Floral Closure in Lesser Celandine (Ficaria verna) Protects Anthers from Pollen Flushing and Preserves Pollen Viability
Abstract
:1. Introduction
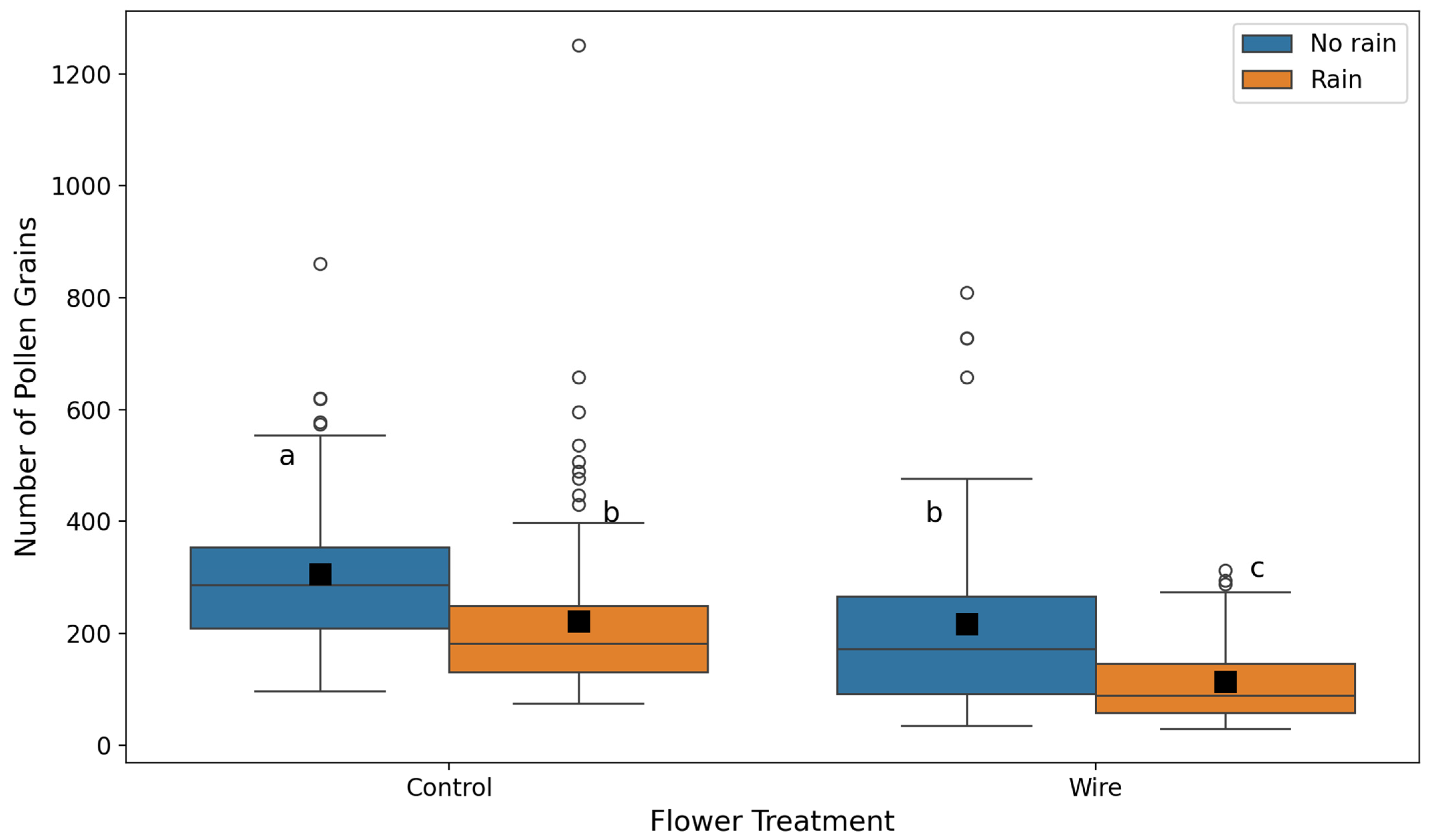
2. Results
2.1. Simulated Rain Experiment
2.2. Natural Rain
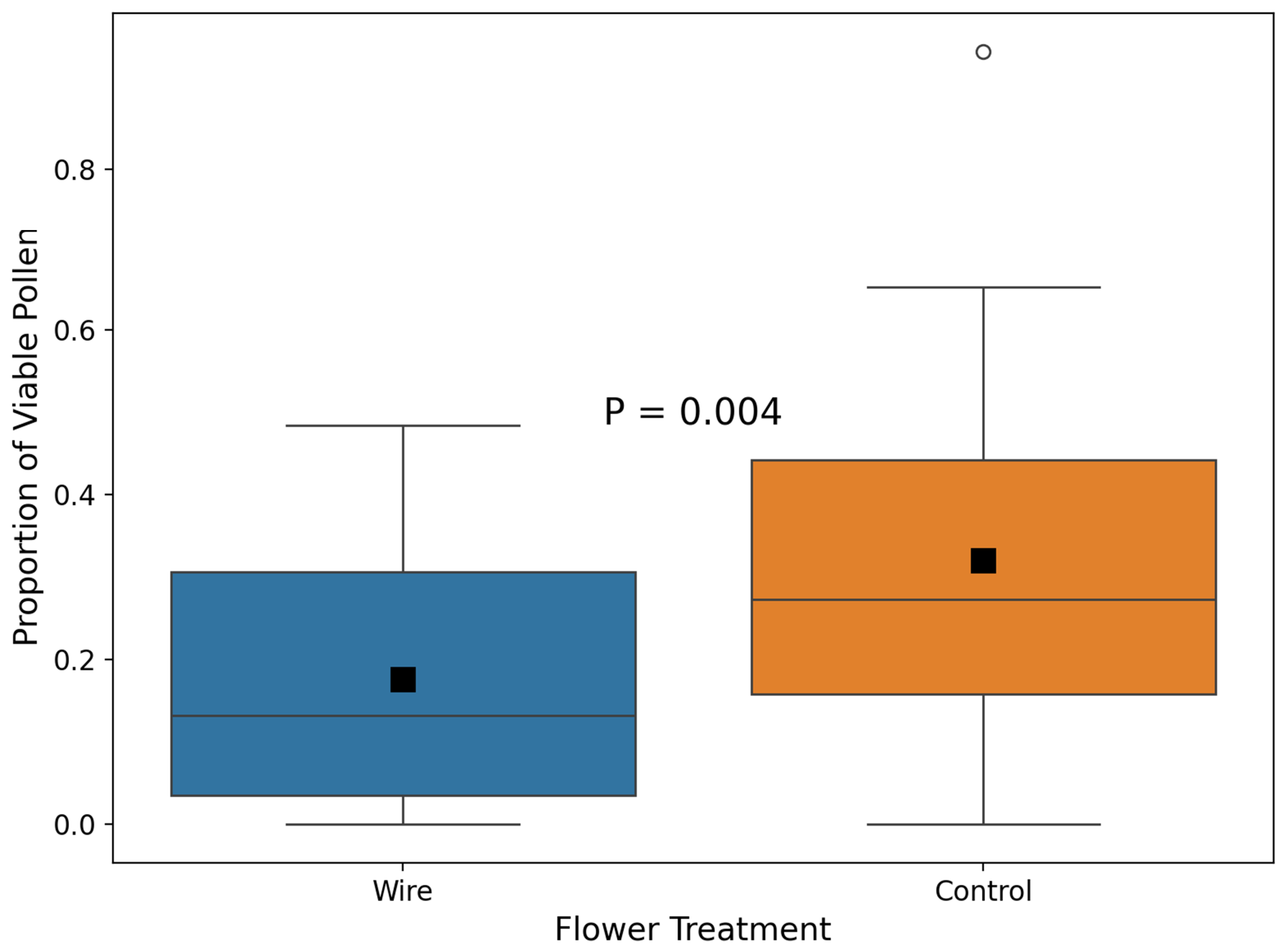
2.3. Pollen Viability
3. Discussion
4. Materials and Methods
4.1. Study Species
4.2. Study Area
4.3. Procedure
4.4. Simulated Rain Experiment
4.5. Natural Rain Experiment
4.6. Pollen Count
4.7. Pollen Viability
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdusalam, A.; Tan, D.Y. Contribution of Temporal Floral Closure to Reproductive Success of the Spring-Flowering Tulipa iliensis. J. Syst. Evol. 2014, 52, 186–194. [Google Scholar] [CrossRef]
- Bynum, M.R.; Smith, W.K. Floral Movements in Response to Thunderstorms Improve Reproductive Effort in the Alpine Species Gentiana algida (Gentianaceae). Am. J. Bot. 2001, 88, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Husband, B.C. Plasticity and Timing of Flower Closure in Response to Pollination in Chamerion angustifolium (Onagraceae). Int. J. Plant Sci. 2007, 168, 619–625. [Google Scholar] [CrossRef]
- Dafni, A.; Firmage, D. Pollen Viability and Longevity: Practical, Ecological and Evolutionary Implications. Plant Syst. Evol. 2000, 222, 113–132. [Google Scholar] [CrossRef]
- Franchi, G.G.; Nepi, M.; Pacini, E. Is Flower/Corolla Closure Linked to Decrease in Viability of Desiccation-Sensitive Pollen? Facts and Hypotheses: A Review of Current Literature with the Support of Some New Experimental Data. Plant Syst. Evol. 2014, 300, 577–584. [Google Scholar] [CrossRef]
- Frankel, R.; Galun, E. Pollination Mechanisms, Reproduction and Plant Breeding; Springer: New York, NY, USA, 2012; Volume 2. [Google Scholar]
- He, Y.P.; Duan, Y.W.; Liu, J.Q.; Smith, W.K. Floral Closure in Response to Temperature and Pollination in Gentiana straminea Maxim. (Gentianaceae), an Alpine Perennial in the Qinghai-Tibetan Plateau. Plant Syst. Evol. 2005, 256, 17–33. [Google Scholar] [CrossRef]
- Huang, S.Q.; Takahashi, Y.; Dafni, A. Why Does the Flower Stalk of Pulsatilla cernua (Ranunculaceae) Bend During Anthesis? Am. J. Bot. 2002, 89, 1599–1603. [Google Scholar] [CrossRef]
- Imbert, E.; Wang, H.; Conchou, L.; Vincent, H.; Talavera, M.; Schatz, B. Positive Effect of the Yellow Morph on Female Reproductive Success in the Flower Colour Polymorphic Iris lutescens (Iridaceae), a Deceptive Species. J. Evol. Biol. 2014, 27, 1965–1974. [Google Scholar] [CrossRef]
- Kemp, J.E.; Ellis, A.G. Cryptic Petal Coloration Decreases Floral Apparency and Herbivory in Nocturnally Closing Daisies. Funct. Ecol. 2019, 33, 2130–2141. [Google Scholar] [CrossRef]
- Lawson, D.A.; Rands, S.A. The Effects of Rainfall on Plant–Pollinator Interactions. Arthropod-Plant Interact. 2019, 13, 561–569. [Google Scholar] [CrossRef]
- Mao, Y.Y.; Huang, S.Q. Pollen Resistance to Water in 80 Angiosperm Species: Flower Structures Protect Rain-Susceptible Pollen. New Phytol. 2009, 183, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Marsden-Jones, E.M. Ranunculus ficaria Linn: Life History and Pollination. J. Linn. Soc. Bot. 1935, 50, 39–55. [Google Scholar] [CrossRef]
- Mauseth, J.D. Structure–Function Relationships in Highly Modified Shoots of Cactaceae. Ann. Bot. 2006, 98, 901–926. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical Factors Responsible for Fungi Growth in Stored Food Grains and Non-Chemical Approaches for Their Control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Nakata, T.; Rin, I.; Yaida, Y.A.; Ushimaru, A. Horizontal Orientation Facilitates Pollen Transfer and Rain Damage Avoidance in Actinomorphic Flowers of Platycodon grandiflorus. Plant Biol. 2022, 24, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Franchi, G.G.; Pacini, E. Pollen Hydration Status at Dispersal: Cytophysiological Features and Strategies. Protoplasma 2001, 216, 171–180. [Google Scholar] [CrossRef]
- Nesse, R.M. The Smoke Detector Principle: Natural Selection and the Regulation of Defensive Responses. Ann. N. Y. Acad. Sci. 2001, 935, 75–85. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Kessler, A. Pollinators Exert Natural Selection on Flower Size and Floral Display in Penstemon digitalis. New Phytol. 2010, 188, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Das, R.; Chetry, K.; Baishnab, B.; Banik, B.; Datta, B.K. Permanent Floral Closure Enhances the Reproductive Efficiency in Merremia umbellata subsp. orientalis (Hallier f.) Ooststr. (Convolvulaceae). Plant Ecol. 2023, 224, 491–500. [Google Scholar]
- Prokop, P.; Fedor, P. Why Do Flowers Close at Night? Experiments with the Lesser Celandine Ficaria verna Huds (Ranunculaceae). Biol. J. Linn. Soc. 2016, 118, 698–702. [Google Scholar] [CrossRef]
- Prokop, P.; Jersáková, J.; Fančovičová, J.; Pipíška, M. Flower Closure Enhances Pollen Viability in Crocus discolor G. Reuss. Flora 2019, 250, 68–71. [Google Scholar] [CrossRef]
- Prokop, P.; Ježová, Z.; Mešková, M.; Vanerková, V.; Zvaríková, M.; Fedor, P. Flower Angle Favors Pollen Export Efficiency in the Snowdrop Galanthus nivalis (Linnaeus, 1753) but Not in the Lesser Celandine Ficaria verna (Huds, 1762). Plant Signal. Behav. 2023, 18, 2163065. [Google Scholar] [CrossRef] [PubMed]
- Prokop, P.; Zvaríková, M.; Ježová, Z.; Fedor, P. Functional Significance of Flower Orientation and Green Marks on Tepals in the Snowdrop Galanthus nivalis (Linnaeus, 1753). Plant Signal. Behav. 2020, 15, 1807153. [Google Scholar] [CrossRef]
- Rodriguez-Riano, T.; Dafni, A. A New Procedure to Assess Pollen Viability. Sex. Plant Reprod. 2000, 12, 241–244. [Google Scholar] [CrossRef]
- Schwarz, B.; Dormann, C.F.; Vázquez, D.P.; Fründ, J. Within-Day Dynamics of Plant–Pollinator Networks Are Dominated by Early Flower Closure: An Experimental Test of Network Plasticity. Oecologia 2021, 196, 781–794. [Google Scholar] [CrossRef]
- Tagawa, K.; Osaki, H.; Watanabe, M. Rapid Flower Closure of Drosera tokaiensis Deters Caterpillar Herbivory. Biol. Lett. 2022, 18, 20220373. [Google Scholar] [CrossRef]
- Taylor, K.; Markham, B. Ranunculus Ficaria L. (Ficaria verna Huds.; F. Ranunculoides Moench). J. Ecol. 1978, 66, 1011–1031. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 2.4) [Computer Software]. 2023. Available online: https://www.jamovi.org (accessed on 10 April 2025).
- van der Kooi, C.J.; Kevan, P.G.; Koski, M.H. The Thermal Ecology of Flowers. Ann. Bot. 2019, 124, 343–353. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.G.; Van Meeteren, U. Flower Opening and Closure: A Review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef]
- Von Hase, A.; Cowling, R.M.; Ellis, A.G. Petal Movement in Cape Wild Flowers Protects Pollen from Exposure to Moisture. Plant Ecol. 2006, 184, 75–87. [Google Scholar] [CrossRef]
- Werker, E. Trichome Diversity and Development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokop, P.; Provazník, Z.; Tučník, K. Floral Closure in Lesser Celandine (Ficaria verna) Protects Anthers from Pollen Flushing and Preserves Pollen Viability. Plants 2025, 14, 1437. https://doi.org/10.3390/plants14101437
Prokop P, Provazník Z, Tučník K. Floral Closure in Lesser Celandine (Ficaria verna) Protects Anthers from Pollen Flushing and Preserves Pollen Viability. Plants. 2025; 14(10):1437. https://doi.org/10.3390/plants14101437
Chicago/Turabian StyleProkop, Pavol, Zuzana Provazník, and Kristián Tučník. 2025. "Floral Closure in Lesser Celandine (Ficaria verna) Protects Anthers from Pollen Flushing and Preserves Pollen Viability" Plants 14, no. 10: 1437. https://doi.org/10.3390/plants14101437
APA StyleProkop, P., Provazník, Z., & Tučník, K. (2025). Floral Closure in Lesser Celandine (Ficaria verna) Protects Anthers from Pollen Flushing and Preserves Pollen Viability. Plants, 14(10), 1437. https://doi.org/10.3390/plants14101437





