The Expression of Key Ethylene and Anthocyanin Biosynthetic Genes of ‘Honeycrisp’ Apples Subjected to the Combined Use of Reflective Groundcovers and Aminoethoxyvinylglycine in the Mid-Atlantic US
Abstract
1. Introduction
2. Results
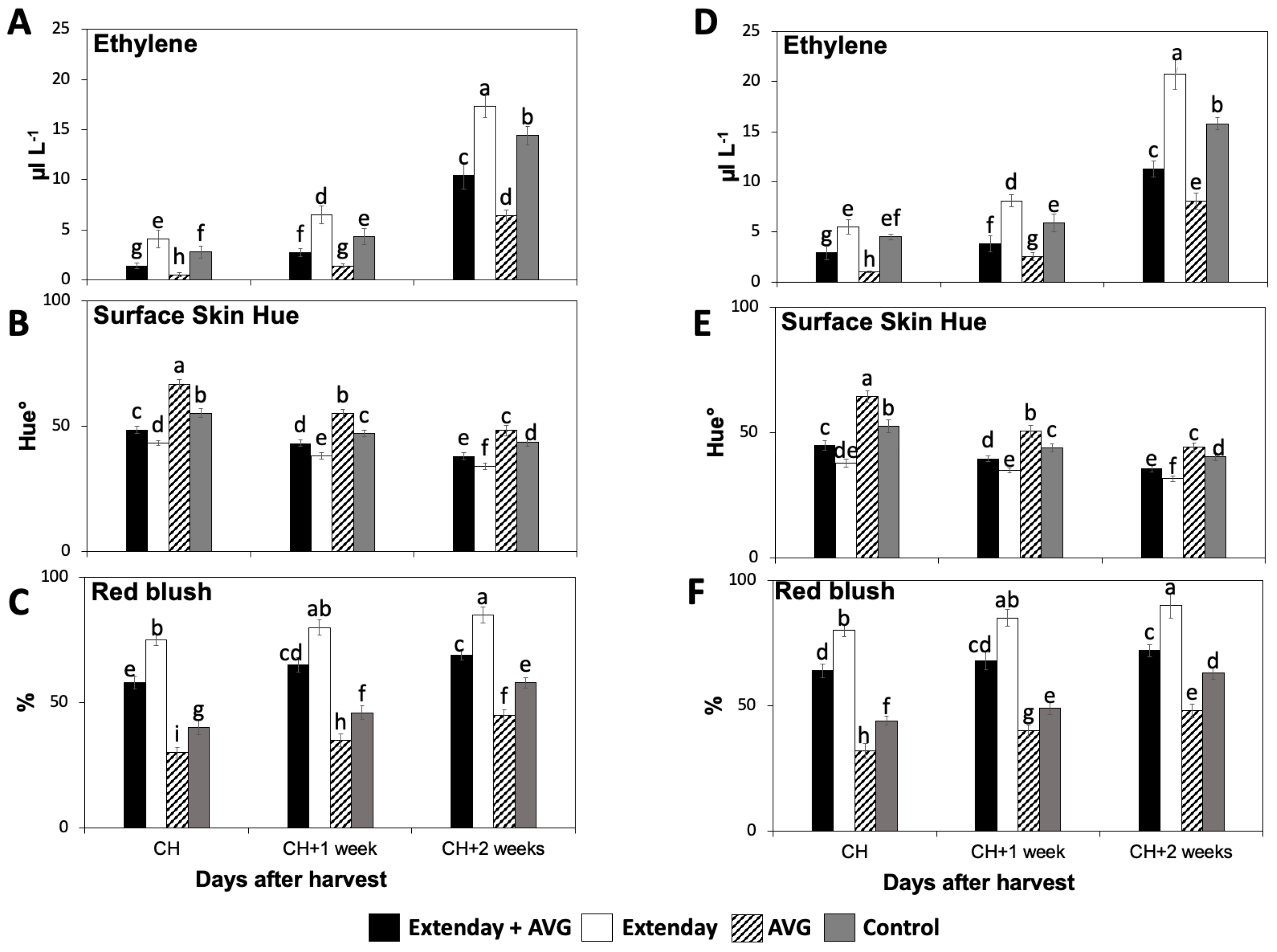
2.1. Effects of Extenday and AVG Treatment Combinations on ‘Honeycrisp’ Internal Ethylene Concentration and Red Skin Coloration
2.2. Effects of Extenday and AVG Treatment Combinations on ‘Honeycrisp’ Fruit Drop
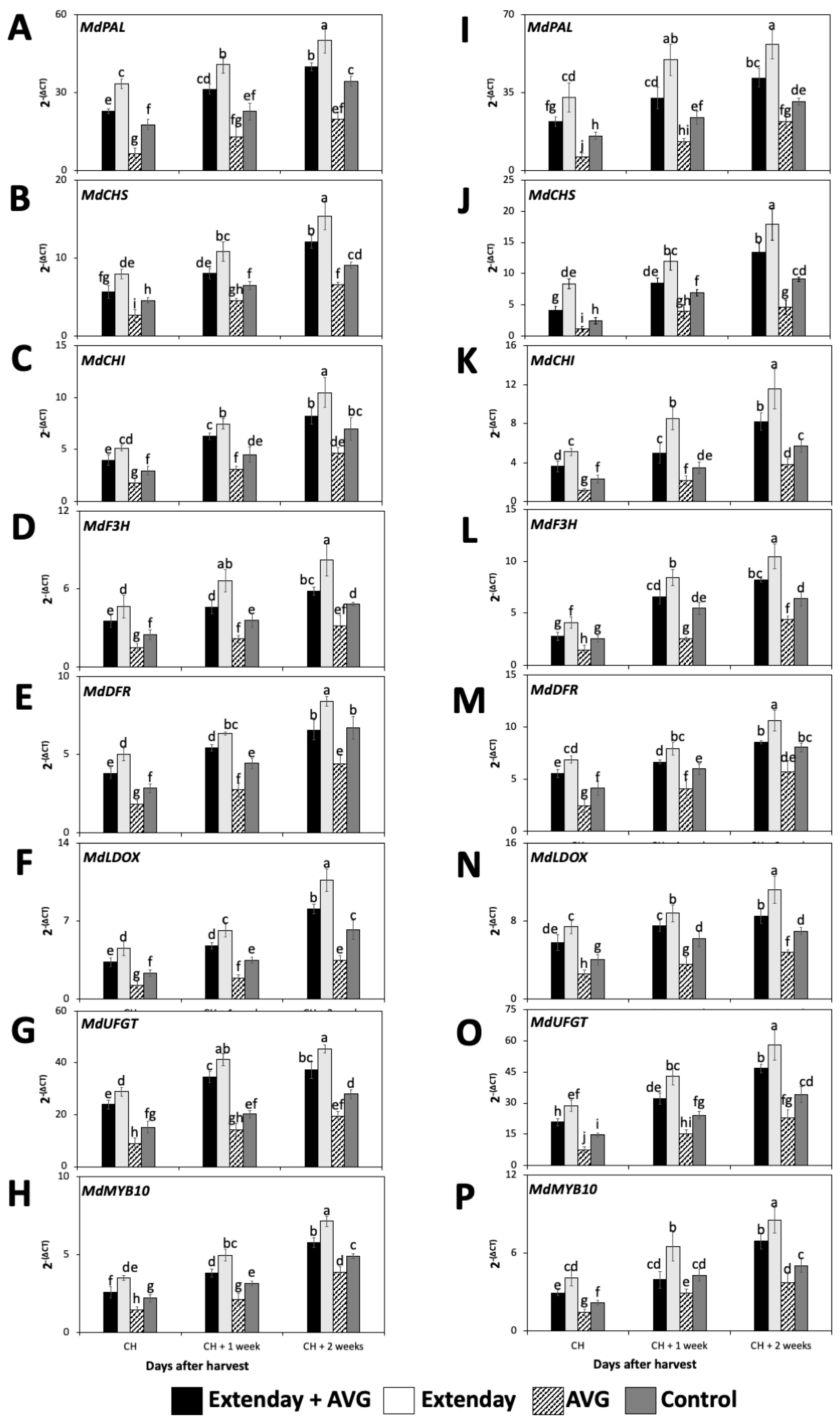
2.3. Effects of Extenday and AVG Treatment Combinations on Key Ethylene and Anthocyanin Biosynthetic Pathway-Related Genes and Transcription Factors Associated with Their Regulation
2.3.1. Ethylene Biosynthetic Genes
2.3.2. Anthocyanin Biosynthetic Genes and Transcription Factors Associated with Its Regulation
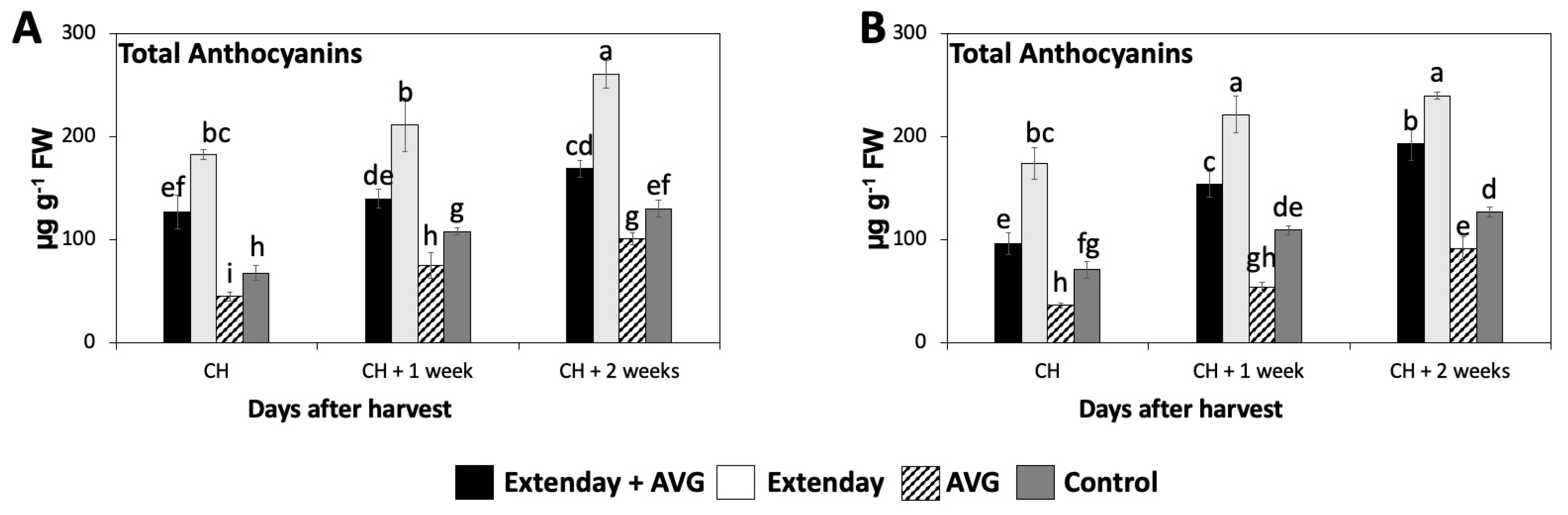
2.4. Effects of Extenday and AVG Treatment Combinations on ‘Honeycrisp’ Total Anthocyanin Concentration
2.5. Relationships among Ethylene Concentration, Red Skin Coloration, Key Ethylene and Anthocyanin Biosynthetic-Related Genes, and Anthocyanin Concentration of ‘Honeycrisp’ Apple Fruit Submitted to Extenday and AVG Treatment Combinations
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preharvest Orchard Treatments
4.2. Fruit Internal Ethylene Concentration and Color Measurements
4.3. Fruit Drop Measurements
4.4. Real-Time Quantitative RT-PCR Analysis
4.5. Total Anthocyanin Quantification
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and Functional Analysis of a MYB Transcription Factor Gene That Is a Key Regulator for the Development of Red Coloration in Apple Skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Musacchi, S.; Serra, S. Apple Fruit Quality: Overview on Pre-Harvest Factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Kon, T.M.; Clavet, C.D. Enhancing Red Fruit Coloration of Apples in the Southeastern US with Reflective Fabrics. Horticulturae 2023, 9, 1125. [Google Scholar] [CrossRef]
- Miah, M.S.; Farcuh, M. Combining the Use of Reflective Groundcovers and Aminoethoxyvinylglycine to Assess Effects on Skin Color, Preharvest Drop, and Quality of ‘Honeycrisp’Apples in the Mid-Atlantic US. Horticulturae 2024, 10, 179. [Google Scholar] [CrossRef]
- USDA Agricultural Marketing Service Apples Grades and Standards. Available online: https://www.ams.usda.gov/grades-standards/apple-grades-standards (accessed on 11 February 2024).
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Phenylpropanoid Metabolites and Expression of Key Genes Involved in Anthocyanin Biosynthesis in the Shaded Peel of Apple Fruit in Response to Sun Exposure. Plant Physiol. Biochem. 2013, 69, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P. Special Issue “Flavonoids and Their Disease Prevention and Treatment Potential”: Recent Advances and Future Perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, L. The Shaded Side of Apple Fruit Becomes More Sensitive to Photoinhibition with Fruit Development. Physiol. Plant. 2008, 134, 282–292. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar Variation in Apple Peel and Whole Fruit Phenolic Composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Kunradi Vieira, F.G.; da Silva Campelo Borges, G.; Copetti, C.; Valdemiro Gonzaga, L.; da Costa Nunes, E.; Fett, R. Activity and Contents of Polyphenolic Antioxidants in the Whole Fruit, Flesh and Peel of Three Apple Cultivars. Arch. Latinoam. Nutr. 2009, 59, 101–106. [Google Scholar]
- Xie, R.; Zheng, L.; He, S.; Zheng, Y.; Yi, S.; Deng, L. Anthocyanin Biosynthesis in Fruit Tree Crops: Genes and Their Regulation. Afr. J. Biotechnol. 2011, 10, 19890–19897. [Google Scholar]
- Espley, R.V.; Brendolise, C.; Chagne, D.; Kutty-Amma, S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P. Multiple Repeats of a Promoter Segment Causes Transcription Factor Autoregulation in Red Apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Telias, A.; Lin-Wang, K.; Stevenson, D.E.; Cooney, J.M.; Hellens, R.P.; Allan, A.C.; Hoover, E.E.; Bradeen, J.M. Apple Skin Patterning Is Associated with Differential Expression of MYB10. BMC Plant Biol. 2011, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-A.; Duan, S.; Gil, C.S.; Jeong, H.Y.; Lee, C.; Kang, I.-K.; Eom, S.H. Combined UV-B and Methyl Jasmonate Treatments Enhance Postharvest Pigmentation of “Fuji” Apples. Postharvest Biol. Technol. 2022, 190, 111938. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red Colouration in Apple Fruit Is Due to the Activity of the MYB Transcription Factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin Biosynthetic Genes Are Coordinately Expressed during Red Coloration in Apple Skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression Analysis of Anthocyanin Biosynthetic Genes in Apple Skin: Effect of UV-B and Temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Honda, C.; Moriya, S. Anthocyanin Biosynthesis in Apple Fruit. Hortic. J. 2018, 87, 305–314. [Google Scholar] [CrossRef]
- Lancaster, J.E. Regulation of Skin Color in Apples. CRC. Crit. Rev. Plant Sci. 1992, 10, 487–502. [Google Scholar] [CrossRef]
- Farcuh, M.; Tajima, H.; Lerno, L.A.; Blumwald, E. Changes in Ethylene and Sugar Metabolism Regulate Flavonoid Composition in Climacteric and Non-Climacteric Plums during Postharvest Storage. Food Chem. Mol. Sci. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Wang, Z.; Dilley, D.R. Aminoethoxyvinylglycine, Combined with Ethephon, Can Enhance Red Color Development without over-Ripening Apples. HortScience 2001, 36, 328–331. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Unrath, C.R. PAL and Ethylene Content during Maturation of Red and Golden Delicious Apples. Phytochemistry 1988, 27, 1001–1003. [Google Scholar] [CrossRef]
- Whale, S.K.; Singh, Z. Endogenous Ethylene and Color Development in the Skin of ‘Pink Lady’Apple. J. Am. Soc. Hortic. Sci. 2007, 132, 20–28. [Google Scholar] [CrossRef]
- Whale, S.K.; Singh, Z.; Behboudian, M.H.; Janes, J.; Dhaliwal, S.S. Fruit Quality in ‘Cripp’s Pink’Apple, Especially Colour, as Affected by Preharvest Sprays of Aminoethoxyvinylglycine and Ethephon. Sci. Hortic. 2008, 115, 342–351. [Google Scholar] [CrossRef]
- Toivonen, P.; Stoochnoff, J.; Usher, K.; Lu, C.; Wiersma, P.; Zhou, C. Biochemical and Gene Expression Involved in Red Blush Color Development in ‘Ambrosia’ Apple. J. Am. Soc. Hortic. Sci. 2019, 144, 164–171. [Google Scholar] [CrossRef]
- Charles, M.T.; Arul, J. UV Treatment of Fresh Fruits and Vegetables for Improved Quality: A Status Report. Stewart Postharvest Rev. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research Progress of Fruit Color Development in Apple (Malus domestica borkh). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Dong, Y.; Mitra, D.; Kootstra, A.; Lister, C.; Lancaster, J. Postharvest Stimulation of Skin Color in Royal Gala Apple. J. Am. Soc. Hortic. Sci. 1995, 120, 95–100. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, D.; Han, Y.; Khan, M.A.; Soria-Guerra, R.E.; Korban, S.S. Transcriptome Analysis of the Exocarp of Apple Fruit Identifies Light-Induced Genes Involved in Red Color Pigmentation. Gene 2014, 534, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Duan, Y.; Ju, Z. Effects of Covering the Orchard Floor with Reflecting Films on Pigment Accumulation and Fruit Coloration InFuji’apples. Sci. Hortic. 1999, 82, 47–56. [Google Scholar] [CrossRef]
- Kondo, S.; Hiraoka, K.; Kobayashi, S.; Honda, C.; Terahara, N. Changes in the Expression of Anthocyanin Biosynthetic Genes during Apple Development. J. Am. Soc. Hortic. Sci. 2002, 127, 971–976. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-Induced Expression of a MYB Gene Regulates Anthocyanin Biosynthesis in Red Apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB Transcription Factors That Colour Our Fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, S.; Jiao, Q.; Liu, C.; Zhang, W.; Chen, W.; Chen, X. Comparison of MdMYB1 Sequences and Expression of Anthocyanin Biosynthetic and Regulatory Genes between Malus Domestica Borkh. Cultivar ‘Ralls’ and Its Blushed Sport. Euphytica 2012, 185, 157–170. [Google Scholar] [CrossRef]
- Funke, K.; Blanke, M. Spatial and Temporal Enhancement of Colour Development in Apples Subjected to Reflective Material in the Southern Hemisphere. Horticulturae 2021, 7, 2. [Google Scholar] [CrossRef]
- Mupambi, G.; Valverdi, N.A.; Camargo-Alvarez, H.; Reid, M.; Kalcsits, L.; Schmidt, T.; Castillo, F.; Toye, J. Reflective Groundcover Improves Fruit Skin Color in ‘Honeycrisp’Apples Grown under Protective Netting. Horttechnology 2021, 31, 607–614. [Google Scholar] [CrossRef]
- Layne, D.R.; Jiang, Z.; Rushing, J.W. The Influence of Reflective Film and ReTain on Red Skin Coloration and Maturity OfGala’Apples. Horttechnology 2002, 12, 640–645. [Google Scholar] [CrossRef]
- Privé, J.-P.; Russell, L.; LeBlanc, A. Impact of Reflective Groundcover on Growth, Flowering, Yield and Fruit Quality in Gala Apples in New Brunswick. Can. J. Plant Sci. 2011, 91, 765–772. [Google Scholar] [CrossRef]
- Toye, J. Reflective Mulches—New Zealand Leads the Way. Orchard. 1995, 68, 58–60. [Google Scholar]
- Iglesias, I.; Alegre, S. The Effects of Reflective Film on Fruit Color, Quality, Canopy Light Distribution, and Profitability of “Mondial Gala” Apples. Horttechnology 2009, 19, 488–498. [Google Scholar] [CrossRef]
- Robinson, T.L.; Gonzalez, L. Effect of Different Reflective Ground Covers on Light Reflection and on the Coloring of Apples at Harvest. Proc. Acta Hortic. 2023, 1366, 385–392. [Google Scholar]
- Privé, J.P.; Russell, L.; Leblanc, A. Use of Extenday Reflective Groundcover in Production of ‘Gala’ Apples (Malus domestica) in New Brunswick, Canada: 1. Impact on Canopy Microclimate and Leaf Gas Exchange. N. Z. J. Crop Hortic. Sci. 2008, 36, 221–231. [Google Scholar] [CrossRef]
- Miller, S.S.; Greene, G.M. The Use of Reflective Film and Ethephon to Improve Red Skin Color of Apples in the Mid-Atlantic Region of the United States. Horttechnology 2003, 13, 90–99. [Google Scholar] [CrossRef]
- Shafiq, M.; Singh, Z.; Khan, A.S. Pre-Harvest Ethephon Application and Training Systems Affect Colour Development, Accumulation of Flavonoids and Fruit Quality of ‘Cripps Pink’ apple. Aust. J. Crop Sci. 2014, 8, 1579–1589. [Google Scholar]
- Faragher, J.D.; Brohier, R.L. Anthocyanin Accumulation in Apple Skin during Ripening: Regulation by Ethylene and Phenylalanine Ammonia-Lyase. Sci. Hortic. 1984, 22, 89–96. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Ethylene Action and the Ripening of Fruits: Ethylene Influences the Growth and Development of Plants and Is the Hormone Which Initiates Fruit Ripening. Science 1965, 148, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.S.; Hinson, C.; Farcuh, M. Assessing Fruit Maturity and Quality of ‘Buckeye Gala’ Grown on a Diverse Panel of Apple (Malus domestica borkh) Rootstocks in Western Maryland. Agronomy 2023, 13, 2528. [Google Scholar] [CrossRef]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.-X.; Van Deynze, A. Sensory, Physicochemical and Volatile Compound Analysis of Short and Long Shelf-Life Melon (Cucumis melo L.) Genotypes at Harvest and after Postharvest Storage. Food Chem. X 2020, 8, 100107. [Google Scholar] [CrossRef]
- Farcuh, M.; Li, B.; Rivero, R.M.; Shlizerman, L.; Sadka, A.; Blumwald, E. Sugar Metabolism Reprogramming in a Non-Climacteric Bud Mutant of a Climacteric Plum Fruit during Development on the Tree. J. Exp. Bot. 2017, 68, 5813–5828. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Toubiana, D.; Sade, N.; Rivero, R.M.; Doron-Faigenboim, A.; Nambara, E.; Sadka, A.; Blumwald, E. Hormone Balance in a Climacteric Plum Fruit and Its Non-Climacteric Bud Mutant during Ripening. Plant Sci. 2019, 280, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, M.H.; Cline, J.A. A Review of Apple Preharvest Fruit Drop and Practices for Horticultural Management. Sci. Hortic. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Brumos, J. Gene Regulation in Climacteric Fruit Ripening. Curr. Opin. Plant Biol. 2021, 63, 102042. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Stella, S.; Van de Weg, W.E.; Guerra, W.; Cecchinel, M.; Dallavia, J.; Koller, B.; Sansavini, S. Role of the Genes Md-ACO1 and Md-ACS1 in Ethylene Production and Shelf Life of Apple (Malus domestica borkh). Euphytica 2005, 141, 181–190. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and Its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and Shakers’ in the Regulation of Fruit Ripening: A Cross-Dissection of Climacteric versus Non-Climacteric Fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, M.H.; Cline, J.A. AVG, NAA, Boron, and Magnesium Influence Preharvest Fruit Drop and Fruit Quality of ‘Honeycrisp’ Apples. Can. J. Plant Sci. 2018, 98, 741–752. [Google Scholar] [CrossRef]
- Chu, C.L. Internal Ethylene Concentration of ‘McIntosh’, ‘Northern Spy’, ‘Empire’, ‘Mutsu’, and ‘Idared’ Apples during the Harvest Season. J. Am. Soc. Hortic. Sci. 1988, 113, 226–229. [Google Scholar] [CrossRef]
- Gussman, C.D.; Goffreda, J.C.; Gianfagna, T.J. Ethylene Production and Fruit-Softening Rates in Several Apple Fruit Ripening Variants. HortScience 1993, 28, 135–137. [Google Scholar] [CrossRef]
- Irish-Brown, A.; Schwallier, P.; Shane, B.; Tritten, B. Why Does Apple Fruit Drop Prematurely? Michigan State University, Extension: East Lansing, MI, USA, 2011. [Google Scholar]
- Liu, J.; Islam, M.T.; Sherif, S.M. Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples. Horticulturae 2022, 8, 1100. [Google Scholar] [CrossRef]
- Schupp, J.R.; Greene, D.W. Effect of Aminoethoxyvinylglycine (AVG) on Preharvest Drop, Fruit Quality, and Maturation of ‘McIntosh’ Apples. I. Concentration and Timing of Dilute Applications of AVG. HortScience 2004, 39, 1030–1035. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Yuan, R. Profiling the Expression of Genes Related to Ethylene Biosynthesis, Ethylene Perception, and Cell Wall Degradation during Fruit Abscission and Fruit Ripening in Apple. J. Am. Soc. Hortic. Sci. 2010, 135, 391–401. [Google Scholar] [CrossRef]
- Greene, D.W.; Schupp, J.R. Effect of Aminoethoxyvinylglycine (AVG) on Preharvest Drop, Fruit Quality, and Maturation of McIntosh’ Apples. II. Effect of Timing and Concentration Relationships and Spray Volume. HortScience 2004, 39, 1036–1041. [Google Scholar] [CrossRef]
- Greene, D.W. Time of Aminoethoxyvinylglycine Application Influences Preharvest Drop and Fruit Quality of ‘McIntosh’ apples. HortScience 2005, 40, 2056–2060. [Google Scholar] [CrossRef]
- Byers, R.E. Effects of Aminoethoxyvinylglycine (AVG) on Preharvest Fruit Drop, Maturity, and Cracking of Several Apple Cultivars. J. Tree Fruit. Prod. 1997, 2, 77–97. [Google Scholar] [CrossRef]
- Yuan, R.; Li, J. Effect of Sprayable 1-MCP, AVG, and NAA on Ethylene Biosynthesis, Preharvest Fruit Drop, Fruit Maturity, and Quality of ‘Delicious’ Apples. HortScience 2008, 43, 1454–1460. [Google Scholar] [CrossRef]
- Boyacı, S. Effect of Aminoethoxyvinylglycine (AVG) Applications on Pre-Harvest Drop and Fruit Quality of ‘Red Delicious, Red Chief’ Apple Cultivar. Erwerbs-Obstbau 2022, 64, 395–400. [Google Scholar] [CrossRef]
- Layne, D.R.; Jiang, Z.; Rushing, J.W. Tree Fruit Reflective Film Improves Red Skin Coloration and Advances Maturity in Peach. Horttechnology 2001, 11, 234–242. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz-Eiberger, M.A.; Blanke, M.M. Reflective Mulch Enhances Ripening and Health Compounds in Apple Fruit. J. Sci. Food Agric. 2013, 93, 2575–2579. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Mitchell, F.G.; Ju, Z. Susceptibility to Chilling Injury of Peach, Nectarine, and Plum Cultivars Grown in California. HortScience 1999, 34, 1116–1118. [Google Scholar] [CrossRef]
- Saure, M.C. External Control of Anthocyanin Formation in Apple. Sci. Hortic. 1990, 42, 181–218. [Google Scholar] [CrossRef]
- An, J.-P.; Liu, Y.-J.; Zhang, X.-W.; Bi, S.-Q.; Wang, X.-F.; You, C.-X.; Hao, Y.-J. Dynamic Regulation of Anthocyanin Biosynthesis at Different Light Intensities by the BT2-TCP46-MYB1 Module in Apple. J. Exp. Bot. 2020, 71, 3094–3109. [Google Scholar] [CrossRef] [PubMed]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.L. Developmental Changes in Enzymes of Flavonoid Biosynthesis in the Skins of Red and Green Apple Cultivars. J. Sci. Food Agric. 1996, 71, 313–320. [Google Scholar] [CrossRef]
- Phan-Thien, K.Y.; Wargo, J.M.; Mitchell, L.W.; Collett, M.G.; Rath, A.C. Delay in Ripening of Gala and Pink Lady Apples in Commercial Orchards Following Pre-Harvest Applications of Aminoethoxyvinylglycine. Aust. J. Exp. Agric. 2004, 44, 807–812. [Google Scholar] [CrossRef]
- Stover, E.; Fargione, M.J.; Watkins, C.B.; Iungerman, K.A. Harvest Management of ‘Marshall McIntosh’ Apples: Effects of AVG, NAA, Ethephon, and Summer Pruning on Preharvest Drop and Fruit Quality. HortScience 2003, 38, 1093–1099. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, K.; Sun, W.; Yang, T.; Wu, T.; Song, T.; Zhang, J.; Yao, Y.; Tian, J. A Long Noncoding RNA Functions in High-Light-Induced Anthocyanin Accumulation in Apple by Activating Ethylene Synthesis. Plant Physiol. 2022, 189, 66–83. [Google Scholar] [CrossRef]
- An, J.-P.; Wang, X.-F.; Li, Y.-Y.; Song, L.-Q.; Zhao, L.-L.; You, C.-X.; Hao, Y.-J. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 Act in a Regulatory Loop That Synergistically Modulates Ethylene Biosynthesis and Anthocyanin Accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef]
- Awad, M.A.; De Jager, A. Formation of Flavonoids, Especially Anthocyanin and Chlorogenic Acid in ‘Jonagold’ Apple Skin: Influences of Growth Regulators and Fruit Maturity. Sci. Hortic. 2002, 93, 257–266. [Google Scholar] [CrossRef]
- Farcuh, M.; Hopfer, H. Aroma Volatiles as Predictors of Chilling Injury Development during Peach (Prunus persica (L) Batsch) Cold Storage and Subsequent Shelf-Life. Postharvestig. Biol. Technol. 2023, 195, 112137. [Google Scholar] [CrossRef]
- Infante, R.; Farcuh, M.; Meneses, C. Monitoring the Sensorial Quality and Aroma through an Electronic Nose in Peaches during Cold Storage. J. Sci. Food Agric. 2008, 88, 2073–2078. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A Simple and Efficient Method for Isolating RNA from Pine Trees. Plant Mol. Biol. Report. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Farcuh, M.; Rivero, R.M.; Sadka, A.; Blumwald, E. Ethylene Regulation of Sugar Metabolism in Climacteric and Non-Climacteric Plums. Postharvestig. Biol. Technol. 2018, 139, 20–30. [Google Scholar] [CrossRef]
- Kim, H.; Farcuh, M.; Cohen, Y.; Crisosto, C.; Sadka, A.; Blumwald, E. Non-Climacteric Ripening and Sorbitol Homeostasis in Plum Fruits. Plant Sci. 2015, 231, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Siegelman, H.W.; Hendricks, S.B. Photocontrol of Anthocyanin Synthesis in Apple Skin. Plant Physiol. 1958, 33, 185. [Google Scholar] [CrossRef]


| Treatment | Fruit Drop (%) | |||||
|---|---|---|---|---|---|---|
| 2021 | 2022 | |||||
| CH | CH + 1 | CH + 2 | CH | CH + 1 | CH + 2 | |
| T1 | 6.7 ± 0.4 d | 12.0 ± 1.1 c | 17.1 ± 0.7 b | 4.5 ± 0.3 d | 9.9 ± 0.3 c | 15.8 ± 0.9 b |
| T2 | 10.9 ± 0.7 c | 16.3 ± 2.2 b | 28.6 ± 2.4 a | 5.3± 0.4 d | 15.1 ± 1.2 b | 25.8 ± 1.9 a |
| T3 | 5.7 ± 0.5 d | 10.4 ± 1.3 c | 16.8 ± 1.8 b | 4.7 ± 0.3 d | 9.3± 0.6 c | 14.9 ± 0.9 b |
| T4 | 10.5 ± 1.1 c | 17.4 ± 0.7 b | 27.1 ± 3.3 a | 5.4 ± 0.4 d | 15.9 ± 1.2 b | 24.8 ± 1.9 a |
| Feature | IEC | Surface Hue | Skin Blush | ACS1 | ACO1 | PAL | CHS | CHI | F3H | DFR | LDOX | UFGT | MYB10 | Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IEC | 1.00 | −0.69 * | 0.63 * | 0.92 * | 0.96 * | 0.80 * | 0.86 * | 0.86 * | 0.83 * | 0.89 * | 0.86 * | 0.80* | 0.91 * | 0.71 * |
| Surface Hue | 1.00 | −0.90 * | −0.80 * | −0.81 * | −0.90 * | −0.90 * | −0.90 * | −0.90 * | −0.90 * | −0.90 * | −0.90 * | −0.90 * | −0.90 * | |
| Skin Blush | 1.00 | 0.75 * | 0.72 * | 0.94 * | 0.83 * | 0.86 * | 0.86 * | 0.87 * | 0.89 * | 0.91 * | 0.81 * | 0.96 * | ||
| ACS1 | 1.00 | 0.98 * | 0.87 * | 0.87 * | 0.87 * | 0.89 * | 0.90 * | 0.88 * | 0.84 * | 0.90 * | 0.83 * | |||
| ACO1 | 1.00 | 0.87 * | 0.89 * | 0.89 * | 0.90 * | 0.92 * | 0.89 * | 0.85 * | 0.93 * | 0.81 * | ||||
| PAL | 1.00 | 0.96 * | 0.97 * | 0.98 * | 0.97 * | 0.98 * | 0.99 * | 0.95 * | 0.98 * | |||||
| CHS | 1.00 | 0.99 * | 0.98 * | 0.97 * | 0.99 * | 0.98 * | 0.99 * | 0.92 * | ||||||
| CHI | 1.00 | 0.98 * | 0.97 * | 0.99 * | 0.99 * | 0.99 * | 0.93 * | |||||||
| F3H | 1.00 | 0.96 * | 0.97 * | 0.98 * | 0.97 * | 0.94 * | ||||||||
| DFR | 1.00 | 0.98 * | 0.97 * | 0.98 * | 0.92 * | |||||||||
| LDOX | 1.00 | 0.99 * | 0.97 * | 0.94 * | ||||||||||
| UFGT | 1.00 | 0.96 * | 0.96 * | |||||||||||
| MYB10 | 1.00 | 0.90 * | ||||||||||||
| Total Anthocyanins | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miah, M.S.; Farcuh, M. The Expression of Key Ethylene and Anthocyanin Biosynthetic Genes of ‘Honeycrisp’ Apples Subjected to the Combined Use of Reflective Groundcovers and Aminoethoxyvinylglycine in the Mid-Atlantic US. Plants 2024, 13, 1141. https://doi.org/10.3390/plants13081141
Miah MS, Farcuh M. The Expression of Key Ethylene and Anthocyanin Biosynthetic Genes of ‘Honeycrisp’ Apples Subjected to the Combined Use of Reflective Groundcovers and Aminoethoxyvinylglycine in the Mid-Atlantic US. Plants. 2024; 13(8):1141. https://doi.org/10.3390/plants13081141
Chicago/Turabian StyleMiah, Md Shipon, and Macarena Farcuh. 2024. "The Expression of Key Ethylene and Anthocyanin Biosynthetic Genes of ‘Honeycrisp’ Apples Subjected to the Combined Use of Reflective Groundcovers and Aminoethoxyvinylglycine in the Mid-Atlantic US" Plants 13, no. 8: 1141. https://doi.org/10.3390/plants13081141
APA StyleMiah, M. S., & Farcuh, M. (2024). The Expression of Key Ethylene and Anthocyanin Biosynthetic Genes of ‘Honeycrisp’ Apples Subjected to the Combined Use of Reflective Groundcovers and Aminoethoxyvinylglycine in the Mid-Atlantic US. Plants, 13(8), 1141. https://doi.org/10.3390/plants13081141






