Pyroxsulam Resistance in Apera spica-venti: An Emerging Challenge in Crop Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Dose–Response Assay
2.3. Cross- and Multiple-Resistance Experiments with Other Herbicides
2.4. In Vitro ALS Activity Assay
2.5. Partial ALS Gene Substitution and Overexpression Studies
2.6. 14C Pyroxsulam Absorption and Translocation Study
2.7. Statistical Analysis
3. Results
3.1. Whole-Plant Dose–Response Experiments
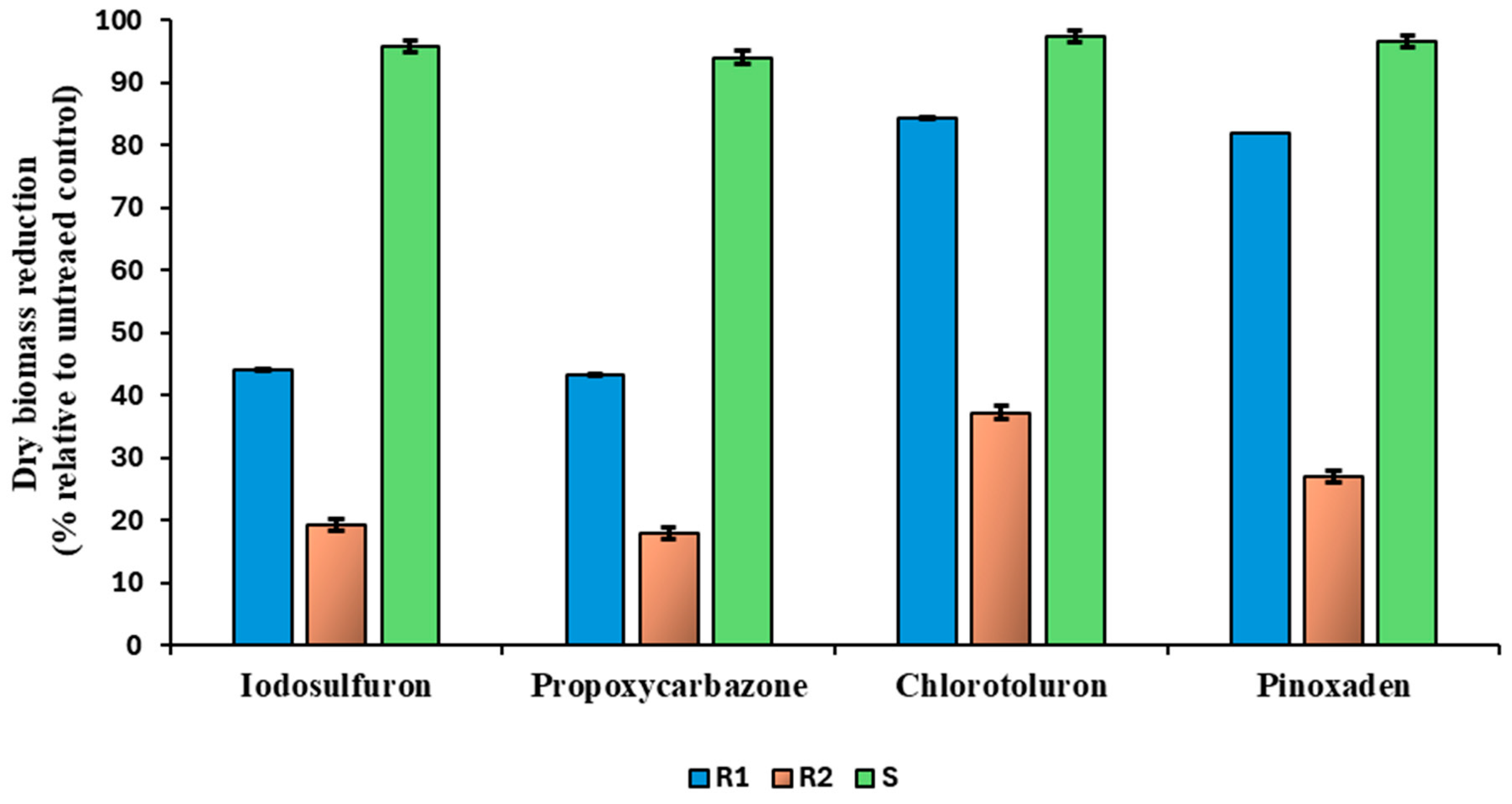
3.2. Cross and Multiple Resistance to Other Herbicides
3.3. ALS Enzyme Activity
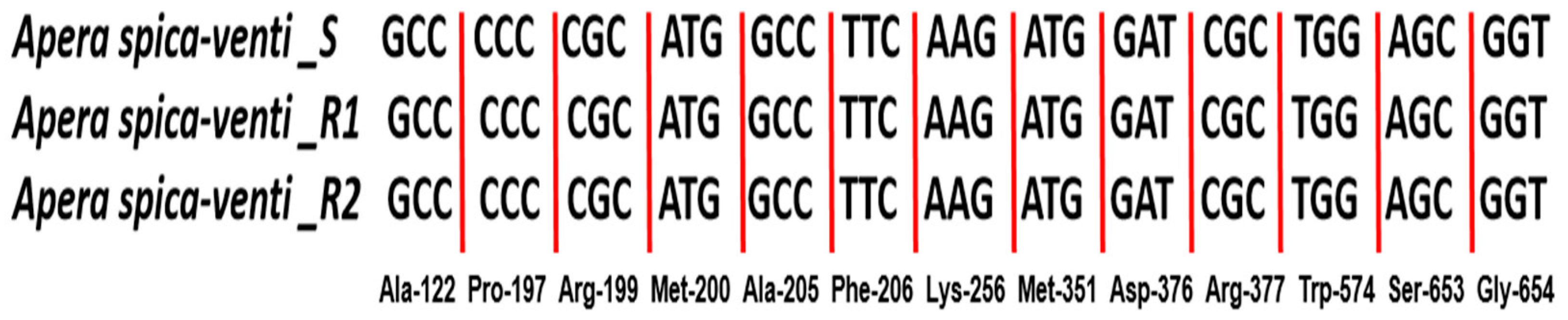
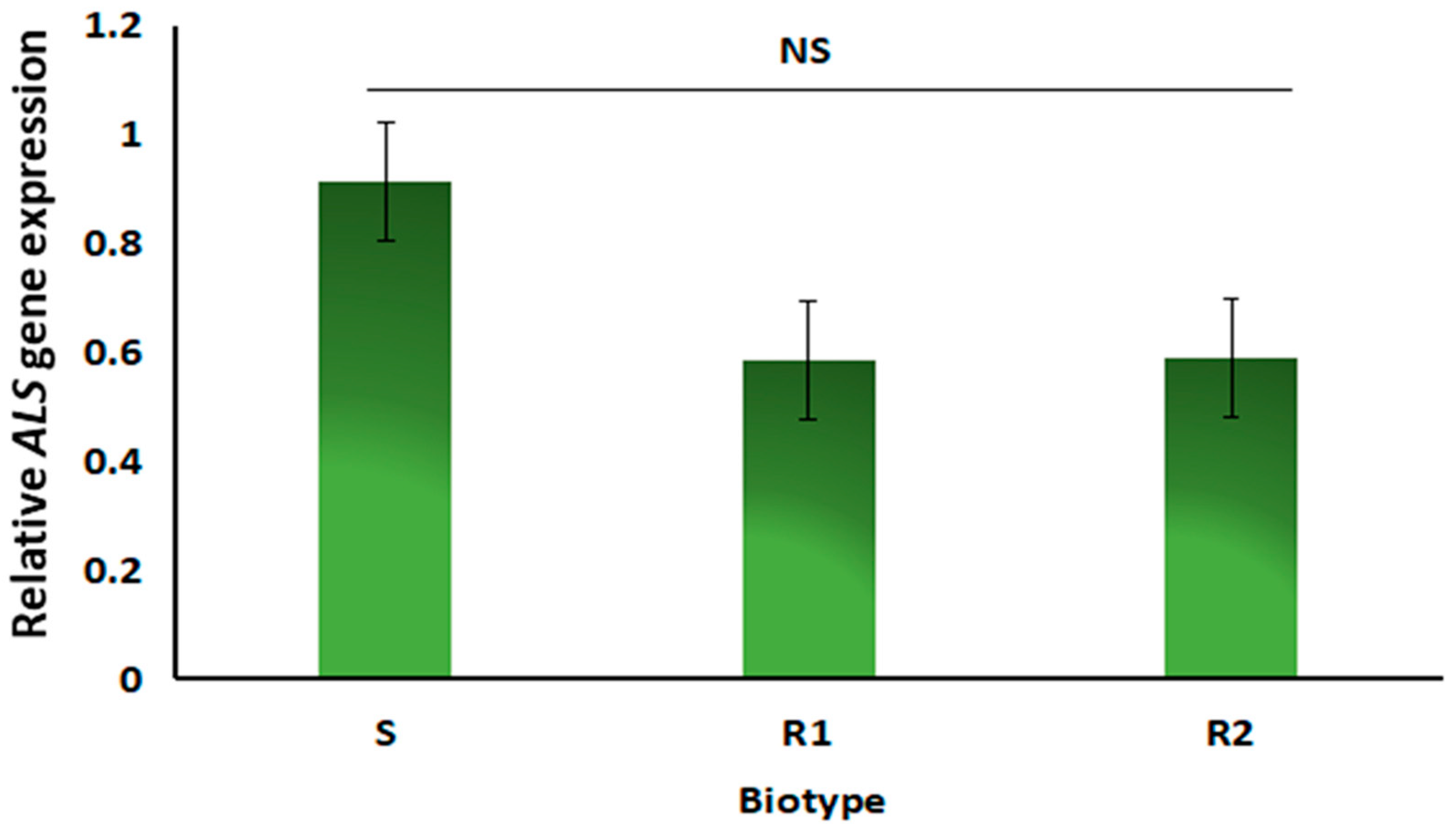
3.4. ALS Gene Mutation and Expression Analysis
3.5. Pyroxsulam Translocation and Penetration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Košnarová, P.; Hamouz, P.; Hamouzová, K.; Linn, A.; Sen, M.K.; Mikulka, J.; Soukup, J. Apera spica-venti in the Czech Republic Develops Resistance to Three Herbicide Modes of Action. Weed Res. 2021, 61, 420–429. [Google Scholar] [CrossRef]
- Hamouzová, K.; Soukup, J.; Jursík, M.; Hamouz, P.; Venclová, V.; Tůmová, P. Cross-Resistance to Three Frequently Used Sulfonylurea Herbicides in Populations of Apera spica-venti from the Czech Republic. Weed Res. 2011, 51, 113–122. [Google Scholar] [CrossRef]
- Massa, D.; Krenz, B.; Gerhards, R. Target-Site Resistance to ALS-Inhibiting Herbicides in Apera spica-venti Populations Is Conferred by Documented and Previously Unknown Mutations. Weed Res. 2011, 51, 294–303. [Google Scholar] [CrossRef]
- Babineau, M.; Mathiassen, S.K.; Kristensen, M.; Holst, N.; Beffa, R.; Kudsk, P. Spatial Distribution of Acetolactate Synthase Resistance Mechanisms in Neighboring Populations of Silky Windgrass (Apera spica-venti). Weed Sci. 2017, 65, 479–490. [Google Scholar] [CrossRef]
- Massa, D.; Kaiser, Y.I.; Andújar-Sánchez, D.; Carmona-Alférez, R.; Mehrtens, J.; Gerhards, R. Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass). Agronomy 2013, 3, 13–27. [Google Scholar] [CrossRef]
- Hamouzová, K.; Košnarová, P.; Salava, J.; Soukup, J.; Hamouz, P. Mechanisms of Resistance to Acetolactate Synthase-Inhibiting Herbicides in Populations of Apera spica-venti from the Czech Republic. Pest Manag. Sci. 2014, 70, 541–548. [Google Scholar] [CrossRef]
- Hulme, P.E. Potential Risks of Future Herbicide-Resistant Weeds in New Zealand Revealed through Machine Learning. N. Z. J. Agric. Res. 2024, 67, 17–27. [Google Scholar] [CrossRef]
- Auškalnienė, O.; Kadžienė, G.; Stefanovičienė, R.; Jomantaitė, B. Development of Herbicides Resistance in Apera spica-venti in Lithuania. Zemdirbyste-Agric. 2020, 107, 71–76. [Google Scholar] [CrossRef]
- Schulz, A.; Mathiassen, S.K.; de Mol, F. Approaches to Early Detection of Herbicide Resistance in Apera spica-venti Regarding Intra- and Inter-Field Situations. J. Plant Dis. Prot. 2014, 121, 138–148. [Google Scholar] [CrossRef]
- Adamczewski, K.; Matysiak, K. The Mechanism of Resistance to ALS-Inhibiting Herbicides in Biotypes of Wind Bent Grass (Apera spica-venti L.) with Cross and Multiple Resistance. Pol. J. Agron. 2012, 10, 3–8. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Online. Friday, 15 December 2023. Available online: www.weedscience.org (accessed on 15 December 2023).
- Torra, J.; Alcántara-De La Cruz, R. Molecular Mechanisms of Herbicide Resistance in Weeds. Genes 2022, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of Evolved Herbicide Resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- De la Cruz, R.A.; Da Silva Amaral, G.; Mendes, K.F.; Rojano-Delgado, A.M.; De Prado, R. Absorption, Translocation, and Metabolism Studies of Herbicides in Weeds and Crops. In Radioisotopes in Weed Research; CRC Press: Boca Raton, FL, USA, 2020; pp. 127–154. [Google Scholar]
- Goldberg-Cavalleri, A.; Onkokesung, N.; Franco-Ortega, S.; Edwards, R. ABC Transporters Linked to Multiple Herbicide Resistance in Alopecurus myosuroides. Front. Plant Sci. 2023, 14, 1082761. [Google Scholar] [CrossRef]
- Wang, J.; Cao, W.; Guo, Q.; Yang, Y.; Bai, L.; Pan, L. Resistance to Mesosulfuron-Methyl in Beckmannia syzigachne May Involve ROS Burst and Non-Target-Site Resistance Mechanisms. Ecotoxicol. Environ. Saf. 2022, 229, 113072. [Google Scholar] [CrossRef]
- Dimaano, N.G.; Iwakami, S. Cytochrome P450-Mediated Herbicide Metabolism in Plants: Current Understanding and Prospects. Pest Manag. Sci. 2021, 77, 22–32. [Google Scholar] [CrossRef]
- Sen, M.K.; Hamouzová, K.; Mikulka, J.; Bharati, R.; Košnarová, P.; Hamouz, P.; Soukup, J. Enhanced Metabolism and Target Gene Overexpression Confer Resistance Against Acetolactate Synthase-Inhibiting Herbicides in Bromus sterilis. Pest Manag. Sci. 2021, 77, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Lorentz, L.; Figge, A.; Herrmann, J.; Maiwald, F.; Ott, M.-C.; Han, H.; Busi, R.; Yu, Q.; Powles, S.B.; et al. RNA-Seq Transcriptome Analysis to Identify Genes Involved in Metabolism-Based Diclofop Resistance in Lolium rigidum. Plant J. 2014, 78, 865–876. [Google Scholar] [CrossRef]
- Délye, C.; Gardin, J.A.C.; Boucansaud, K.; Chauvel, B.; Petit, C. Non-Target-Site-Based Resistance Should Be the Centre of Attention for Herbicide Resistance Research: Alopecurus myosuroides as an Illustration. Weed Res. 2011, 51, 433–437. [Google Scholar] [CrossRef]
- Han, Y.; Gao, H.; Sun, Y.; Wang, Y.; Yan, C.; Ma, H.; Huang, Z. Target Gene Overexpression and Enhanced Metabolism Confer Resistance to Nicosulfuron in Eriochloa villosa (Thunb.). Pestic. Biochem. Physiol. 2024, 202, 105946. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Buddenhagen, C.E.; Griffiths, A.G.; Harrington, K.C.; Ngow, Z. Target-Site and non-target site resistance mechanisms are associated with iodosulfuron resistance in Lolium perenne L. N. Z. J. Agric. Res. 2024, 67, 40–53. [Google Scholar] [CrossRef]
- Wrzesińska, B.; Kościelniak, K.; Frąckowiak, P.; Praczyk, T.; Obrępalska-Stęplowska, A. The Analysis of Reference Genes Expression Stability in Susceptible and Resistant Apera spica-venti Populations under Herbicide Treatment. Sci. Rep. 2021, 11, 22145. [Google Scholar] [CrossRef] [PubMed]
- Palma-Bautista, C.; Vázquez-García, J.G.; de Portugal, J.; Bastida, F.; Alcántara-De La Cruz, R.; Osuna-Ruiz, M.D.; Torra, J.; De Prado, R. Enhanced Detoxification via Cyt-P450 Governs Cross-Tolerance to ALS-Inhibiting Herbicides in Weed Species of Centaurea. Environ. Pollut. 2023, 322, 121140. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; Ferreira Mendes, K.; Alcántara De La Cruz, R.; Torra, J.; De Prado, R. Non-Target-Site Resistance Mechanisms Endow Multiple Herbicide Resistance to Five Mechanisms of Action in Conyza bonariensis. J. Agric. Food Chem. 2021, 69, 14792–14801. [Google Scholar] [CrossRef]
- Torra, J.; Rojano-Delgado, A.M.; Menéndez, J.; Salas, M.; De Prado, R. Cytochrome P450 Metabolism-Based Herbicide Resistance to Imazamox and 2,4-D in Papaver rhoeas. Plant Physiol. Biochem. 2021, 160, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Délye, C. Unravelling the Genetic Bases of Non-Target-Site-Based Resistance (NTSR) to Herbicides: A Major Challenge for Weed Science in the Forthcoming Decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Powles, S. Metabolism-Based Herbicide Resistance and Cross-Resistance in Crop Weeds: A Threat to Herbicide Sustainability and Global Crop Production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Tardif, F.J. Herbicide Cross Resistance in Weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Alcántara-De La Cruz, R.; Rojano-Delgado, A.M.; Giménez, M.J.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. First Resistance Mechanisms Characterization in Glyphosate-Resistant Leptochloa virgata. Front. Plant Sci. 2016, 7, 1742. [Google Scholar] [CrossRef]
- Zhong, V.; Archibald, B.N.; Brophy, J.A. Transcriptional and Post-Transcriptional Controls for Tuning Gene Expression in Plants. Curr. Opin. Plant Biol. 2023, 71, 102315. [Google Scholar] [CrossRef]
- Hada, Z.; Menchari, Y.; Rojano-Delgado, A.M.; Torra, J.; Menéndez, J.; Palma-Bautista, C.; Souissi, T. Point Mutations as Main Resistance Mechanism Together with P450-Based Metabolism Confer Broad Resistance to Different ALS-Inhibiting Herbicides in Glebionis coronaria from Tunisia. Front. Plant Sci. 2021, 12, 626702. [Google Scholar] [CrossRef] [PubMed]
- Palma-Bautista, C.; Vázquez-García, J.G.; Osuna, M.D.; Garcia-Garcia, B.; Torra, J.; Portugal, J.; De Prado, R. An Asp376Glu Substitution in ALS Gene and Enhanced Metabolism Confers High Tribenuron-Methyl Resistance in Sinapis alba. Front. Plant Sci. 2022, 13, 1011596. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Hamouzová, K.; Onkokesung, N.; Menendez, J.; Torra, J.; Košnarová, P.; Sellamuthu, G.; Gupta, A.; Bharati, R.; Sur, V.P.; et al. Transcriptomic Response in Pyroxsulam-Resistant and Susceptible Bromus sterilis Identified Three Distinct Mechanisms of Resistance. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Souza, A.d.S.; Leal, J.F.L.; Montgomery, J.S.; Ortiz, M.F.; Simões Araujo, A.L.; Morran, S.; de Figueiredo, M.R.A.; Langaro, A.C.; Zobiole, L.H.S.; Nissen, S.J.; et al. Nontarget-Site Resistance Due to Rapid Physiological Response in 2,4-D Resistant Conyza sumatrensis: Reduced 2,4-D Translocation and Auxin-Induced Gene Expression. Pest Manag. Sci. 2023, 79, 3581–3592. [Google Scholar] [CrossRef]
- Nandula, V.K.; Ray, J.D.; Ribeiro, D.N.; Pan, Z.; Reddy, K.N. Glyphosate Resistance in Tall Waterhemp (Amaranthus tuberculatus) from Mississippi Is Due to Both Altered Target-Site and Nontarget-Site Mechanisms. Weed Sci. 2013, 61, 374–383. [Google Scholar] [CrossRef]
- Nalin, D.; Munhoz-Garcia, G.V.; Witter, A.P.W.; Takeshita, V.; Oliveira, C.; Adegas, F.S.; Tornisielo, V.L.; Oliveira Junior, R.S.D.; Constantin, J. Absorption, Translocation, and Metabolism of Glyphosate and Imazethapyr in Smooth Pigweed with Multiple Resistance. Agronomy 2023, 13, 1720. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′ to 3′) | Amplicon Size (in bp) | Annealing Temperature (in °C) | Mutation Points Covered (Numbers According to Arabidopsis thaliana) | |
|---|---|---|---|---|---|
| Forward primer (F1) | ATGGCCACAGCCACGTCCAC | 710 | 62.8 | Ala-122, Pro-197, Arg-199, Met-200, Ala-205, Phe-206 | |
| Reverse primer (R1) | CCTCTACTATGGGCGTCTCC | ||||
| Forward primer (F2) | TCTGTATGTTGGTGGTGGCT | 266 | 59.6 | Met-351, Asp-376, Arg-377 | |
| Reverse primer (R2) | CAATCTTGGACCTGCTTGCA | ||||
| Forward primer (F3) | TGATGGGGATGGTAGCTTCC | 409 | 56.9 | Trp-574, Ser-653, Gly-654 | |
| Reverse primer (R3) | TTAATAAGAAACCCTGCCAT | ||||
| Primers for quantitative real-time PCR | ALS_ Forward primer | CACAACTACCTGGTCCTCGA | 166 | 57 | _ |
| ALS_ Reverse primer | ATCCTGGCAGACTCATTGGA | ||||
| GAPDH_ Forward primer | CAGTCACTGTCTTCGGTGTCA | 150 | |||
| GAPDH_ Reverse primer | GCAGAGATGACCACCTTCTTG | ||||
| TBP_ Forward primer | GGCTCTTGTGATGTCAAATTTCC | 126 | 58.7 | ||
| TBP_ Reverse primer | GAACAATCTTCGGTTGCTTCA | ||||
| Chemical Ingredients | Biotype | b (±SE) | d (±SE) | GR50 (±SE) | p-Value | RF |
|---|---|---|---|---|---|---|
| Pyroxsulam | R1 | 0.62 (±0.2) | 98.97 (±5.7) | 7.5 (±2.3) | 6.7 | |
| R2 | 0.78 (±0.1) | 98.4 (±3.6) | 158.4 (±26.7) | 141.7 | ||
| S | 0.73 (±0.04) | 95.92 (±6.5) | 1.1 (±0.2) | NA | ||
| Malathion + pyroxsulam | R1 | 0.53 (±0.1) | 100 (±5.1) | 3.1 (±2) | <2e-16 | 2.8 |
| R2 | 0.76 (±0.1) | 99.1 (±3.2) | 127.5 (±11.9) | 0.0085 | 112.7 | |
| S | 0.83 (±0.04) | 98.02 (±4.7) | 1.1 (±0.1) | 0.10 | NA | |
| NBD-Cl + pyroxsulam | R1 | 0.41 (±0.1) | 98.58 (±9.5) | 5.4 (±2.6) | 0.93 | 5.2 |
| R2 | 0.84 (±0.1) | 97.83 (±2.4) | 84.3 (±8.6) | 0.0384 | 81.8 | |
| S | 0.82 (±0.04) | 96.55 (±4.5) | 1 (±0.1) | 0.77 | NA |
| Time Duration | Biotype | Penetration % | Translocation % | ||
|---|---|---|---|---|---|
| Average (±SD) | Leaves (±SD) | Systemic Leaves (±SD) | Roots (±SD) | ||
| 12 HAT | S | 44.9 (±4.5) a | 92.4 (±4.7) a | 4.1 (±2.1) a | 3.5 (±2.5) b |
| R1 | 39.8 (±1.8) a | 97.8 (±0.5) a | 1.6 (±0.4) b | 0.6 (±0.2) a | |
| R2 | 37 (±9.1) a | 98.4 (±0.1) a | 1.1 (±0.1) b | 0.5 (±0.0) a | |
| 24 HAT | S | 41.1 (±5.4) bc | 96.3 (±0.9) a | 1.8 (±0.4) a | 1.9 (±0.7) a |
| R1 | 38.8 (±5) b | 93.3 (±2.8) a | 4.5 (±1.8) a | 2.2 (±1.3) a | |
| R2 | 27.4 (±2.0) ab | 92.2 (±1.3) a | 5.1 (±1.2) a | 2.7 (±0.5) a | |
| 48 HAT | S | 40.0 (±5.5) a | 96.4 (±0.6) a | 2.5 (±0.4) a | 1.1 (±0.2) a |
| R1 | 47.3 (±7.4) a | 97.6 (±0.8) a | 1.5 (±0.5) a | 0.9 (±0.4) a | |
| R2 | 37.8 (±4.7) a | 96.3 (±1.0) a | 2.5 (±0.7) a | 1.2 (±0.3) a | |
| 72 HAT | S | 55.5 (±6.2) a | 95.4 (±1.9) a | 3.5 (±1.6) a | 1.1 (±0. 4) a |
| R1 | 57.5 (±7.2) a | 97.4 (±0.4) a | 2.0 (±0.4) a | 0.6 (±0.1) a | |
| R2 | 48.0 (±7.0) a | 94.9 (±1.4) a | 3.3 (±0.8) a | 1.8 (±0.6) a | |
| 96 HAT | S | 58.0 (±4.718) a | 95.5 (±1.6) a | 3.6 (±1.4) a | 0.8 (±0.2) a |
| R1 | 48.31 (±5.2) a | 95.4 (±1.5) a | 3.5 (±1.1) a | 1.1 (±0.4) a | |
| R2 | 53.4 (±4.0) a | 97.5 (±0.9) a | 1.8 (±0.6) a | 0.7 (±0.3) a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, S.; Sen, M.K.; Hamouzová, K.; Košnarová, P.; Bharati, R.; Menendez, J.; Soukup, J. Pyroxsulam Resistance in Apera spica-venti: An Emerging Challenge in Crop Protection. Plants 2025, 14, 74. https://doi.org/10.3390/plants14010074
Bhattacharya S, Sen MK, Hamouzová K, Košnarová P, Bharati R, Menendez J, Soukup J. Pyroxsulam Resistance in Apera spica-venti: An Emerging Challenge in Crop Protection. Plants. 2025; 14(1):74. https://doi.org/10.3390/plants14010074
Chicago/Turabian StyleBhattacharya, Soham, Madhab Kumar Sen, Katerina Hamouzová, Pavlína Košnarová, Rohit Bharati, Julio Menendez, and Josef Soukup. 2025. "Pyroxsulam Resistance in Apera spica-venti: An Emerging Challenge in Crop Protection" Plants 14, no. 1: 74. https://doi.org/10.3390/plants14010074
APA StyleBhattacharya, S., Sen, M. K., Hamouzová, K., Košnarová, P., Bharati, R., Menendez, J., & Soukup, J. (2025). Pyroxsulam Resistance in Apera spica-venti: An Emerging Challenge in Crop Protection. Plants, 14(1), 74. https://doi.org/10.3390/plants14010074







