Changes in Metabolites Produced in Wheat Plants Against Water-Deficit Stress
Abstract
1. Introduction
2. Results
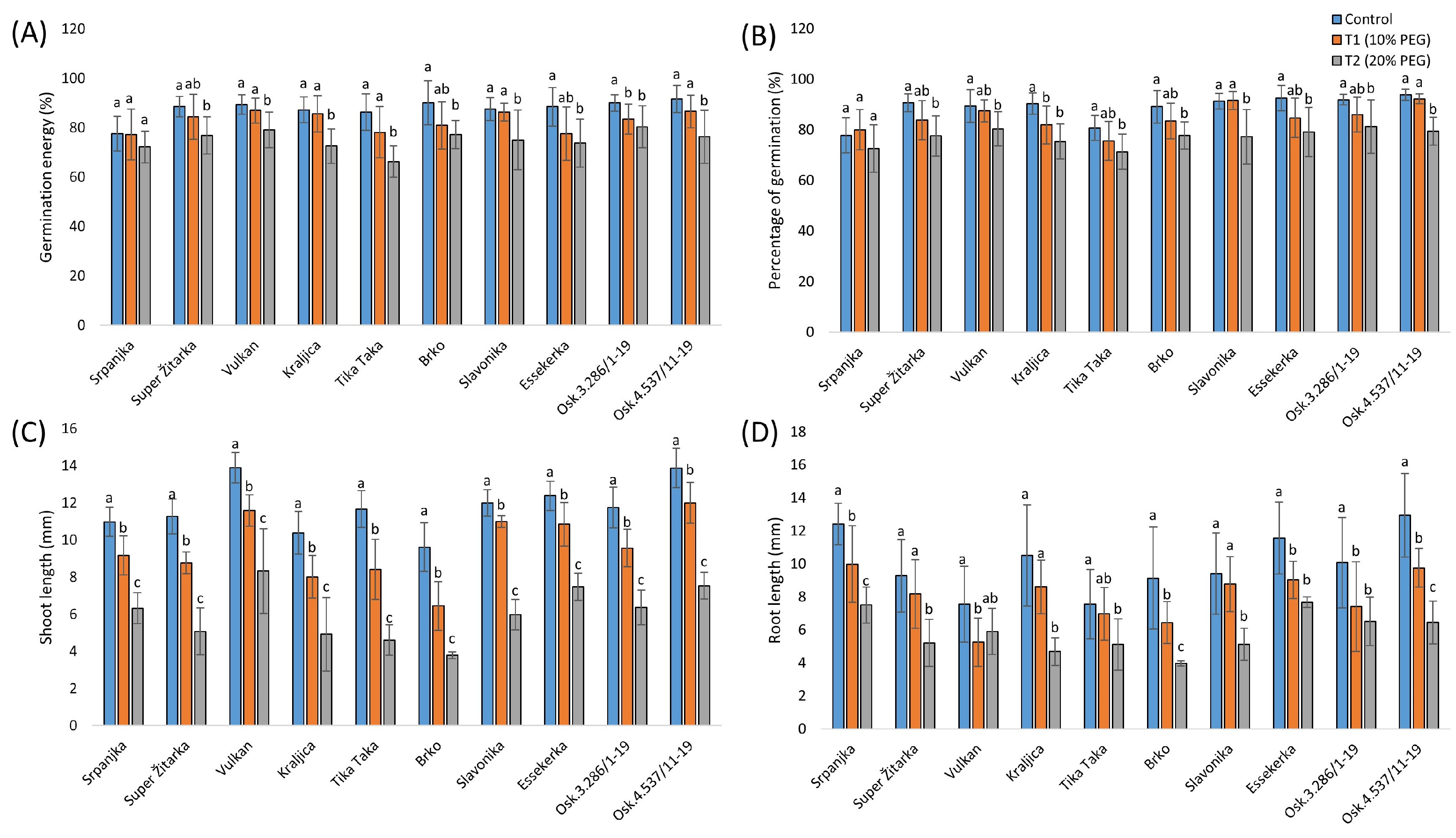
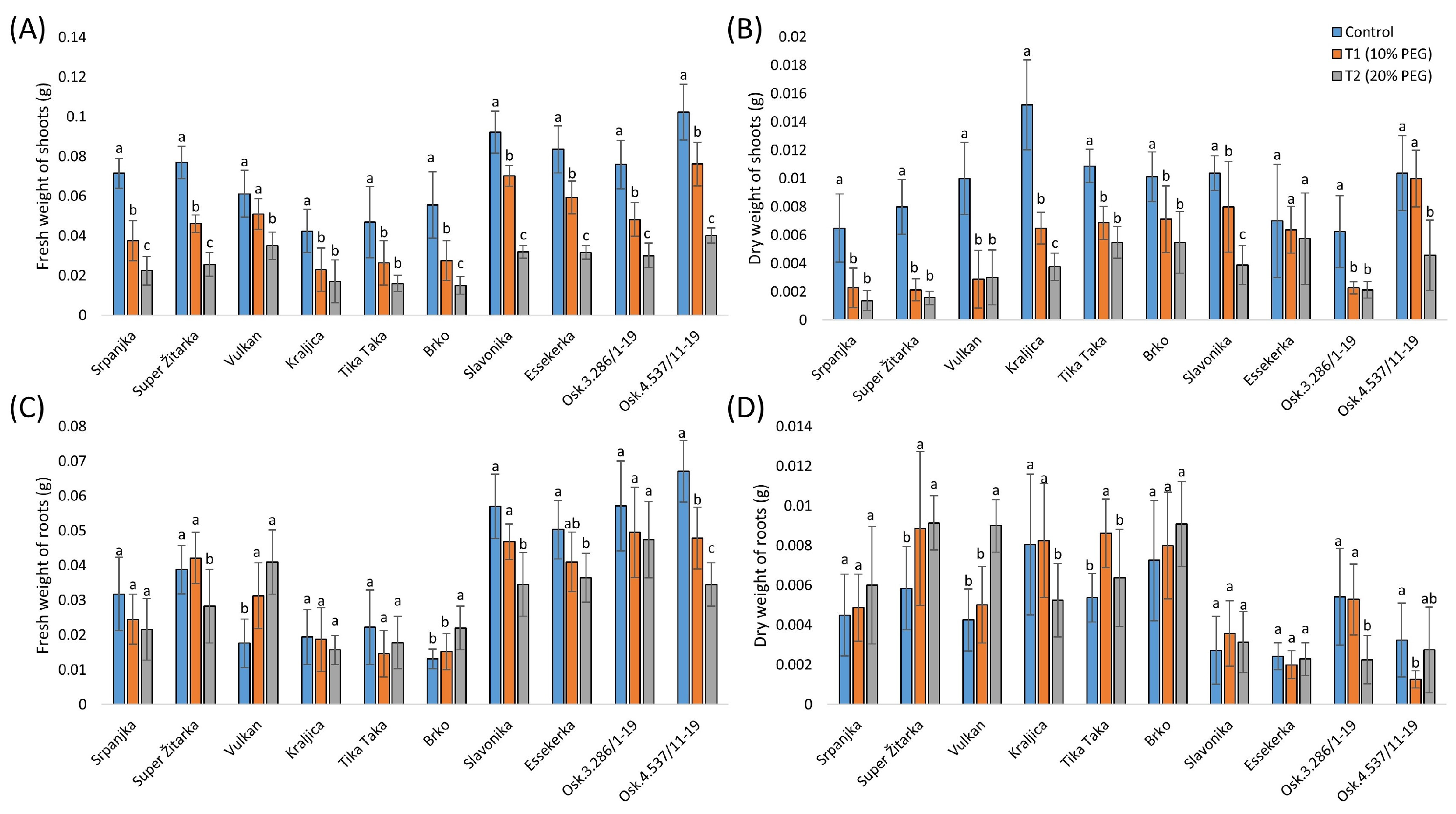
2.1. Morphological Effects of Two Drought Stress Treatments in Wheat
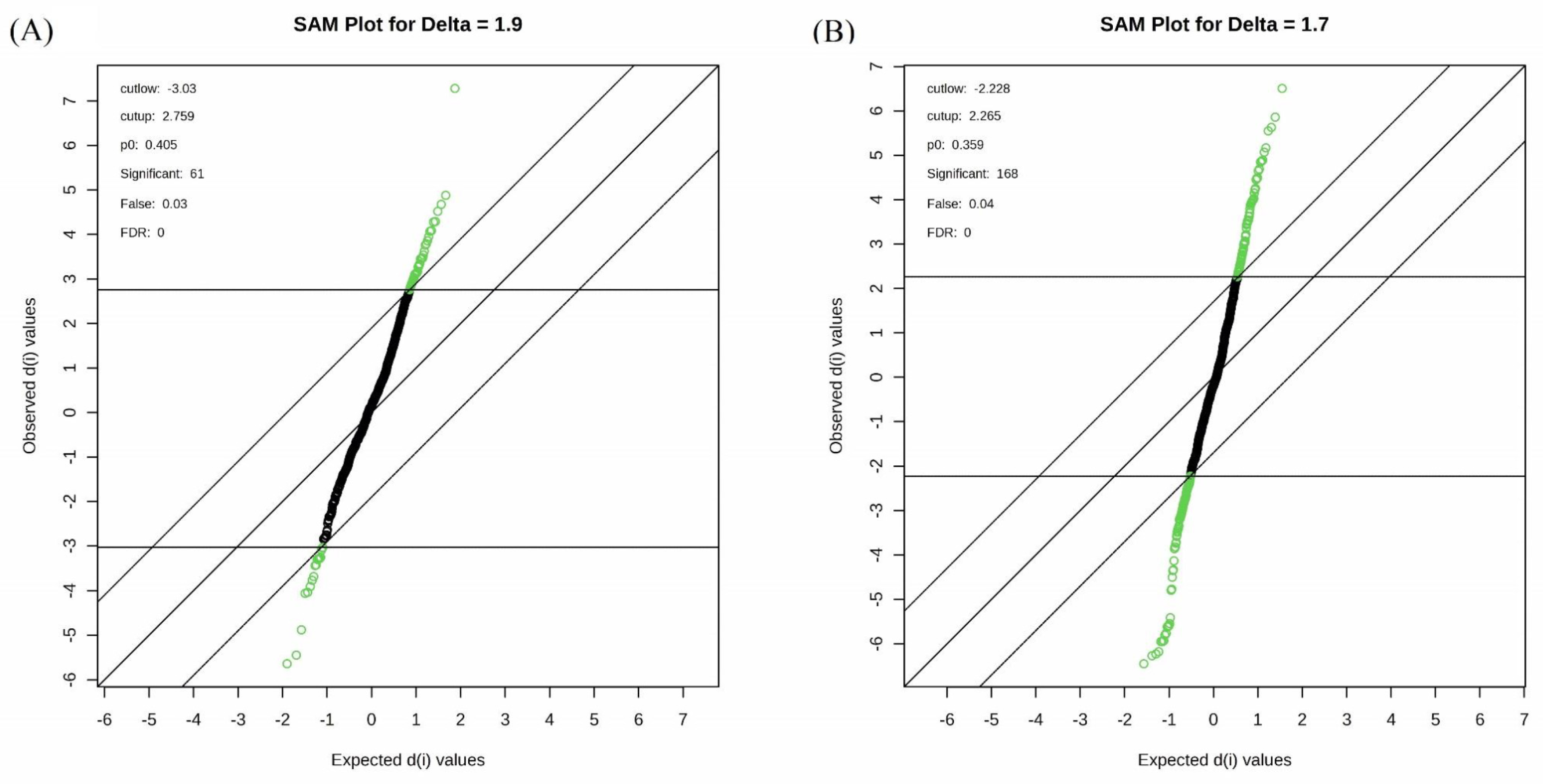
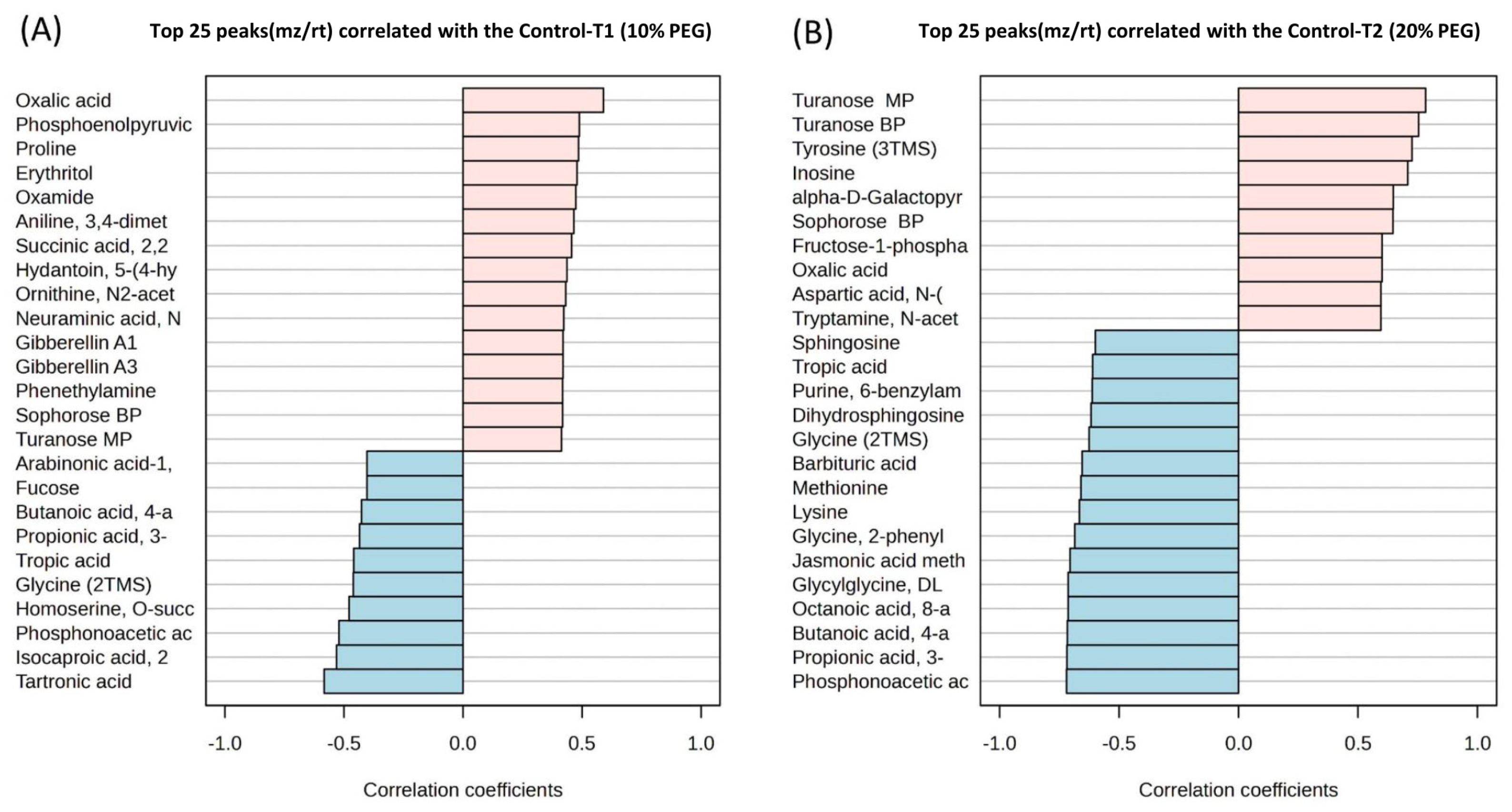
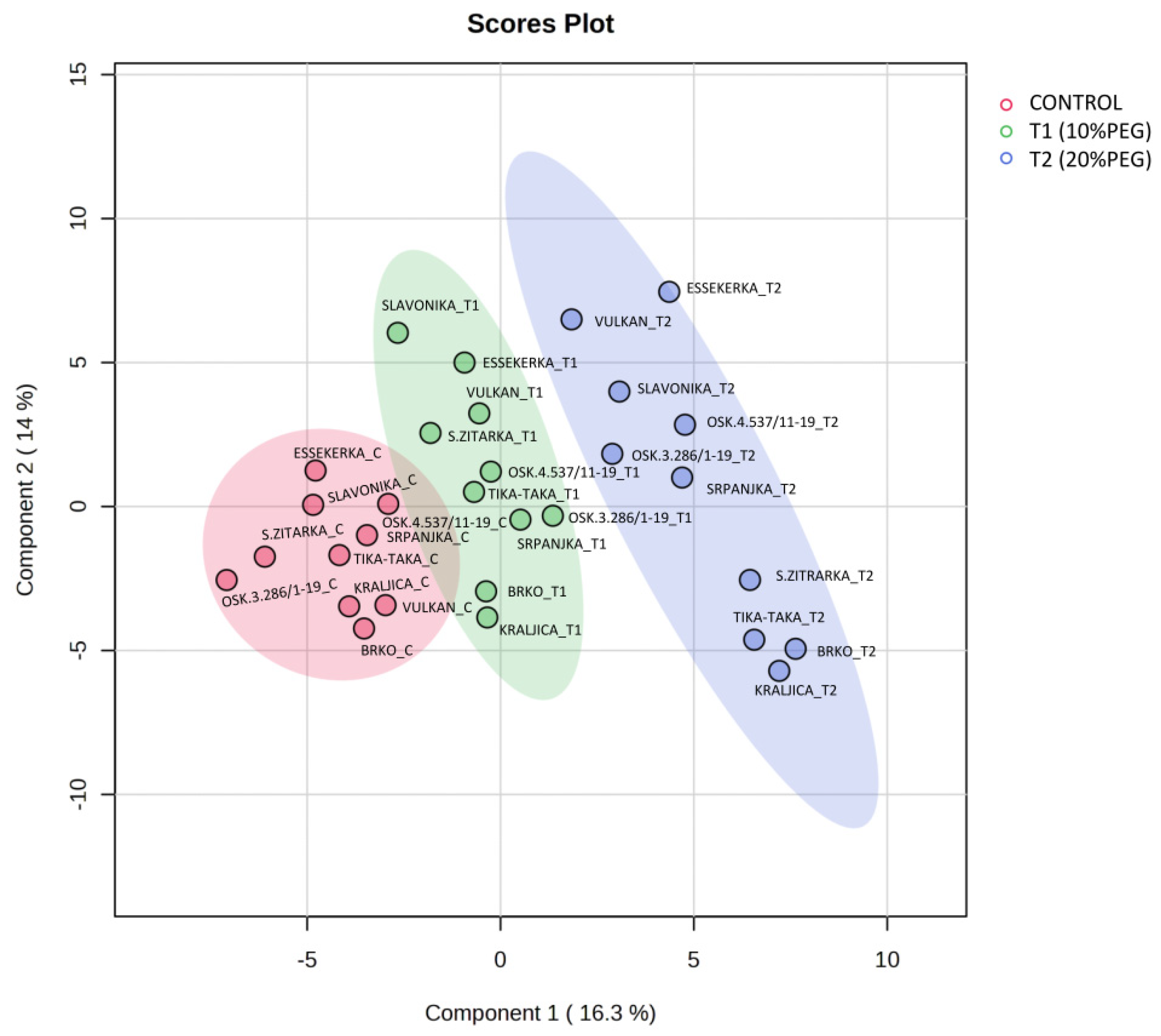
2.2. Total Metabolite Profiling Under Two Drought Intensities in Wheat
3. Discussion
3.1. Morphological Changes in Wheat Seedlings from This Study
3.2. Metabolic Changes in Two Drought Intensities
3.2.1. Organic Acids and Compounds
3.2.2. Amino Acids, Hormones, and Benzenoids
3.2.3. Sugars, Amino Sugars, and Carboxylic Acids
3.2.4. Fatty Acids
4. Materials and Methods
4.1. Wheat Material
4.2. Experimental Layout
4.3. Morphological Traits
4.4. Metabolic Profiling
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajanna, G.A.; Suman, A.; Venkatesh, P. Mitigating drought stress effects in arid and semi-arid agro-ecosystems through bioirrigation strategies-A review. Sustainability 2023, 15, 3542. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Szablińska-Piernik, J.; Lahuta, L.B. Polar metabolites profiling of wheat shoots (Triticum aestivum L.) under repeated short-term soil drought and rewatering. Int. J. Mol. Sci. 2023, 24, 8429. [Google Scholar] [CrossRef]
- UN. World Population Prospects: Data Booklet. 2019. Available online: https://population.un.org/wpp/ (accessed on 23 July 2024).
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. CMLS 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Hu, X.W.; Fan, Y.; Baskin, C.C.; Baskin, J.M.; Wang, Y.R. Comparison of the effects of temperature and water potential on seed germination of Fabaceae species from desert and subalpine grassland. Am. J. Bot. 2015, 102, 649–660. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Jolánkai, M.; Kovács, G.P.; Gyuricza, C.; Tarnawa, Á. Impact of temperature and water on seed germination and seedling growth of maize (Zea mays L.). Agronomy 2022, 12, 397. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Han, G.; Sun, X.; Yang, X. Seed osmopriming with polyethylene glycol (PEG) enhances seed germination and seedling physiological traits of Coronilla varia L. under water stress. PLoS ONE 2024, 19, e0303145. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef]
- Khakwani, A.A.; Dennett, M.D.; Munir, M. Drought tolerance screening of wheat varieties by inducing water stress conditions. SJST 2011, 33, 135–142. [Google Scholar]
- Saeidi, M.; Ahmadi, A.; Moradi, F.; Hajirezaei, M.-R. Comparative metabolome profiling of two contrasting wheat cultivars in late-season water deficit. Front. Plant Physiol. 2024, 2, 1386473. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Yang, X.; Feng, K.; Wang, G.; Shi, Q.; Wang, X.; Yuan, X.; Ren, J. Hydrogen sulfide increases drought tolerance by modulating carbon and nitrogen metabolism in Foxtail Millet seedlings. Agronomy 2024, 14, 1080. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar Patel, M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 24, 9108. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 11, e0213502. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics response for drought stress tolerance in chinese wheat genotypes (Triticum aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef]
- Fadoul, H.E.; Martínez Rivas, F.J.; Neumann, K.; Balazadeh, S.; Fernie, A.R.; Alseekh, S. Comparative molecular and metabolic profiling of two contrasting wheat cultivars under drought stress. Int. J. Mol. Sci. 2021, 22, 13287. [Google Scholar] [CrossRef] [PubMed]
- Aldesuquy, H.S.; Ibraheem, F.I.; Gahnem, H.E. Comparative morpho-biochemical responses of wheat cultivars sensitive and tolerant to water stress. J. Stress Physiol. Biochem. 2014, 10, 168–189. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Wolny, E.; Betekhtin, A.; Rojek, M.; Braszewska-Zalewska, A.; Lusinska, J.; Hasterok, R. Germination and the early stages of seedling development in Brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 2916. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Chen, Y.; Zhang, L.; Kudusi, K.; Song, J. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest China. Front. Ecol. Evol. 2022, 10, 1026095. [Google Scholar] [CrossRef]
- Harris, D.; Tripathi, R.S.; Joshi, A. On-farm seed priming to improve crop establishment and yield in dry direct-seeded rice. In Direct Seeding: Research Strategies and Opportunities; Pandey, S., Mortimer, M., Wade, L., Tuong, T.P., Lopes, K., Hardy, B., Eds.; International Research Institute: Manila, Philippines, 2002; pp. 231–240. [Google Scholar]
- Vuković, R.; Štolfa Čamagajevac, I.; Vuković, A.; Šunić, K.; Begović, L.; Mlinarić, S.; Sekulić, R.; Sabo, N.; Španić, V. Physiological, biochemical and molecular response of different winter wheat varieties under drought stress at germination and seedling growth stage. Antioxidants 2022, 11, 693. [Google Scholar] [CrossRef]
- Duvnjak, J.; Brkljačić, L.; Salopek Sondi, B.; Španić, V. Morpho-physiological and hormonal response of different winter wheat varieties to drought stress at germination and seedling stage. Agron. Glas. 2023, 84, 277–294. [Google Scholar] [CrossRef]
- Duan, H.; Zhu, Y.; Li, J.; Ding, W.; Wang, H.; Jiang, L.; Zhou, Y. Effects of drought stress on growth and development of wheat seedlings. Int. J. Agric. Biol. 2017, 19, 1119–1124. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhou, B.Z.; Zhou, Y.; Ge, X.G.; Wang, X.M.; Cao, Y.H. Effects of drought stress simulated by peg on seed germination and growth physiological characteristics of Phyllostachys edulis. For. Res. 2018, 31, 47–54. [Google Scholar] [CrossRef]
- Evamoni, F.Z.; Nulit, R.; Yap, C.K.; Ibrahim, M.F.; Sidek, N.B. Assessment of germination performance and early seedling growth of Malaysian indica rice genotypes under drought conditions for strategic cropping during water scarcity. Chil. J. Agric. Res. 2023, 83, 281–292. [Google Scholar] [CrossRef]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Nonami, H. Plant water relations and control of cell elongation at low water potentials. J. Plant Res. 1998, 111, 373–382. [Google Scholar] [CrossRef]
- Kerbauy, G.B. Fisiologia Vegetal, 2nd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2012; 431p. [Google Scholar]
- Ahmed, H.G.M.; Zeng, Y.; Shah, A.N.; Yar, M.M.; Ullah, A.; Ali, M. Conferring of drought tolerance in wheat (Triticum aestivum L.) genotypes using seedling indices. Front. Plant Sci. 2022, 13, 961049. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.S.; Fujita, D.B.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Zhao, P.; Zhou, Q.; Zhao, X. The abundance of certain metabolites responds to drought stress in the highly drought tolerant plant Caragana korshinskii. Acta Physiol. Plant. 2017, 39, 116. [Google Scholar] [CrossRef]
- Lv, L.; Chen, X.; Li, H.; Huang, J.; Liu, Y.; Zhao, A. Different adaptive patterns of wheat with different drought tolerance under drought stresses and rehydration revealed by integrated metabolomic and transcriptomic analysis. Front. Plant Sci. 2022, 13, 1008624. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Singh, A.; Kamal, A. Osmoprotective role of sugar in mitigating abiotic stress in plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress. Biochemical and Molecular Perspectives; Wiley: Hoboken, NJ, USA, 2020; pp. 53–70. [Google Scholar] [CrossRef]
- Tatar, Ö.; Gevrek, M.N. Lipid peroxidation and water content of wheat. Asian J. Plant Sci. 2008, 7, 409–412. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Rivero, R.M.; Lopez-Cantarero, I.; Romero, L. Role of Ca2+ in the metabolism of phenolic compounds in tobacco leaves (Nicotiana tabacum L.). Plant Growth Regul. 2003, 41, 173–177. [Google Scholar] [CrossRef]
- Kleiber, T.; Chadzinikolau, T.; Formela-Luboińska, M.; Lartey, J.L.; Kosiada, T. Enhancing lettuce drought tolerance: The role of organic acids in photosynthesis and oxidative defense. Appl. Sci. 2024, 14, 5119. [Google Scholar] [CrossRef]
- Xie, H.; Bai, G.; Lu, P.; Li, H.; Fei, M.; Xiao, B.G.; Chen, X.J.; Tong, Z.J.; Wang, Z.Y.; Yang, D.H. Exogenous citric acid enhances drought tolerance in tobacco (Nicotiana tabacum). Plant Biol. 2022, 24, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Rossi, S.; Huang, B. Metabolic and physiological regulation of aspartic acid-mediated enhancement of heat stress tolerance in Perennial ryegrass. Plants 2022, 13, 199. [Google Scholar] [CrossRef]
- Khosravi-nejad, F.; Khavari-nejad, R.A.; Moradi, F.; Najafi, F. Cytokinin and abscisic acid alleviate drought stress through changing organic acids profile, ion immolation, and fatty acid profile to improve yield of wheat (Triticum aestivum L.) cultivars. Physiol. Mol. Biol. Plants 2022, 28, 1119–1129. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.; Schroeder, S.; Schachtman, D. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef]
- Mungi, C.V.; Singh, S.K.; Chugh, J.; Rajamani, S. Synthesis of barbituric acid containing nucleotides and their implications for the origin of primitive informational polymers. Phys. Chem. Chem. Phys. 2016, 18, 20144–20152. [Google Scholar] [CrossRef] [PubMed]
- Ribarova, F. Amino acids: Carriers of nutritional and biological value foods. In Food Processing for Increased Quality and Consumption; Academic Press: London, UK, 2018; pp. 287–311. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, A. Kernel biochemical aspects in stressed wheat in response to glycine betaine and salicylic scid. Agric. Res. Technol. 2017, 7, 555724. [Google Scholar] [CrossRef]
- Rahman, M.; Akond, M.; Babar, M.A.; Beecher, C.; Erickson, J.; Thomason, K.; De Jong, F.A.; Mason, R.E. LC-HRMS Based non-targeted metabolomic profiling of wheat (Triticum aestivum L.) under post-anthesis drought stress. Am. J. Plant Sci. 2017, 8, 3024–3061. [Google Scholar] [CrossRef]
- Eirini, S.; Paschalina, C.; Loannis, T.; Kortessa, D.T. Effect of drought and salinity on volatile organic compounds and other cecondary metabolites of Citrus aurantium leaves. Nat. Prod. Commun. 2017, 12, 193–196. [Google Scholar]
- Sharma, B.; Yadav, L.; Shrestha, A.; Shrestha, S.; Subedi, M.; Subedi, S.; Shrestha, J. Drought stress and its management in wheat (Triticum aestivum L.): A review. Agric. Sci. Technol. 2022, 14, 3–14. [Google Scholar] [CrossRef]
- Ali, E.F.; Aljarani, A.M.; Mohammed, F.A.; Desoky, E.M.; Mohamed, I.A.A.; El-Sharnouby, M.; Tammam, S.A.; Hassan, F.A.S.; Rady, M.M.; Shaaban, A. Exploring the potential enhancing effects of trans-zeatin and silymarin on the productivity and antioxidant defense capacity of cadmium-stressed wheat. Biology 2022, 11, 1173. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Jiang, Y.; Zhang, J.; Ni, Y.; Zhang, P.; Yao, Z.; Jiao, Z.; Li, H.; Li, L.; et al. Wheat gibberellin oxidase genes and their functions in regulating tillering. PeerJ 2023, 11, e15924. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Baltensperger, D.D.; Santra, D.K.; Hergert, G.W.; Knox, S. Gibberellic acid promotes early growth of winter wheat and rye. Am. J. Plant Sci. 2014, 5, 2984–2996. [Google Scholar] [CrossRef][Green Version]
- Lenton, J.R.; Appleford, N.E.J.; Croker, S.J. Gibberellins and αamylase gene expression in germinating wheat grains. Plant Growth Regul. 1994, 15, 261–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, Z.; Yao, Y.; Nie, X.; Sun, Q. Gibberellins and heterosis of plant height in wheat (Triticum aestivum L.). BMC Genet. 2007, 8, 40. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Novi, G.; Loreti, E.; Paolicchi, F.; Poggi, A.; Alpi, A.; Perata, P. A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 2005, 44, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Weed, R.A.; Savchenko, K.G.; Lessin, L.M.; Carris, L.M.; Gang, D.R. Untargeted metabolomic investigation of wheat infected with stinking smut Tilletia caries. Phytopathology 2021, 111, 2343–2354. [Google Scholar] [CrossRef]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- Zeleny, R.; Kolarich, D.; Strasser, R.; Altmann, F. Sialic acid concentrations in plants are in the range of inadvertent contamination. Planta 2006, 224, 222–227. [Google Scholar] [CrossRef]
- Lehner, A.; Meimoun, P.; Errakhi, R.; Madiona, K.; Barakate, M.; Bouteau, F. Toxic and signaling effects of oxalic acid: Natural born killer or natural born protector? Plant Signal. Behav. 2008, 3, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Meena, H.M.; Kumar, K. Production of oxalic acid as influenced by the application of organic residue and its effect on phosphorus uptake by wheat (Triticum aestivum L.) in an inceptisol of North India. Natl. Acad. Sci. Lett. 2014, 37, 401–405. [Google Scholar] [CrossRef][Green Version]
- Liu, E.E.; Luo, W.; Zhou, H.; Peng, X.X. Determination of oxalate in plant tissues with oxalate oxidase prepared from wheat. Biol. Plant 2009, 53, 129–132. [Google Scholar] [CrossRef]
- Delisle, G.; Champoux, M.; Houde, M. Characterization of oxalate oxidase and cell death in al-sensitive and tolerant wheat roots. Plant Cell Physiol. 2001, 42, 324–333. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, M.N.; Lodhi, S.S.; Ahmed, I.; Tayyab, M.; Mehmood, T.; Din, I.U.; Khan, M.; Sohail, Q.; Akram, M. Targeted metabolomics reveals fatty acid abundance adjustments as playing a crucial role in drought-stress response and post-drought recovery in wheat. Front. Genet. 2022, 13, 972696. [Google Scholar] [CrossRef]
- Sharma, P.; Lakra, N.; Goyal, A.; Ahlawat, Y.K.; Zaid, A.; Siddique, K.H.M. Drought and heat stress mediated activation of lipid signaling in plants: A critical review. Front. Plant Sci. 2023, 14, 1216835. [Google Scholar] [CrossRef] [PubMed]
- Gigon, A.; Matos, A.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- Moradi, P.; Ford-Lloyd, B.; Pritchard, J. Metabolomic approach reveals the biochemical mechanisms underlying drought stress tolerance in thyme. Anal. Biochem. 2017, 527, 49–62. [Google Scholar] [CrossRef]
- Sarsenbayev, K. Determination of Signal Molecules, Proteins, and Low-Molecular-Weight Organic Compounds in Wheat Varieties Infected by leaf Rust Disease; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the correlations between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Riewe, D.; Jeon, H.J.; Lisec, J.; Heuermann, M.C.; Schmeichel, J.; Seyfarth, M.; Meyer, R.C.; Willmitzer, L.; Altmann, T. A naturally occurring promoter polymorphism of the Arabidopsis FUM2 gene causes expression variation, and is associated with metabolic and growth traits. Plant J. 2016, 88, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Erban, A.; Schauer, N.; Fernie, A.R.; Kopka, J. Nonsupervised construction and application of massspectral and retention time index libraries from time-of-flight gaschromatography–mass spectrometry metabolite profiles. Methods Mol. 2007, 358, 19–38. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Inostroza, A.; Caldana, C.; Redestig, H.; Kusano, M.; Lisec, J.; Peña-Cortés, H.; Willmitzer, L.; Hannah, M.A. TargetSearch—A bioconductor package for the efficient preprocessing of GC-MS metabolite profiling data. BMC Bioinform. 2009, 10, 428. [Google Scholar] [CrossRef]
| No. | Wheat Genotype | Origin | Registration Year |
|---|---|---|---|
| 1 | Srpanjka | AIO *, Croatia | 1989 |
| 2 | Super Žitarka | AIO, Croatia | 1997 |
| 3 | Vulkan | AIO, Croatia | 2009 |
| 4 | Kraljica | AIO, Croatia | 2010 |
| 5 | Tika Taka | AIO, Croatia | 2014 |
| 6 | Brko | AIO, Croatia | 2020 |
| 7 | Slavonika | AIO, Croatia | 2023 |
| 8 | Essekerka | AIO, Croatia | 2023 |
| 9 | Osk.3.286/1-19 | AIO, Croatia | - |
| 10 | Osk.4.537/11-19 | AIO, Croatia | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Duvnjak, J.; Hefer, D.; D’Auria, J.C. Changes in Metabolites Produced in Wheat Plants Against Water-Deficit Stress. Plants 2025, 14, 10. https://doi.org/10.3390/plants14010010
Spanic V, Duvnjak J, Hefer D, D’Auria JC. Changes in Metabolites Produced in Wheat Plants Against Water-Deficit Stress. Plants. 2025; 14(1):10. https://doi.org/10.3390/plants14010010
Chicago/Turabian StyleSpanic, Valentina, Jurica Duvnjak, Dubravka Hefer, and John C. D’Auria. 2025. "Changes in Metabolites Produced in Wheat Plants Against Water-Deficit Stress" Plants 14, no. 1: 10. https://doi.org/10.3390/plants14010010
APA StyleSpanic, V., Duvnjak, J., Hefer, D., & D’Auria, J. C. (2025). Changes in Metabolites Produced in Wheat Plants Against Water-Deficit Stress. Plants, 14(1), 10. https://doi.org/10.3390/plants14010010








