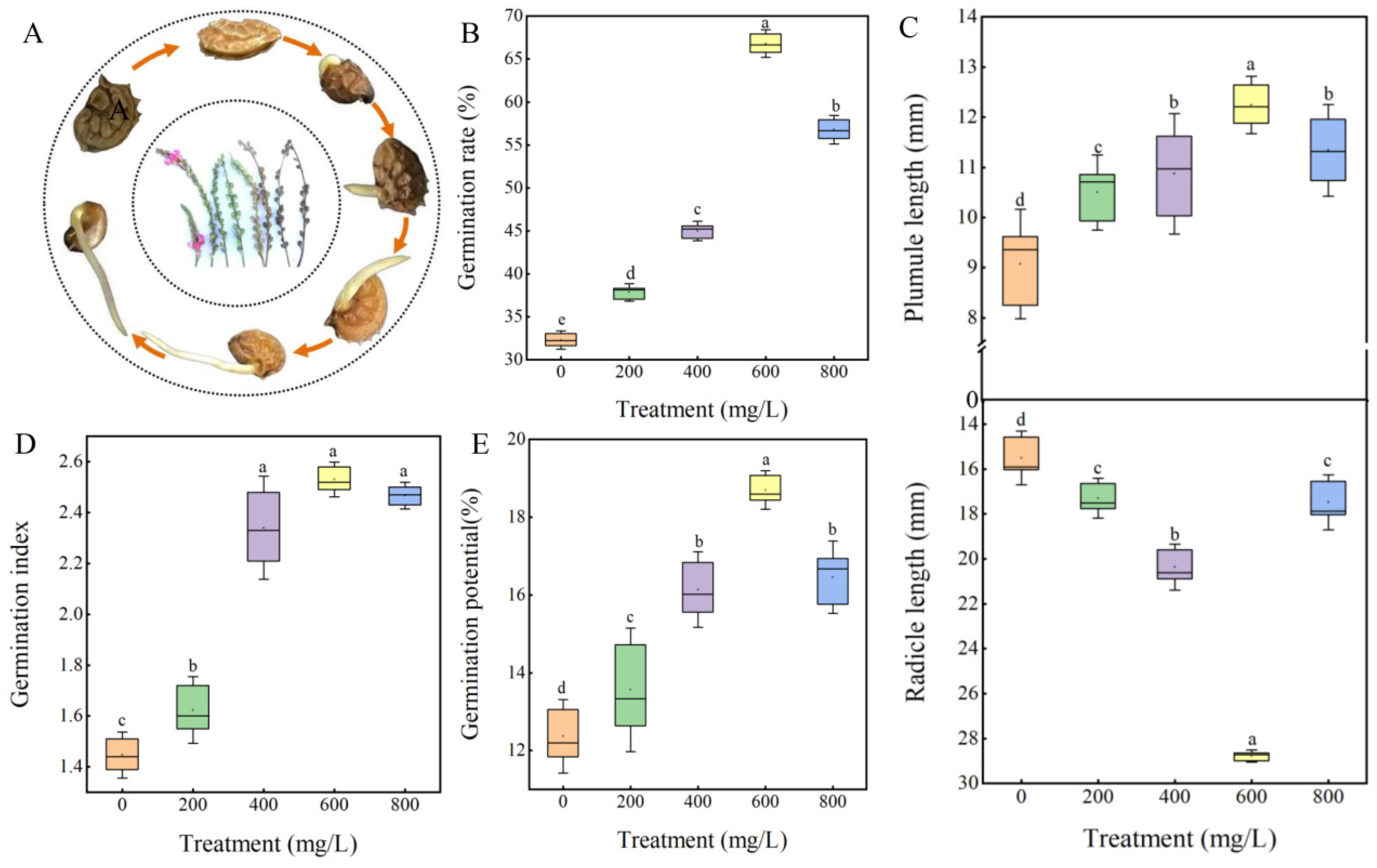
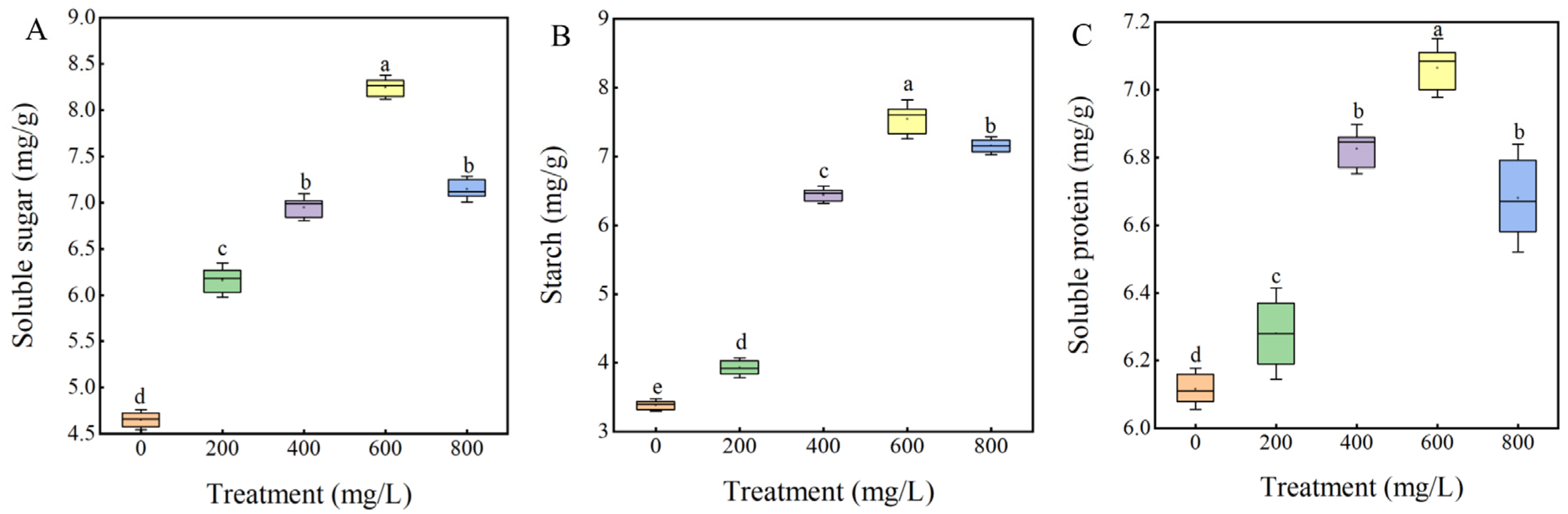
2.6. Sainfoin Seed Germination and Measurement of Physiological Indicators
2.7. Statistical Analysis
3. Results
3.1. Determination of the Biological Activities of the Isolated Phases Using Cabbage
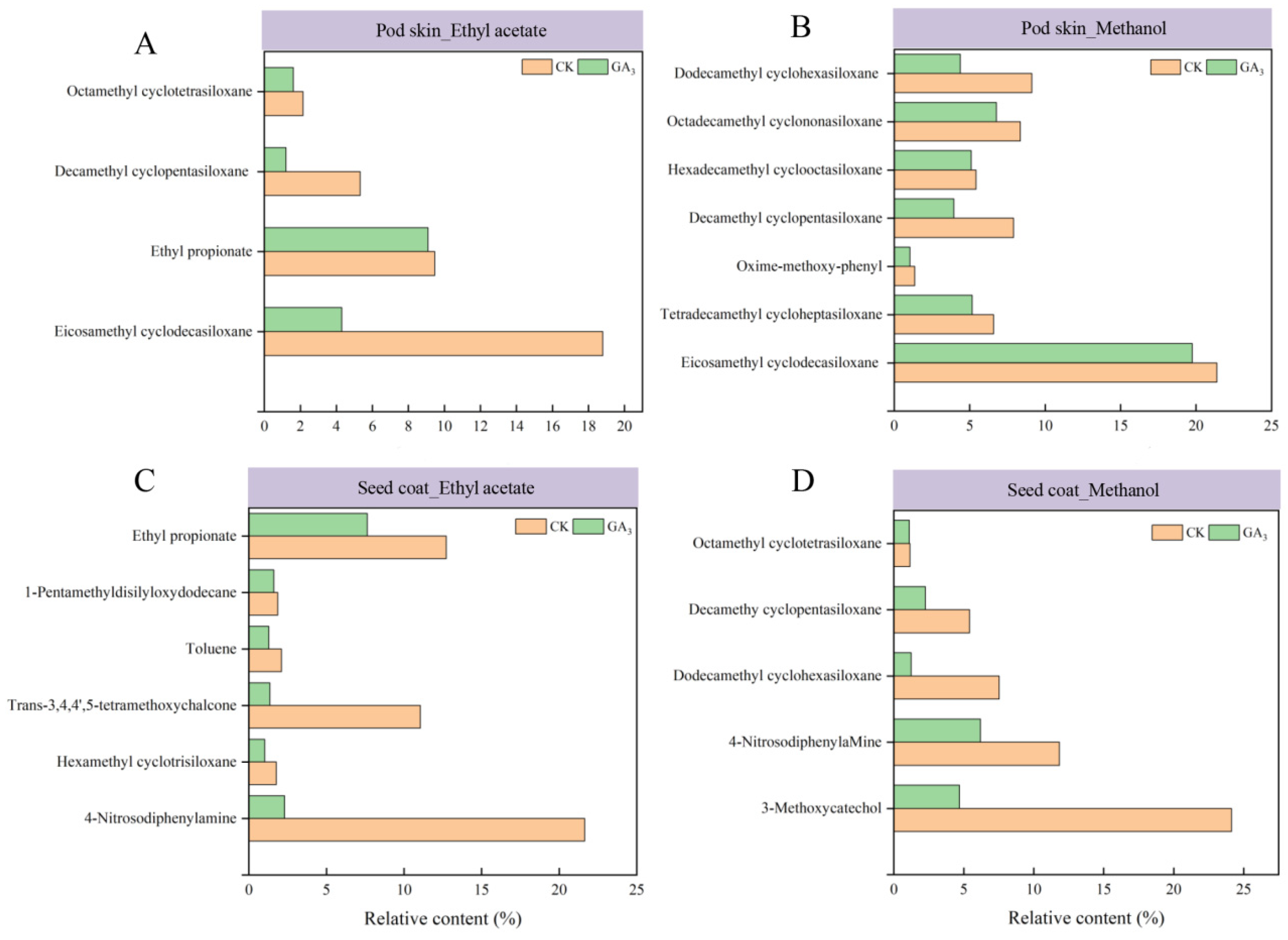
3.2. Identification of the Chemical Composition of the Isolated Phases from Sainfoin Seeds
3.3. Effect of GA3 on the Seed Germination of Sainfoin
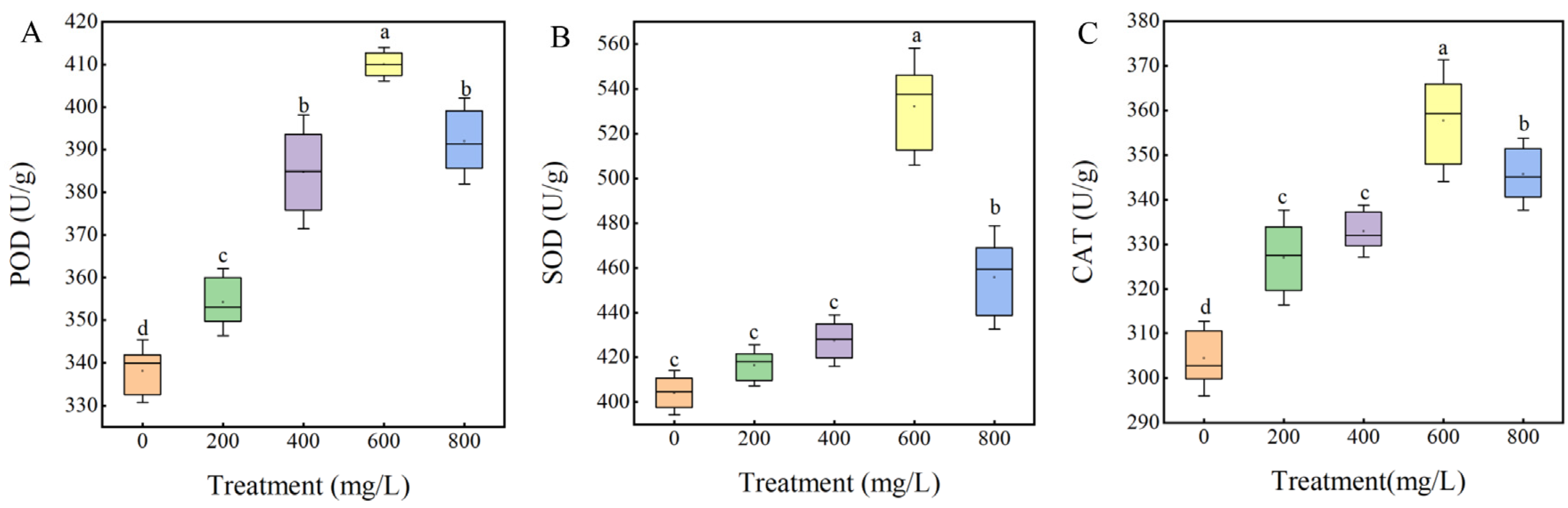
3.4. Effect of GA3 on Physiological Indexes of Sainfoin Seeds
3.5. Effect of GA3 on Endogenous Inhibitors in Sainfoin Seeds
3.6. Effect of GA3 on Endogenous Hormones in Sainfoin Seeds
4. Discussion
4.1. Sainfoin Seeds Contain Endogenous Inhibitors
4.2. GA3 Promotes Sainfoin Seed Germination by Altering the Content Levels of Storage Substances and Antioxidant Enzymes
4.3. GA3 May Regulate the Levels of Endogenous Inhibitors and Endogenous Hormones of Sainfoin Seeds
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, Z.; Jiang, W.; Luo, Y.; Huang, H.; Yi, D.; Pang, Y. Analyses on flavonoids and transcriptome reveals key MYB gene for proanthocyanidins regulation in Onobrychis viciifolia. Front. Plant Sci. 2022, 13, 941918. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tian, D.; Li, X.; Wang, X.; Wang, T.; Wang, Z.; Zang, H.; He, X.; Zhang, T.; Yun, Q.; et al. A chromosome-level genome assembly for Onobrychis viciifolia reveals gene copy number gain underlying enhanced proanthocyanidin biosynthesis. Commun. Biol. 2024, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Wang, X.; Ma, C.; Zhang, F. Effects of intrinsic tannins on proteolysis dynamics, protease activity, and metabolome during sainfoin ensiling. Front. Microbiol. 2022, 13, 976118. [Google Scholar] [CrossRef]
- Huyen, N.T.; Verstegen, M.W.; Hendriks, W.H.; Pellikaan, W.F. Sainfoin (Onobrychis viciifolia) silage in dairy cow rations reduces ruminal biohydrogenation and increases transfer efficiencies of unsaturated fatty acids from feed to milk. Animal Nutr. 2020, 6, 333–341. [Google Scholar] [CrossRef]
- Rivaroli, D.; Prunier, A.; Meteau, K.; Prache, S. Tannin-rich sainfoin pellet supplementation reduces fat volatile indoles content and delays digestive parasitism in lambs grazing alfalfa. Animal 2019, 13, 1883–1890. [Google Scholar] [CrossRef]
- Biligetu, B.; Jefferson, P.G.; Lardner, H.A.; Acharya, S.N. Evaluation of sainfoin (Onobrychis viciifolia) for forage yield and persistence in sainfoin–alfalfa (Medicago sativa) mixtures and under different harvest frequencies. Can. J. Plant Sci. 2021, 101, 525–535. [Google Scholar] [CrossRef]
- Qiao, Y.; Cheng, Q.; Zhang, Y.; Yan, W.; Yi, F.; Shi, F. Transcriptomic and chemical analyses to identify candidate genes involved in color variation of sainfoin flowers. BMC Plant Biol. 2021, 21, 61. [Google Scholar] [CrossRef]
- Peng, C.Y.; Wu, Y.; Shi, F.H.; Shen, Y.B. Review of the current research progress of seed germination inhibitors. Horticulturae 2023, 9, 462. [Google Scholar] [CrossRef]
- Bingöl, Ö.; Battal, A.; Aslan, A.; Emre, E. Investigation of the allelopathic effects of lyophilized ethanol extract of Xanthoparmelia somloensis (Gyelnik) Hale lichen on tomato plant. Anatol. J. Bot. 2022, 6, 39–43. [Google Scholar] [CrossRef]
- Evenari, M. Germination inhibitors. Bot. Rev. 1949, 15, 153–194. [Google Scholar] [CrossRef]
- Shi, S.; Cheng, J.; Ahmad, N.; Zhao, W.; Tian, M.; Yuan, Z.; Li, C.; Zhao, C. Effects of potential allelochemicals in a water extract of Abutilon theophrasti Medik. on germination and growth of Glycine max L., Triticum aestivum L., and Zea mays L. J. Sci. Food Agric. 2019, 103, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, M.; Wang, H.; Li, S. Dynamic changes to endogenous germination inhibitors in Cercis chinensis seeds during dormancy release. HortScience 2021, 56, 557–562. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, S.; Lingakumar, K. Role of salicylic acid (SA) in plants—A review. Int. J. Appl. Res. 2017, 3, 33–37. [Google Scholar]
- Zahid, Z.; Khan, M.K.R.; Hameed, A.; Akhtar, M.; Ditta, A.; Hassan, H.M.; Farid, G. Dissection of drought tolerance in upland cotton through morpho-physiological and biochemical traits at seedling stage. Front. Plant Sci. 2021, 12, 627107. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, H.; Hou, L.; Zhang, C.; Bo, W.; Pang, X.; Li, Y. HPLC-MS/MS-based and transcriptome analysis reveal the effects of ABA and MeJA on jujube (Ziziphus jujuba Mill.) cracking. Food Chem. 2023, 421, 136155. [Google Scholar] [CrossRef]
- Benítez, S.P.; Mario, L.; Delgado, O.A.; Medina, C.I. Highland papayas Vasconcellea cundinamarcensis and Vasconcellea goudotiana seed germination and dormancy release studies. Cienc. Y Tecnol. Agropecuaria 2013, 14, 187–197. [Google Scholar] [CrossRef]
- Graeber, K.A.I.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.E.R.H.A.R.D.; Soppe, W.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef]
- Murdoch, A.J. Seeds: Ecology, biogeography, and evolution of dormancy and germination. Crop Sci. 2000, 40, 564. [Google Scholar] [CrossRef]
- Shah, F.A.; Ni, J.; Chen, J.; Wang, Q.; Liu, W.; Chen, X.; Tang, C.; Fu, S.; Wu, L. Proanthocyanidins in seed coat tegmen and endospermic cap inhibit seed germination in Sapium sebiferum. PeerJ 2018, 6, e4690. [Google Scholar] [CrossRef]
- Zhao, T.; Qian, C.; Gao, Y.; Chen, L.; Zhu, M.; Pan, Y.; Li, S. Germination inhibitors detected in Sapium sebiferum seeds. J. For. Res. 2019, 30, 2305–2312. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Z.; Cheng, C.; Wang, T.; Ji, H.; Zhao, Y.; Deng, Z.; Zhi, L.; Lu, J.; Wu, X.; et al. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis. Mol. Plant 2020, 13, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, L.; Huang, M.; He, X.; Yang, Y.; Liu, X.; Li, Y.; Hou, X. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nat. Plants 2018, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Lei, N.F.; Su, Z.X.; Chen, J.S.; Guo, J.H. Germination inhibitors in fruit of rare and endangered Davidia involucrate. Chin. J. Appl. Environ. Biol. 2003, 9, 607–610. [Google Scholar]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of hormonal regulation in rice seed germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Seed dormancy and germination. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar]
- Yan, H.; Chen, H.; Xia, M.; Liao, Q.; Zhao, J.; Peng, L.; Zou, L.; Zhao, G. The impacts of plant hormones on the growth and quality of sprouts. Food Bioprocess Tech. 2024, 17, 2913–2942. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.S.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Tang, S.Y.; Cui, N.J.; Feng, Y.C.; Zhang, J.; Liu, Y. Effect of gibberellin concentration on seed germination of Acer mono Maxim. Chin. J. Appl. Environ. Biol. 2021, 27, 555–559. [Google Scholar]
- Guo, Y.H.; Lin, H.M. Regulatory effect of GA and KT on seeds germination of Nitraria tangutorum Bobr. Chin. J. Eco-Agric. 2009, 17, 1196–1199. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, J.L.; Ramos, A.R.G.; Lorente, G.G.Y.; González-Olmedo, J.L.; Martínez Montero, M.E. ROS production and antioxidant enzyme activity in relation to germination and vigor during tobacco seed development. Vegetos 2023, 36, 506–515. [Google Scholar] [CrossRef]
- Tonguç, M.; Güler, M.; Önder, S. Germination, reserve metabolism and antioxidant enzyme activities in safflower as affected by seed treatments after accelerated aging. S. Afr. J. Bot. 2023, 153, 209–218. [Google Scholar] [CrossRef]
- Ghannad, R.; Haghjou, M.M.; Raza, A.; Hasanuzzaman, M. Induction of hydrolytic enzyme activities in dormant seeds of Dracocephalum kotschyi Boiss. causes improvement of germination and seedling vigor indices. Acta Physiol. Plant. 2022, 44, 48. [Google Scholar] [CrossRef]
- Bailly, C. ROS in seed germination. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2023; Volume 105, pp. 177–204. [Google Scholar]
- Weerasekara, I.; Sinniah, U.R.; Namasivayam, P.; Nazli, M.H.; Abdurahman, S.A.; Ghazali, M.N. The influence of seed production environment on seed development and quality of Soybean (Glycine max (L.) Merrill). Agronomy 2021, 11, 1430. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Bioch. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, Z.; Zhao, Y.Z. Effect of exogenous GA3 on the growth and physiological basis of rice seedling under NaCl stress. North. Rice 2013, 43, 4–7. [Google Scholar]
- Ke, B.Y.; Zhou, C.Y.; Li, J.Y.; Pan, J.; Zhou, P. Study on methods of seed dormancy and dormancy-release for rare species Acrocarpus fraxinifolius. Seed 2020, 39, 139–143. [Google Scholar]
- Song, J.; Zhang, H.; Zhang, Y.; Yue, H. Effect of stratification after immersion of gibberellin on seed germination of Plagiorhegma dubia Maxim. J. Northeast. For. Univ. 2021, 12, 34–39. [Google Scholar]
- Chen, B.X.; Peng, Y.X.; Yang, X.Q.; Liu, J. Delayed germination of Brassica parachinensis seeds by coumarin involves decreased GA4 production and a consequent reduction of ROS accumulation. Seed Sci. Res. 2021, 31, 224–235. [Google Scholar] [CrossRef]
- Eiuhellin, E.A. Mechanism of action of allelochem icals in allelpothy. Allelopathy 1995, 1, 97–115. [Google Scholar]
- Chen, B.X.; Peng, Y.X.; Gao, J.D.; Zhang, Q.; Liu, Q.J.; Fu, H.; Liu, J. Coumarin-induced delay of rice seed germination is mediated by suppression of abscisic acid catabolism and reactive oxygen species production. Front. Plant Sci. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.R. Seed Physiology; Agricultural Press: Beijing, China, 1992. [Google Scholar]
- Xu, Y.X.; Tang, X.-H.; Fu, J.-R. Progress of Research on Seed Physiology; Zhongshan University Press: Guangzhou, China, 1987. [Google Scholar]
- Yu, F.; Li, M.; He, D.; Yang, P. Advances on post-translational modifications involved in seed germination. Front. Plant Sci. 2021, 12, 642979. [Google Scholar] [CrossRef] [PubMed]
- Do, B.H.; Phuong, V.T.B.; Tran, G.B.; Nguyen, N.H. Emerging functions of chromatin modifications in auxin biosynthesis in response to environmental alterations. Plant Growth Regul. 2019, 87, 165–174. [Google Scholar] [CrossRef]
- Lv, B.; Yu, Q.; Liu, J.; Wen, X.; Yan, Z.; Hu, K.; Li, H.; Kong, X.; Li, C.; Tian, H.; et al. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J. 2020, 39, e101515. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Chen, A.; Saleem, N.; Jia, Q.; Zhao, C.; Li, W.; Zhang, M. FUSCA3-induced AINTEGUMENTA-like 6 manages seed dormancy and lipid metabolism. Plant Physiol. 2023, 193, 1091–1108. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Rep. 2017, 36, 689–703. [Google Scholar] [CrossRef]
- Meng, H.; Chen, Y.; Li, T.; Shi, H.; Yu, S.; Gao, Y.; Wang, Z.; Wang, X.; Zhu, J.K.; Hong, Y.; et al. APETALA2 is involved in ABA signaling during seed germination. Plant Mol. Biol. 2023, 112, 99–103. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, S.C. Arabidopsis SnRK2.3/SRK2I plays a positive role in seed germination under cold stress conditions. Environ. Exp. Bot. 2023, 212, 105399. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic regulation of ABA and GA in metabolism and signaling pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef]
- Awale, P.; McSteen, P. Hormonal regulation of inflorescence and intercalary meristems in grasses. Curr. Opin. Plant Biol. 2023, 76, 102451. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wang, K.; Yao, Y.; Nan, L. Exogenous GA3 Promotes Germination by Reducing Endogenous Inhibitors in Sainfoin (Onobrychis viciifolia) Seeds. Plants 2025, 14, 1464. https://doi.org/10.3390/plants14101464
Luo Y, Wang K, Yao Y, Nan L. Exogenous GA3 Promotes Germination by Reducing Endogenous Inhibitors in Sainfoin (Onobrychis viciifolia) Seeds. Plants. 2025; 14(10):1464. https://doi.org/10.3390/plants14101464
Chicago/Turabian StyleLuo, Yanyan, Kun Wang, Yuheng Yao, and Lili Nan. 2025. "Exogenous GA3 Promotes Germination by Reducing Endogenous Inhibitors in Sainfoin (Onobrychis viciifolia) Seeds" Plants 14, no. 10: 1464. https://doi.org/10.3390/plants14101464
APA StyleLuo, Y., Wang, K., Yao, Y., & Nan, L. (2025). Exogenous GA3 Promotes Germination by Reducing Endogenous Inhibitors in Sainfoin (Onobrychis viciifolia) Seeds. Plants, 14(10), 1464. https://doi.org/10.3390/plants14101464






