Intermittent Supplementation with Far-Red Light Accelerates Leaf and Bud Development and Increases Yield in Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Light Design
2.3. Determination of Lettuce Morphology
2.4. Determination of Photosynthetic Parameters and Photosynthetic Pigment Contents
2.5. Determination of Lettuce Quality
2.6. Quantitation of Hormone Levels of Lettuce Leaves and Petioles
2.7. RNA-Sequencing Analysis
2.8. WGCNA Network Analysis
2.9. RT-qPCR Experiment
2.10. Statistical Analyses
3. Results
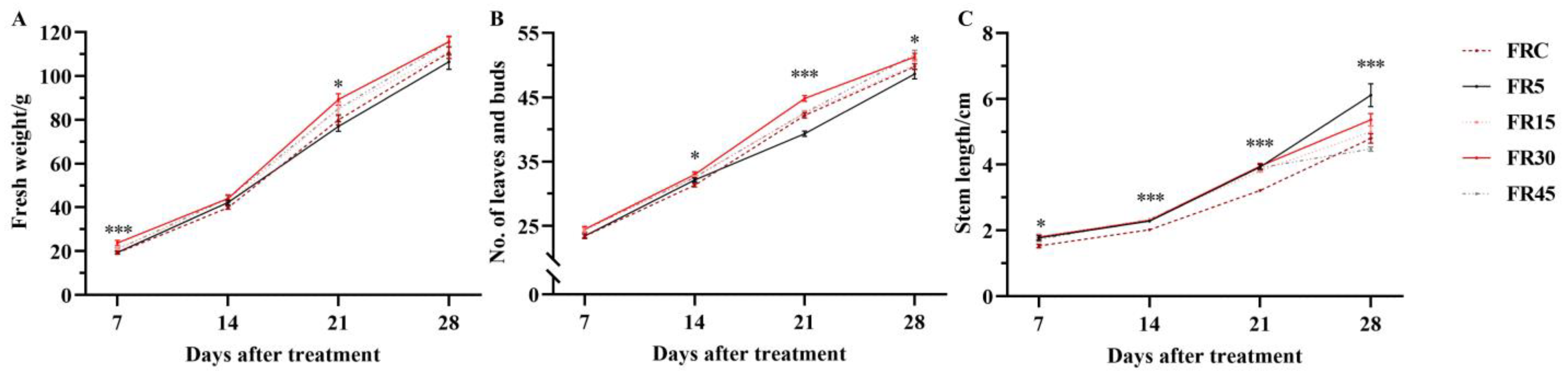
3.1. Intermittent Supplemental Irradiation Enhances the Effect of Far-Red Light on Lettuce Growth and Development
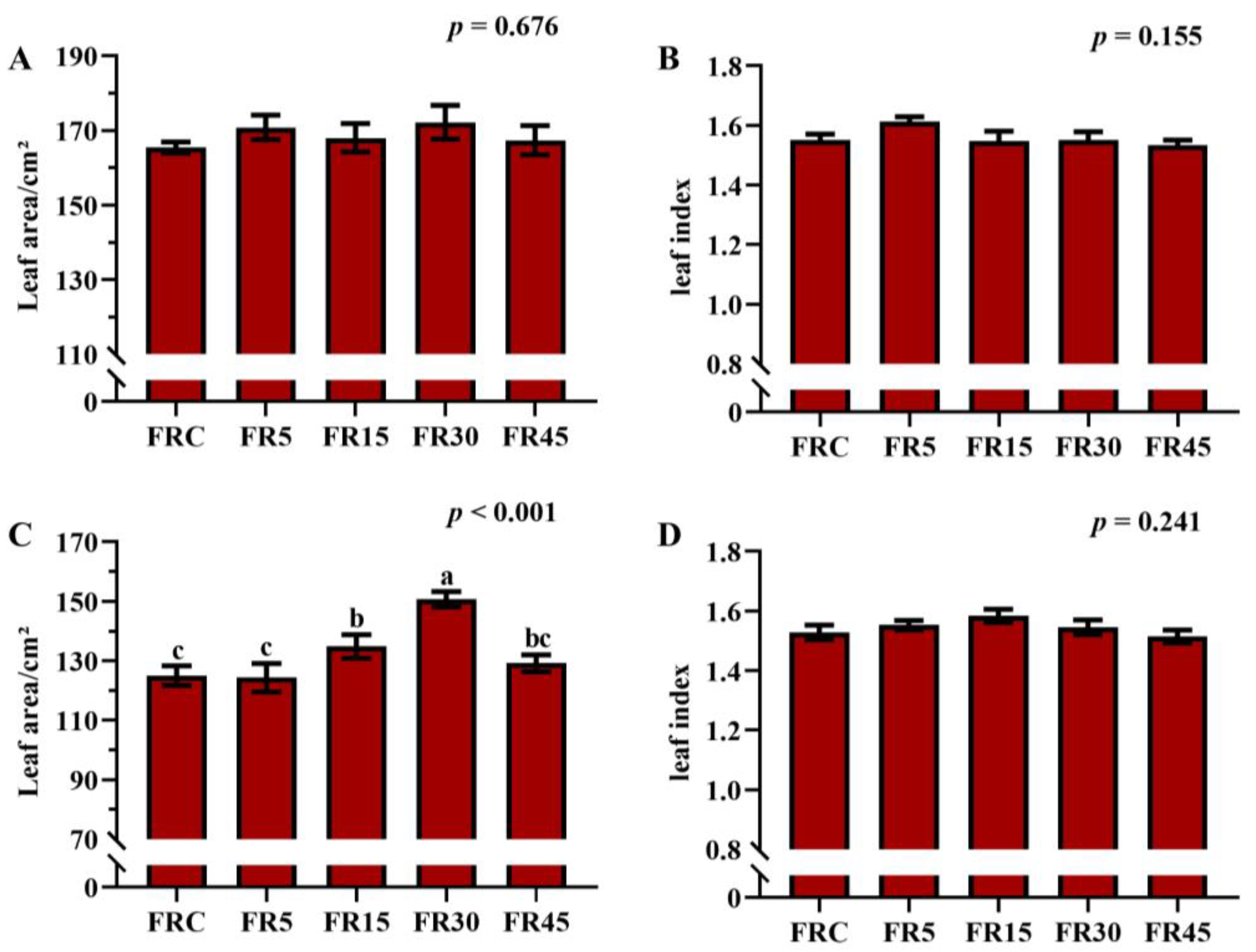
3.2. Overgrowth Caused by Intermittent Supplemental Light Reduces Assimilation of Nutrients
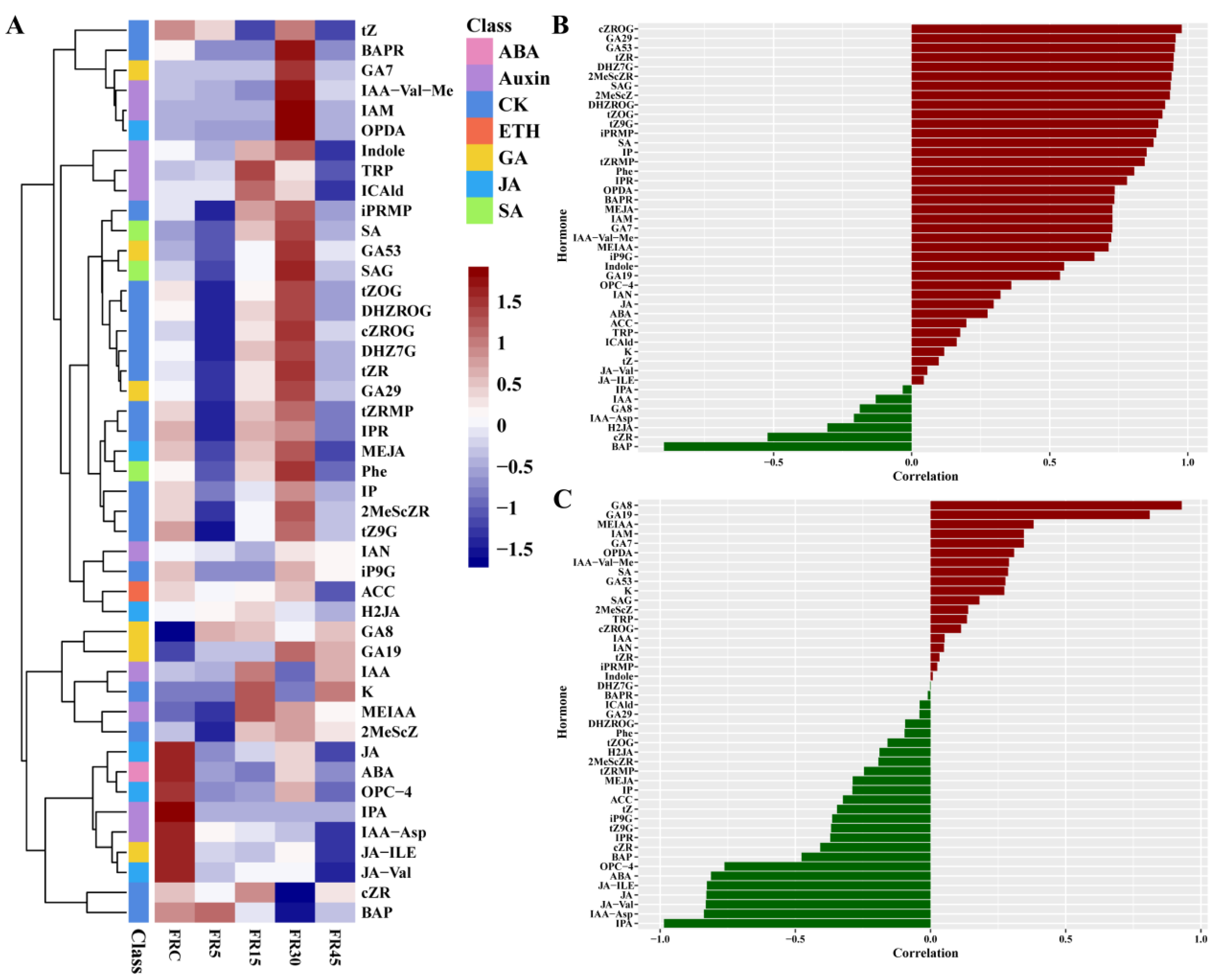
3.3. Hormone Accumulation at the Lettuce Stem Tip Correlates Strongly with Leaf Bud Development and Stem Length
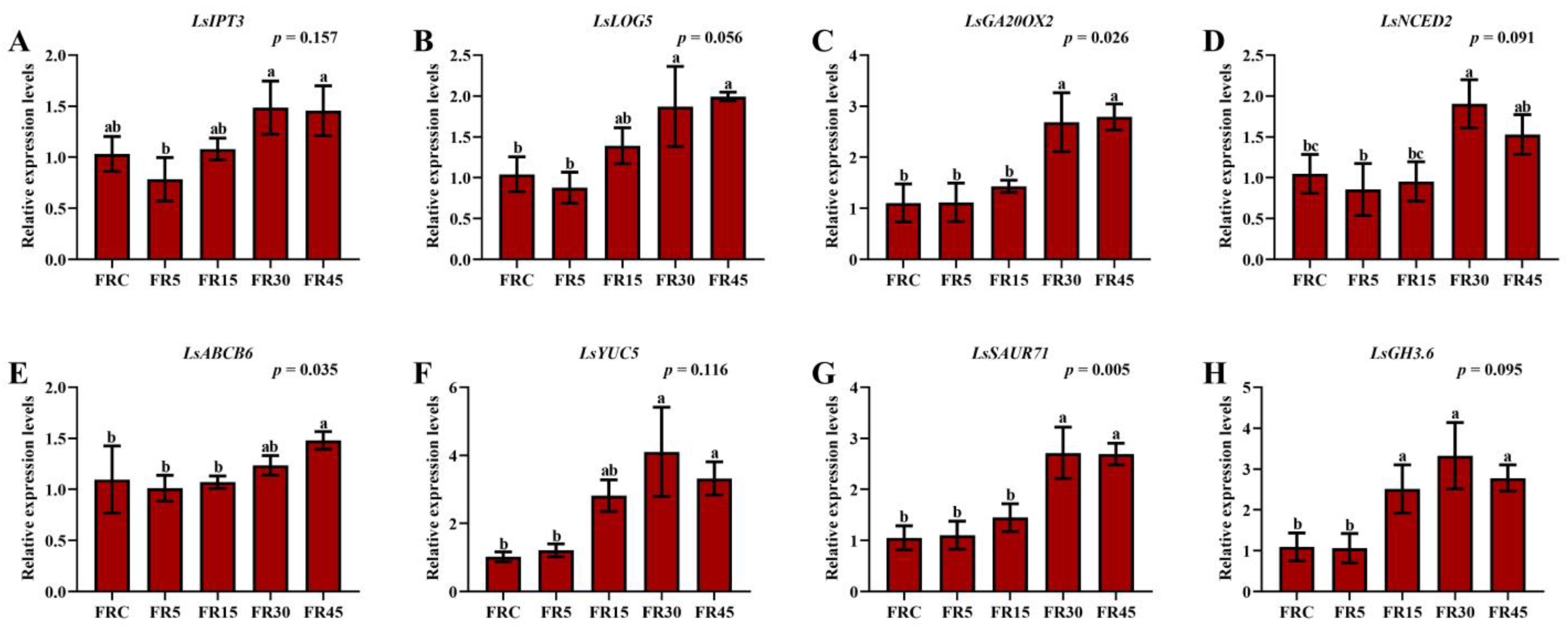
3.4. Expression of Genes Related to Hormone Metabolism and Transport in Lettuce Stem Tips
3.5. Transcriptome Analysis Reveals That Intermittent Supplemental Irradiation with Far-Red Light Primarily Induces Signaling-Related Differences
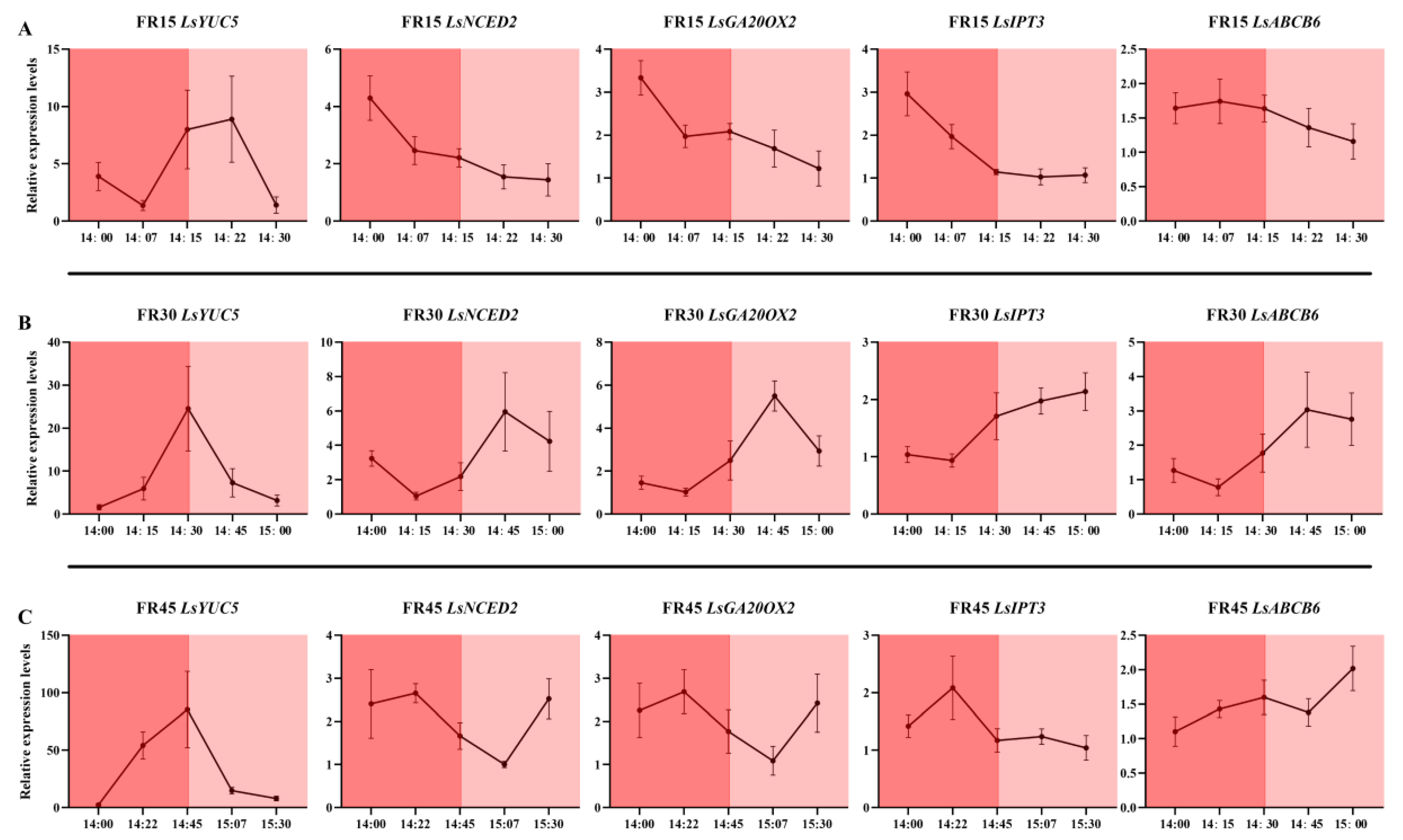
3.6. Dynamic Expression of Genes Related to Far-Red Light Response and Hormone Metabolism and Transport
3.7. Principal Component Analysis
4. Discussion
4.1. Intermittent Supplementation of Far-Red Light Enhances the Effect of Constant Far-Red Light Supplementation
4.2. Differential Response of Signals Under Far-Red Light Irradiation with Varying Interval Times
4.3. Hypothesis for an Artificial Lighting Model That Fully Leverages Both the Signaling and Energetic Effects of Light on Plant
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H. Phytochrome photosensory signalling networks. Nat. Rev. Mol. Cell Biol. 2002, 3, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Parks, B.M.; Spalding, E.P. Sequential and coordinated action of phytochromes A and B during arabidopsis stem growth revealed by kinetic analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 14142–14146. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, D.J.; Hiltbrunner, A. Molecular mechanisms and ecological function of far-red light signalling. Plant Cell Environ. 2017, 40, 2509–2529. [Google Scholar] [CrossRef]
- Kirchenbauer, D.; Viczián, A.; Ádám, É.; Hegedűs, Z.; Klose, C.; Leppert, M.; Hiltbrunner, A.; Kircher, S.; Schäfer, E.; Nagy, F. Characterization of photomorphogenic responses and signaling cascades controlled by phytochrome-A expressed in different tissues. New Phytol. 2016, 211, 584–598. [Google Scholar] [CrossRef]
- Bae, G.; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef]
- Wagner, J.R.; Brunzelle, J.S.; Forest, K.T.; Vierstra, R.D. A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 2005, 438, 325–331. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Ngcobo, B.L.; Bertling, I.; Clulow, A.D. Preharvest illumination of cherry tomato reduces ripening period, enhances fruit carotenoid concentration and overall fruit quality. J. Hortic. Sci. Biotechnol. 2020, 95, 617–627. [Google Scholar] [CrossRef]
- Deram, P.; Lefsrud, M.; Orsat, V. Supplemental lighting orientation and red-to-blue ratio of light-emitting diodes for greenhouse tomato production. HortScience 2014, 49, 448–452. [Google Scholar] [CrossRef]
- Kim, D.; Son, J.E. Adding far-red to red, blue supplemental light-emitting diode interlighting improved sweet pepper yield but attenuated carotenoid content. Front. Plant Sci. 2022, 13, 938199. [Google Scholar] [CrossRef] [PubMed]
- Pola, W.; Sugaya, S.; Photchanachai, S. Color development and phytochemical changes in mature green chili (Capsicum annuum L.) exposed to red and blue light-emitting diodes. J. Agric. Food Chem. 2020, 68, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, K.; Wang, X.; Yan, J.; Zhu, H.; Zhang, N.; Wang, Y.; Zhao, Q.; Liu, Y.; Bu, X.; et al. Manipulation of artificial light environment improves plant biomass and fruit nutritional quality in tomato. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef]
- Lanoue, J.; Little, C.; Hawley, D.; Hao, X. Addition of green light improves fruit weight and dry matter content in sweet pepper due to greater light penetration within the canopy. Sci. Hortic. 2022, 304, 111350. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. LED-induced carotenoid synthesis and related gene expression in brassica microgreens. J. Agric. Food Chem. 2021, 69, 4674–4685. [Google Scholar] [CrossRef]
- Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Miliauskienė, J.; Sutulienė, R.; Laužikė, K.; Viršilė, A.; Samuolienė, G.; Runkle, E.S. Photon distribution of sole-source lighting affects the mineral nutrient content of microgreens. Agriculture 2022, 12, 1086. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, C.; Li, Z.; Li, X.; Cheng, F.; Lin, D.; Yang, Y. Supplementary white, UV-A, and far-red radiation differentially regulates growth and nutritional qualities of greenhouse lettuce. Plants 2023, 12, 3234. [Google Scholar] [CrossRef]
- Van Brenk, J.B.; Courbier, S.; Kleijweg, C.L.; Verdonk, J.C.; Marcelis, L.F.M. Paradise by the far-red light: Far-red and red:blue ratios independently affect yield, pigments, and carbohydrate production in lettuce, Lactuca sativa. Front. Plant Sci. 2024, 15, 1383100. [Google Scholar]
- Liu, J.; van Iersel, M.W. Far-red light effects on lettuce growth and morphology in indoor production are cultivar specific. Plants 2022, 11, 2714. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Monostori, I.; Heilmann, M.; Kocsy, G.; Rakszegi, M.; Ahres, M.; Altenbach, S.B.; Szalai, G.; Pál, M.; Toldi, D.; Simon-Sarkadi, L.; et al. LED lighting—modification of growth, metabolism, yield and flour composition in wheat by spectral quality and intensity. Front. Plant Sci. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Marcelis, L.F.M.; Offringa, R.; Kohlen, W.; Heuvelink, E. Far-red light-enhanced apical dominance stimulates flower and fruit abortion in sweet pepper. Plant Physiol. 2024, 195, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, S.; Finazzi, G.; Wollman, F.A. The dynamics of photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Silva, C.R.; Simoni, J.A.; Collins, C.H.; Volpe, P.L.O. Ascorbic acid as a standard for iodometric titrations. An analytical experiment for general chemistry. J. Chem. Educ. 1999, 76, 1421. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef]
- Linkosalo, T.; Lechowicz, M.J. Twilight far-red treatment advances leaf bud burst of silver birch (Betula pendula). Tree Physiol. 2006, 26, 1249–1256. [Google Scholar] [CrossRef]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef]
- Mor, Y.; Halevy, A.H. Dual effect of light on flowering and sprouting of rose shoots. Physiol. Plant. 1984, 61, 119–124. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Emery, R.N.; Pharis, R.P.; Reid, D.M. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: Putative roles for plant hormones in leaf and internode growth. J. Exp. Bot. 2007, 58, 2145–2157. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Walton, L.J.; Reid, D.M. Interaction of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regul. 2007, 51, 53–61. [Google Scholar] [CrossRef]
- Demotes Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi Travier, S.; Crespel, L.; Morel, P.; Huché Thélier, L.; Boumaza, R. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Samach, A.; Smith, H.M. Constraints to obtaining consistent annual yields in perennials. II: Environment and fruit load affect induction of flowering. Plant Sci. 2013, 207, 168–176. [Google Scholar] [PubMed]
- Deitzer, G.F.; Hayes, R.; Jabben, M. Kinetics and time dependence of the effect of far red light on the photoperiodic induction of flowering in Wintex barley. Plant Physiol. 1979, 64, 1015–1021. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems integrators in plant development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef]
- Li, G.; Kazmi, A.; Feng, M.; Hou, H. Phytochrome-interacting factors (PIFs) regulate phytohormone-mediated plant environmental adaptation. Environ. Exp. Bot. 2024, 218, 105610. [Google Scholar] [CrossRef]
- Song, X.; Gu, X.; Chen, S.; Qi, Z.; Yu, J.; Zhou, Y.; Xia, X. Far-red light inhibits lateral bud growth mainly through enhancing apical dominance independently of strigolactone synthesis in tomato. Plant Cell Environ. 2024, 47, 429–441. [Google Scholar] [CrossRef]
- Lei, K.; Tan, Q.; Zhu, L.; Xu, L.; Yang, S.; Hu, J.; Gao, L.; Hou, P.; Shao, Y.; Jiang, D.; et al. Low red/far-red ratio can induce cytokinin degradation resulting in the inhibition of tillering in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 971003. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Ali Raza, M.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Huang, K.; Liu, Y.; Liu, M.; Wang, J. Effect of LED spectrum on the quality and nitrogen metabolism of lettuce under recycled hydroponics. Front. Plant Sci. 2021, 12, 678197. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Song, W.; Wang, L.; Guo, W.; Xue, X. Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue LED irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Shao, M.; Liu, W.; Zha, L.; Zhou, C.; Zhang, Y.; Li, B. Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Sci. Hortic. 2020, 268, 109366. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, M.; Liu, Y.; Li, C.; Zhang, S.; Duan, J.; Chen, J.; Qi, L.; Liu, Y.; Li, H.; et al. Liquid–liquid phase separation of TZP promotes PPK-mediated phosphorylation of the phytochrome A photoreceptor. Nat. Plants 2024, 10, 798–814. [Google Scholar] [CrossRef]
- Lin, C.; Shalitin, D. Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 2003, 54, 469–496. [Google Scholar] [CrossRef]

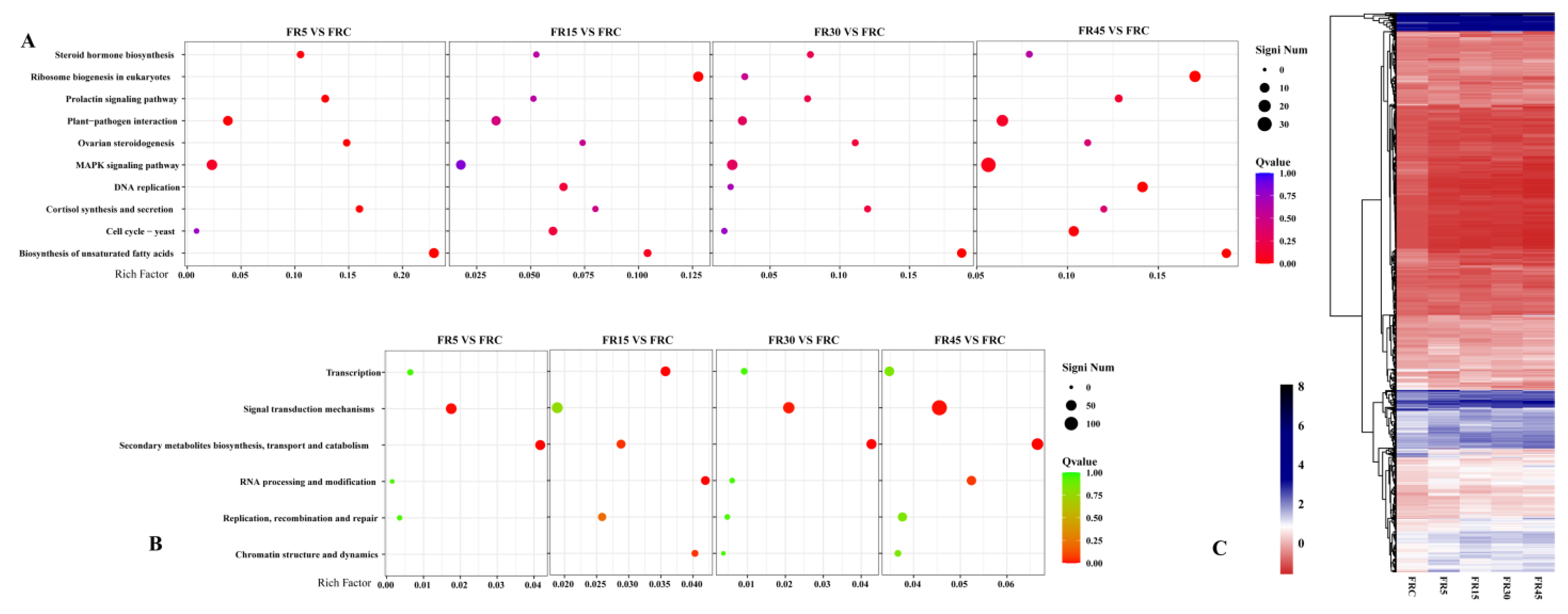
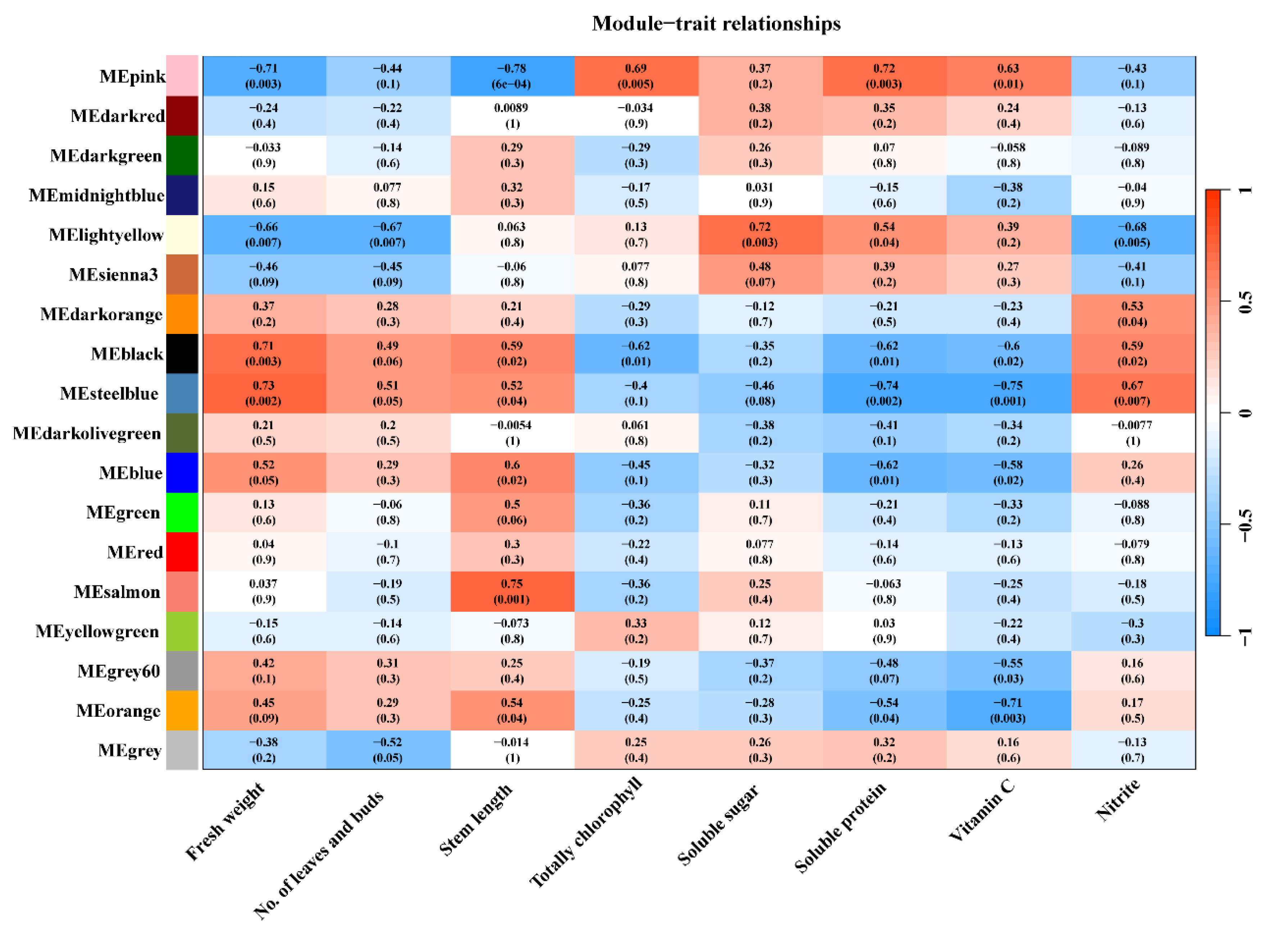
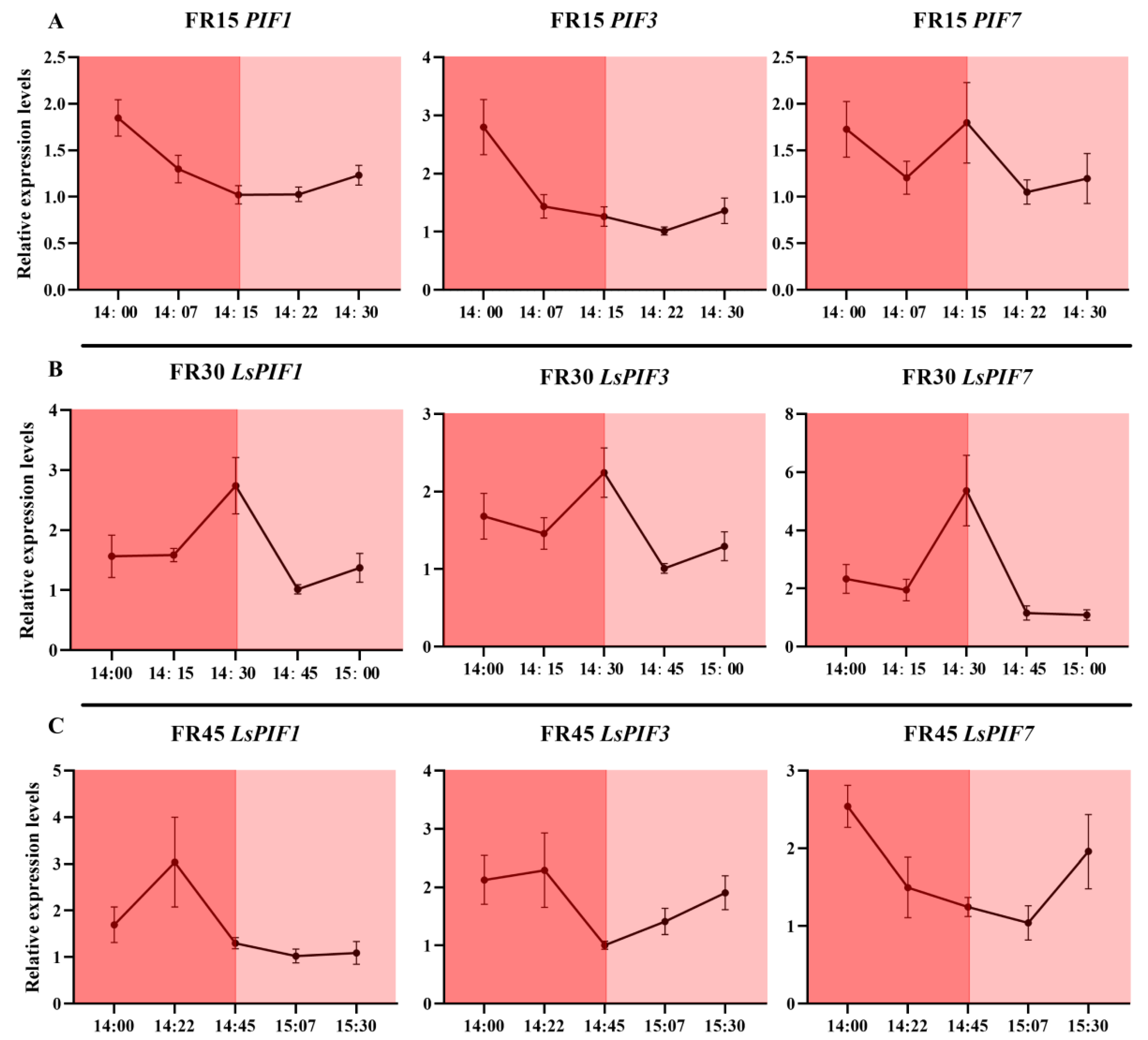
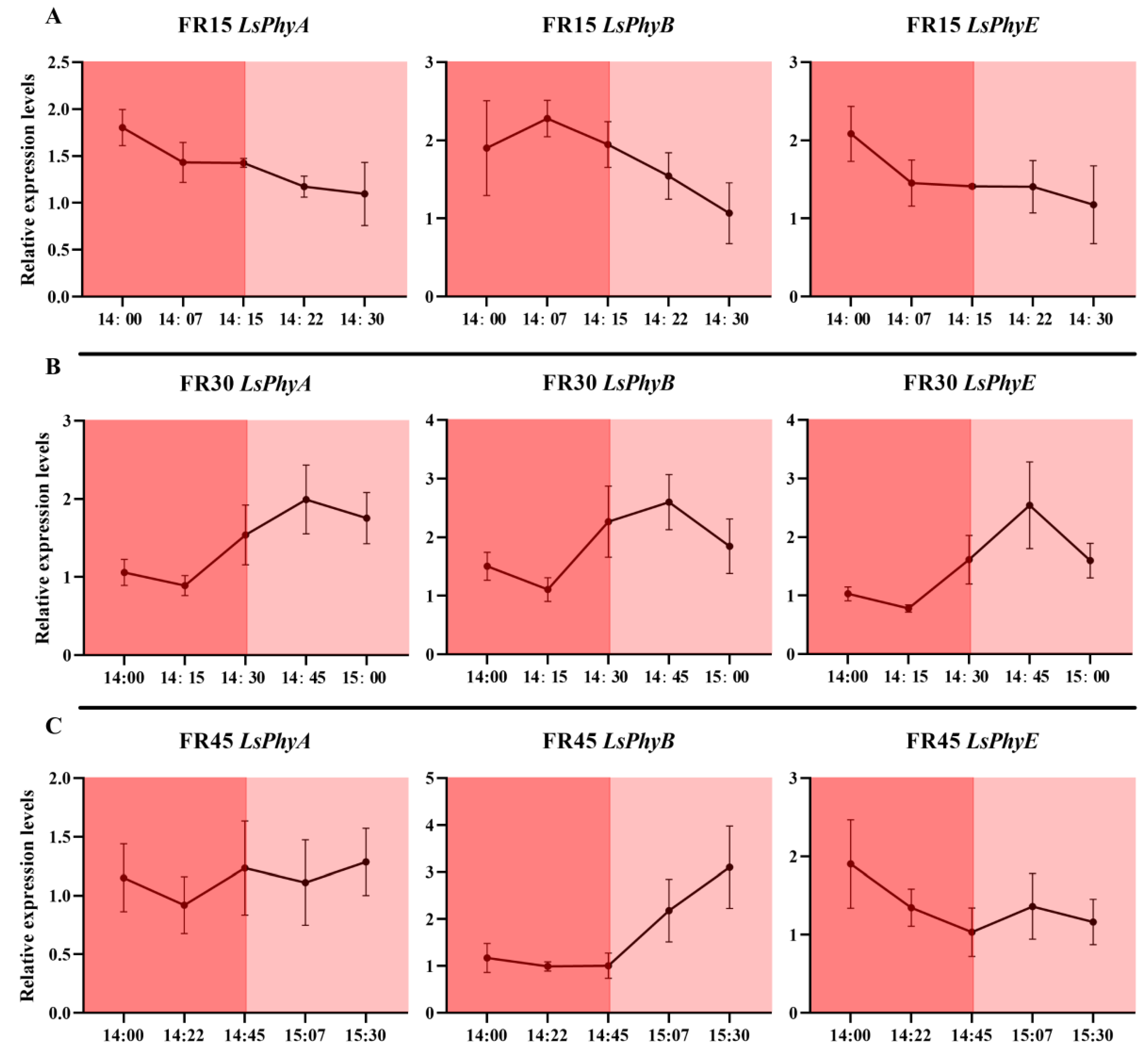

| Treatment Code | Red LEDs Photon Flux Density (µmol·m−2·s−1) | Far-Red LEDs Photon Flux Density (µmol·m−2·s−1) | Supplemental Frequency | Red/Far-Red Ratio (on, off) | PSS Value (on, off) |
|---|---|---|---|---|---|
| FRC | 180 ± 10 | 30 ± 2 | Constant irradiation | 6 | 0.84 |
| FR5 | 180 ± 10 | 60 ± 5 | 5 min on, 5 min off | 3, N/A | 0.82, 0.88 |
| FR15 | 180 ± 10 | 60 ± 5 | 15 min on, 15 min off | 3, N/A | 0.82, 0.88 |
| FR30 | 180 ± 10 | 60 ± 5 | 30 min on, 30 min off | 3, N/A | 0.82, 0.88 |
| FR45 | 180 ± 10 | 60 ± 5 | 45 min on, 45 min off | 3, N/A | 0.82, 0.88 |
| Treatment Code | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Chlorophyll a:b | Total Chlorophyll (mg/g) | Carotenoid (mg/g) |
|---|---|---|---|---|---|
| FRC | 0.709 ± 0.008 a | 0.216 ± 0.002 a | 3.281 ± 0.011 | 0.926 ± 0.010 a | 0.171 ± 0.002 a |
| FR5 | 0.614 ± 0.012 b | 0.185 ± 0.003 c | 3.311 ± 0.016 | 0.800 ± 0.015 c | 0.149 ± 0.003 b |
| FR15 | 0.575 ± 0.014 c | 0.176 ± 0.006 d | 3.264 ± 0.026 | 0.751 ± 0.020 d | 0.140 ± 0.004 c |
| FR30 | 0.558 ± 0.020 c | 0.174 ± 0.004 d | 3.206 ± 0.038 | 0.731 ± 0.024 d | 0.129 ± 0.005 d |
| FR45 | 0.638 ± 0.010 b | 0.196 ± 0.002 b | 3.258 ± 0.011 | 0.834 ± 0.012 b | 0.152 ± 0.002 b |
| p < 0.001 | p < 0.001 | p = 0.078 | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ye, R.; Gao, X.; Lin, R.; Li, Y. Intermittent Supplementation with Far-Red Light Accelerates Leaf and Bud Development and Increases Yield in Lettuce. Plants 2025, 14, 139. https://doi.org/10.3390/plants14010139
Liu Y, Ye R, Gao X, Lin R, Li Y. Intermittent Supplementation with Far-Red Light Accelerates Leaf and Bud Development and Increases Yield in Lettuce. Plants. 2025; 14(1):139. https://doi.org/10.3390/plants14010139
Chicago/Turabian StyleLiu, Yanke, Rong Ye, Xinying Gao, Rongcheng Lin, and Yang Li. 2025. "Intermittent Supplementation with Far-Red Light Accelerates Leaf and Bud Development and Increases Yield in Lettuce" Plants 14, no. 1: 139. https://doi.org/10.3390/plants14010139
APA StyleLiu, Y., Ye, R., Gao, X., Lin, R., & Li, Y. (2025). Intermittent Supplementation with Far-Red Light Accelerates Leaf and Bud Development and Increases Yield in Lettuce. Plants, 14(1), 139. https://doi.org/10.3390/plants14010139





