In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis
Abstract
1. Introduction
2. Results and Discussion
2.1. 240 and Gen12 Initial Chemical Composition Comparison
2.2. Drying Process Efficiency
2.3. Impact of the Drying Process on the Cannabinoid Content Preservation
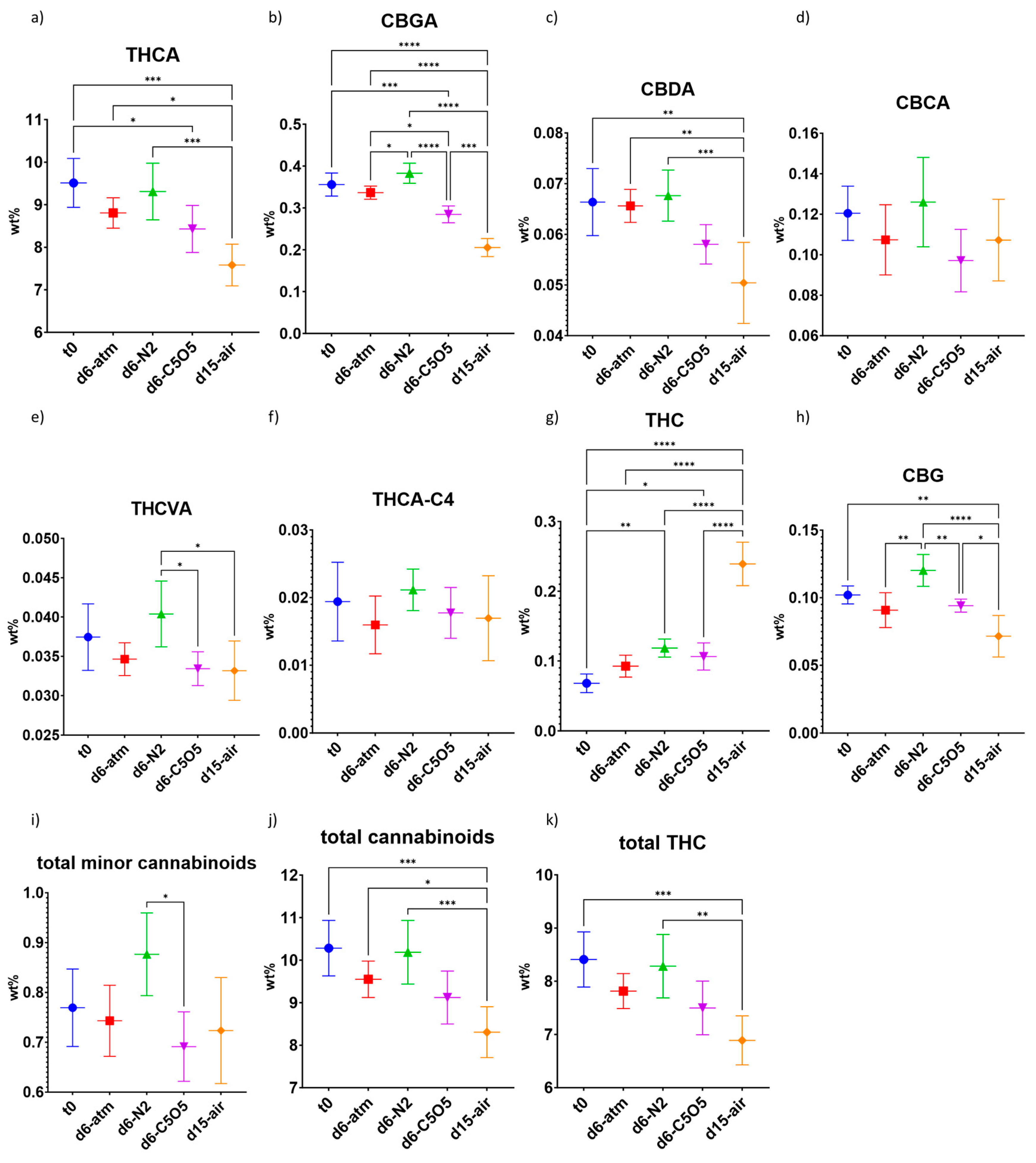
2.3.1. High-THCA Chemovar—240
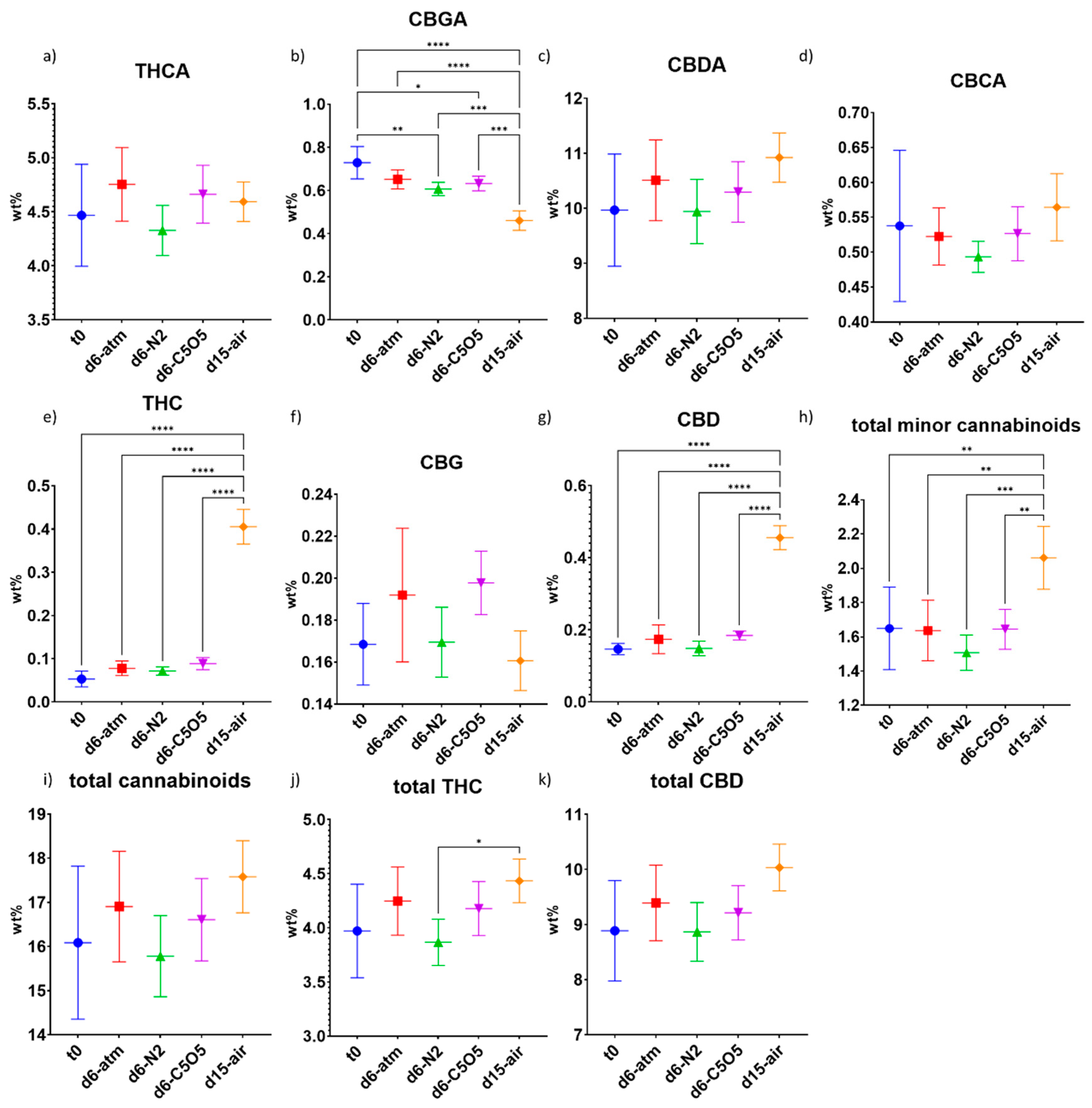
2.3.2. Hybrid Chemovar—Gen12
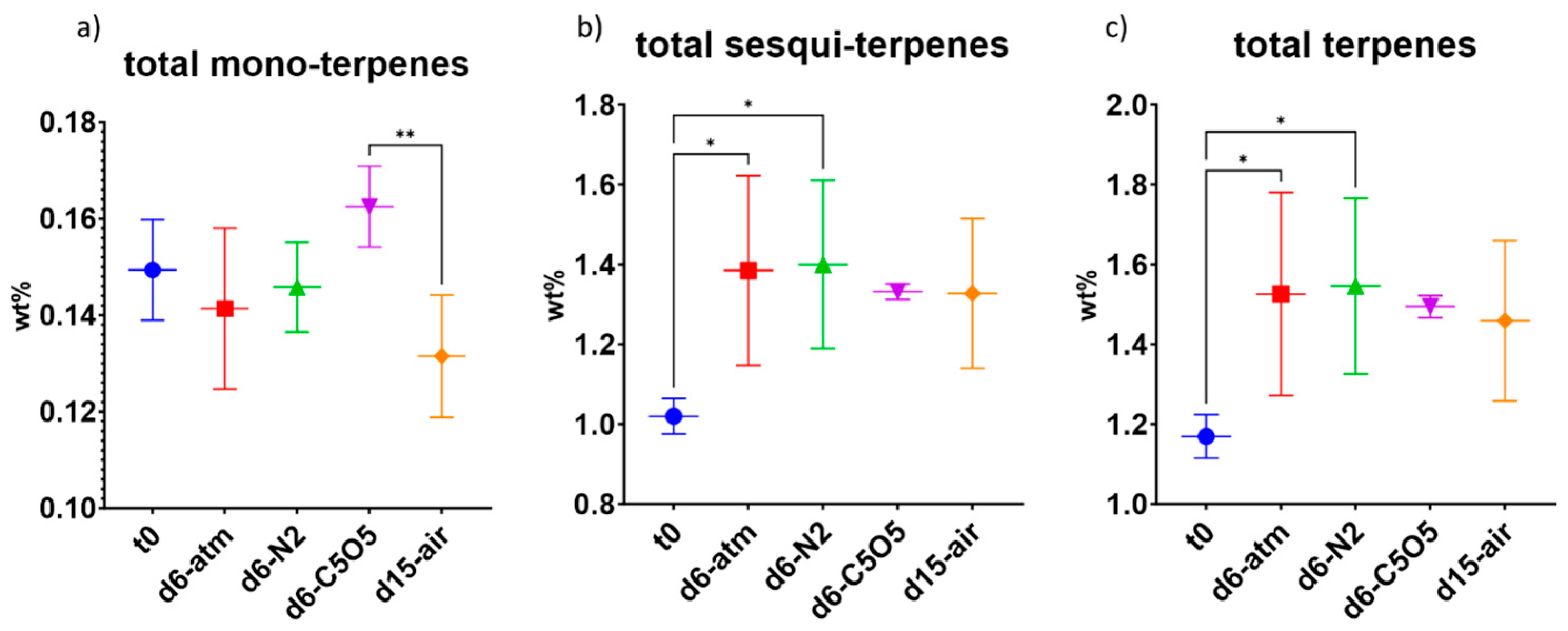
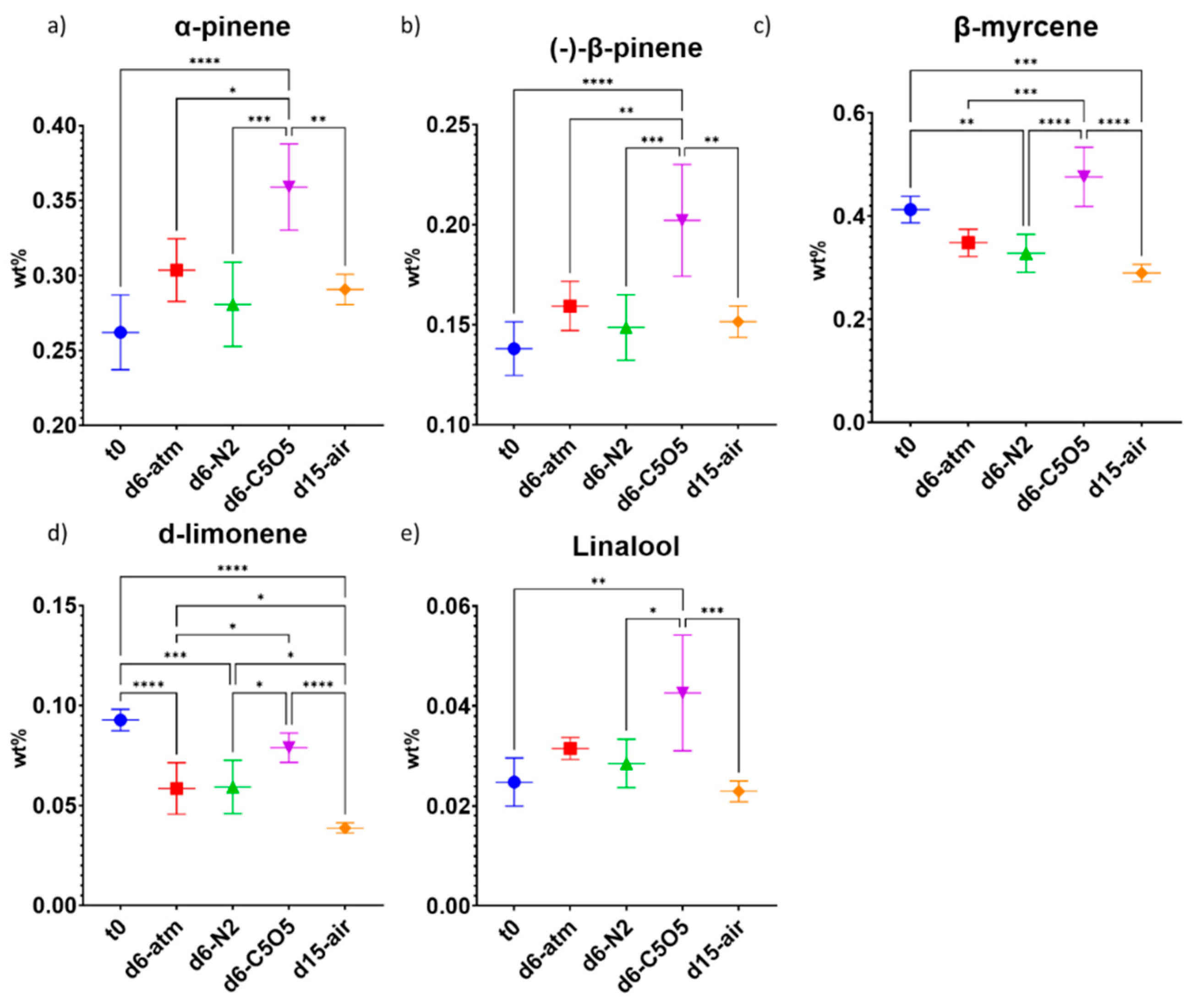
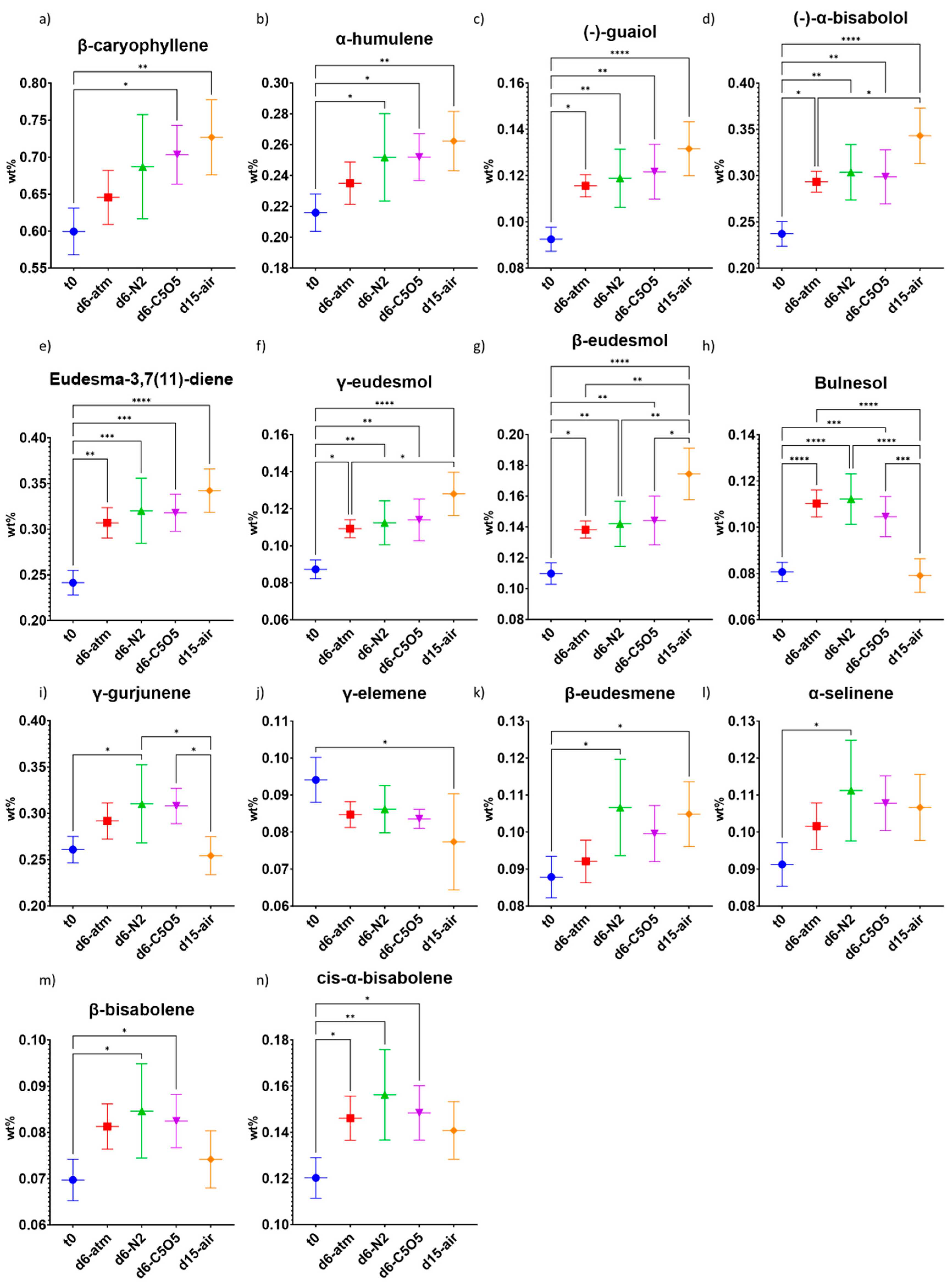
2.4. Impact of the Drying Process on the Terpene Content Preservation
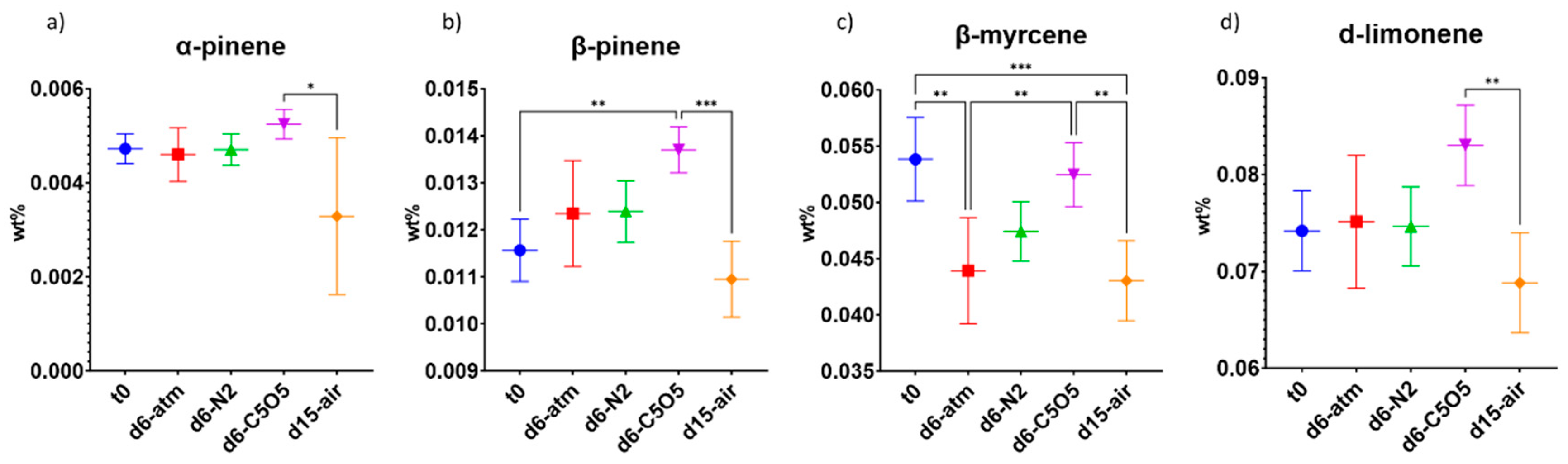
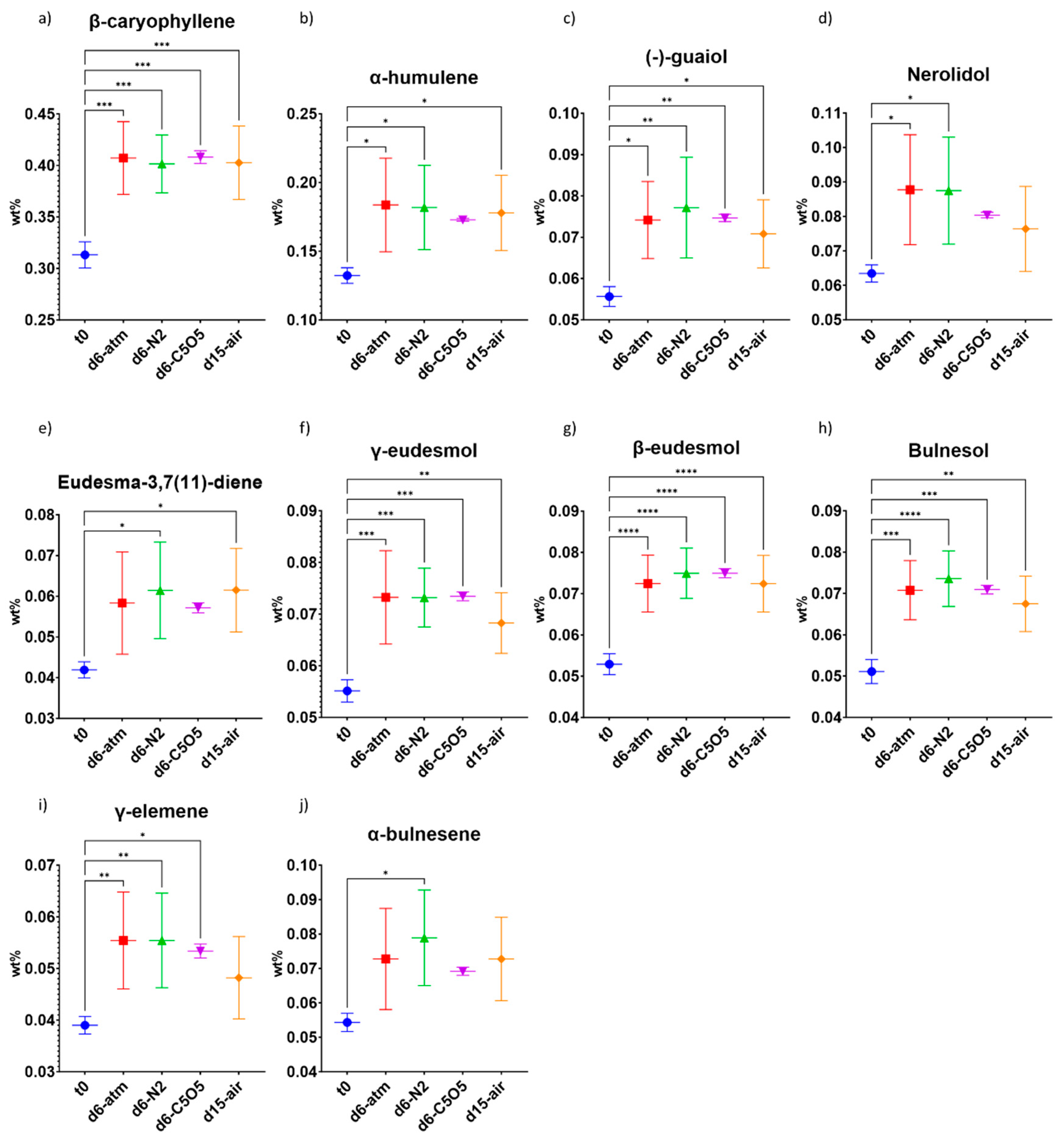
2.4.1. High-THCA Chemovar—240
2.4.2. Hybrid Chemovar—Gen12
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Drying Process
3.4. Sample Preparation
3.5. Quantification of Cannabinoids by HPLC–PDA and Terpenes by GC/MS
3.6. Microbiological Assay
3.7. Statistical Analysis and Cannabinoid/Terpene Content Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Upton, R.; ElSohly, M. Cannabis Inflorescence: Cannabis spp.; Standards of Identity, Analysis, and Quality Control; American Herbal Pharmacopoeia: Scotts Valley, CA, USA, 2014. [Google Scholar]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091. [Google Scholar] [CrossRef] [PubMed]
- Stockings, E.; Zagic, D.; Campbell, G.; Weier, M.; Hall, W.D.; Nielsen, S.; Herkes, G.K.; Farrell, M.; Degenhardt, L. Evidence for Cannabis and cannabinoids for epilepsy: A systematic review of controlled and observational evidence. J. Neurol. Neurosurg Psychiatry 2018, 89, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Aviram, J.; Lewitus, G.M.; Vysotski, Y.; Yellin, B.; Berman, P.; Shapira, A.; Meiri, D. Prolonged medical Cannabis treatment is associated with quality of life improvement and reduction of analgesic medication consumption in chronic pain patients. Front. Pharmacol. 2021, 12, 613805. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, penolics, trpenes and akaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. The case for the entourage effect and conventional breeding of clinical Cannabis: No “strain”, no gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Zhou, R.; Jacobson, C.; Weng, J.; Cheng, E.; Lay, J.; Hung, P.; Lerner, J.T.; Sankar, R. Perceived efficacy of cannabidiol-enriched Cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and Lennox–Gastaut syndrome. Epilepsy Behav. 2015, 47, 138–141. [Google Scholar] [CrossRef]
- Gilbert, A.N.; DiVerdi, J.A. Consumer perceptions of strain differences in Cannabis aroma. PLoS ONE 2018, 13, e0192247. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and Phytocannabinoids Co-Produced in Cannabis sativa Strains Show Specific Interaction for Cell Cytotoxic Activity. Molecules 2019, 24, 3031. [Google Scholar] [CrossRef]
- Cerrato, A.; Citti, C.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Grassi, G.; Marini, F.; Montone, C.M.; Paris, R.; Piovesana, S. Phytocannabinomics: Untargeted metabolomics as a tool for Cannabis chemovar differentiation. Talanta 2021, 230, 122313. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Russo, E.B.; Smith, K.M. Pharmacological foundations of Cannabis chemovars. Planta Med. 2018, 84, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Medical Cannabis Unit, Israel. Medicinal Cannabis, Information Booklet and Medical Guide. 2019. Available online: https://www.health.gov.il/hozer/mmk154_2016.pdf (accessed on 10 June 2019).
- Birenboim, M.; Kengisbuch, D.; Chalupowicz, D.; Maurer, D.; Barel, S.; Chen, Y.; Fallik, E.; Paz-Kagan, T.; Shimshoni, J.A. Use of near-infrared spectroscopy for the classification of medicinal Cannabis cultivars and the prediction of their cannabinoid and terpene contents. Phytochemistry 2022, 204, 113445. [Google Scholar] [CrossRef] [PubMed]
- Birenboim, M.; Chalupowicz, D.; Maurer, D.; Barel, S.; Chen, Y.; Fallik, E.; Paz-Kagan, T.; Rapaport, T.; Sadeh, A.; Kengisbuch, D. Multivariate classification of Cannabis chemovars based on their terpene and cannabinoid profiles. Phytochemistry 2022, 200, 113215. [Google Scholar] [CrossRef] [PubMed]
- Uziel, A.; Milay, L.; Procaccia, S.; Cohen, R.; Burstein, A.; Sulimani, L.; Shreiber-Livne, I.; Lewitus, D.; Meiri, D. Solid-state microwave drying for medical Cannabis inflorescences: A rapid and controlled alternative to traditional drying. Cannabis Cannabinoid Res. 2022, 9, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Milay, L.; Berman, P.; Shapira, A.; Guberman, O.; Meiri, D. Metabolic profiling of Cannabis secondary metabolites for evaluation of optimal postharvest storage conditions. Front. Plant Sci. 2020, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Al Ubeed, H.M.S.; Wills, R.B.; Chandrapala, J. Post-harvest operations to generate high-quality medicinal Cannabis products: A systemic review. Molecules 2022, 27, 1719. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. The role of biotechnology in Cannabis sativa propagation for the production of phytocannabinoids. In Biotechnology for Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 123–148. [Google Scholar] [CrossRef]
- Challa, S.K.R.; Misra, N.; Martynenko, A. Drying of cannabis—State of the practices and future needs. Dry. Technol. 2021, 39, 2055–2064. [Google Scholar] [CrossRef]
- Chen, C.; Wongso, I.; Putnam, D.; Khir, R.; Pan, Z. Effect of hot air and infrared drying on the retention of cannabidiol and terpenes in industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2021, 172, 114051. [Google Scholar] [CrossRef]
- Das, P.C.; Vista, A.R.; Tabil, L.G.; Baik, O.-D. Postharvest operations of Cannabis and their effect on cannabinoid content: A review. Bioengineering 2022, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Oduola, A.A.; Bruce, R.M.; Shafiekhani, S.; Atungulu, G.G. Impacts of industrial microwave and infrared drying approaches on hemp (Cannabis sativa L.) quality and chemical components. Food Bioprod. Process. 2023, 137, 20–27. [Google Scholar] [CrossRef]
- Chasiotis, V.; Tsakirakis, A.; Termentzi, A.; Machera, K.; Filios, A. Drying and quality characteristics of Cannabis sativa L. inflorescences under constant and time-varying convective drying temperature schemes. Therm. Sci. Eng. Prog. 2022, 28, 101076. [Google Scholar] [CrossRef]
- Addo, P.W.; Chauvin-Bossé, T.; Taylor, N.; MacPherson, S.; Paris, M.; Lefsrud, M. Freeze-drying Cannabis sativa L. using real-time relative humidity monitoring and mathematical modeling for the cannabis industry. Ind. Crops Prod. 2023, 199, 116754. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczyński, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Różański, H. Volatile composition and sensory properties as quality attributes of fresh and dried hemp flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Bin, Y.; Wenxue, Z.; Guangyue, R.; Xu, D.; Xinyan, K. Degradation kinetics of functional components of honeysuckle flowers during controlled-atmosphere heat pump drying. Int. J. Agric. Biol. Eng. 2016, 9, 159–168. [Google Scholar]
- Braga, A.M.; Silva, M.A.; Pedroso, M.P.; Augusto, F.; Barata, L.E. Volatile composition changes of pineapple during drying in modified and controlled atmosphere. Int. J. Food Eng. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Kanayama, Y.; Kochetov, A. Abiotic Stress Biology in Horticultural Plants; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bahar, A.; Lichter, A. Effect of controlled atmosphere on the storage potential of Ottomanit fig fruit. Sci. Hortic. 2018, 227, 196–201. [Google Scholar] [CrossRef]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Stephane, F.F.Y.; Jules, B.K.J. Terpenoids as important bioactive constituents of essential oils. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020; Volume 1, pp. 1–15. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’Connell, M.A. Accumulation of bioactive metabolites in cultivated medical Cannabis. PLoS ONE 2018, 13, e0201119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hartman, G.; Domier, L.; Boykin, D. Quantification of Fusarium solani f. sp. glycines isolates in soybean roots by colony-forming unit assays and real-time quantitative PCR. Theor. Appl. Genet. 2008, 117, 343–352. [Google Scholar] [CrossRef] [PubMed]


| Cannabinoid | Absolute Concentration ± SE at t0 | Absolute Concentration ± SE after 6 Days of Controlled Atmospheric Drying | Absolute Concentration ± SE after 15 Days of Drying and Curing (Traditional Drying) | ||
|---|---|---|---|---|---|
| 240 | |||||
| Atm | N2 | C5O5 | Open-air | ||
| CBDVA | <LOD | ||||
| CBDA | 0.066 ± 0.007 | 0.066 ± 0.003 (ns) a | 0.067 ± 0.005 (ns) | 0.058 ± 0.004 (ns) | 0.050 ± 0.008 (**) b |
| CBGA | 0.36 ± 0.03 | 0.34 ± 0.02 (ns) | 0.38 ± 0.02 (ns) | 0.28 ± 0.02 (***) | 0.20 ± 0.02 (****) |
| CBG | 0.102 ± 0.007 | 0.09 ± 0.01 (ns) | 0.12 ± 0.01 (ns) | 0.094 ± 0.005 (ns) | 0.07 ± 0.02 (**) |
| CBD | <LOD | ||||
| THCVA | 0.037 ± 0.004 | 0.035 ± 0.002 (ns) | 0.040 ± 0.004 (ns) | 0.033 ± 0.002 (ns) | 0.033 ± 0.004 (ns) |
| THCA-C4 | 0.020 ± 0.006 | 0.016 ± 0.004 (ns) | 0.021 ± 0.003 (ns) | 0.018 ± 0.004 (ns) | 0.017 ± 0.006 (ns) |
| THC | 0.07 ± 0.01 | 0.09 ± 0.02 (ns) | 0.12 ± 0.01 (**) | 0.10 ± 0.02 (*) | 0.24 ± 0.03 (****) |
| THCA | 9.5 ± 0.7 | 8.8 ± 0.4 (ns) | 9.3 ± 0.7 (ns) | 8.4 ± 0.6 (*) | 7.6 ± 0.5 (***) |
| CBCA | 0.12 ± 0.01 | 0.11 ± 0.02 (ns) | 0.13 ± 0.02 (ns) | 0.10 ± 0.02 (ns) | 0.11 ± 0.02 (ns) |
| Total cannabinoids | 10.3 ± 0.6 | 9.5 ± 0.47 (ns) | 10.2 ± 0.78 (ns) | 9.1 ± 0.67 (ns) | 8.3 ± 0.6 (***) |
| Total minor cannabinoids | 0.80 ± 0.08 | 0.74 ± 0.07 (ns) | 0.90 ± 0.08 (ns) | 0.70 ± 0.07 (ns) | 0.7 ± 0.1 (ns) |
| total THC | 8.4 ± 0.5 | 7.8 ± 0.3 (ns) | 8.3 ± 0.6 (ns) | 7.5 ± 0.5 (ns) | 6.9 ± 0.5 (***) |
| Gen12 | |||||
| Atm | N2 | C5O5 | Open-air | ||
| CBDVA | 0.015 ± 0.004 | 0.020 ± 0.003 (ns) | 0.018 ± 0.004 (ns) | 0.016 ± 0.003 (ns) | 0.016 ± 0.003 (ns) |
| CBDA | 10.0 ± 1.0 | 10.5 ± 0.7 (ns) | 10.0 ± 0.6 (ns) | 10.3 ± 0.5 (ns) | 10.9 ± 0.4 (ns) |
| CBGA | 0.73 ± 0.08 | 0.65 ± 0.04 (ns) | 0.61 ± 0.03 (**) | 0.63 ± 0.03 (*) | 0.46 ± 0.04 (****) |
| CBG | 0.17 ± 0.02 | 0.19 ± 0.03 (ns) | 0.17 ± 0.02 (ns) | 0.20 ± 0.02 (ns) | 0.16 ± 0.01 (ns) |
| CBD | 0.15 ± 0.02 | 0.17 ± 0.04 (ns) | 0.15 ± 0.02 (ns) | 0.18 ± 0.01 (ns) | 0.45 ± 0.03 (****) |
| THCVA | <LOD | ||||
| THCA-C4 | <LOD | ||||
| THC | 0.05 ± 0.02 | 0.08 ± 0.02 (ns) | 0.07 ± 0.01 (ns) | 0.09 ± 0.01 (ns) | 0.40 ± 0.04 (****) |
| THCA | 4.5 ± 0.5 | 4.8 ± 0.3 (ns) | 4.3 ± 0.2 (ns) | 4.7 ± 0.3 (ns) | 4.6 ± 0.2 (ns) |
| CBCA | 0.54 ± 0.11 | 0.52 ± 0.04 (ns) | 0.49 ± 0.02 (ns) | 0.53 ± 0.04 (ns) | 0.56 ± 0.05 (ns) |
| Total cannabinoids | 16.1 ± 1.7 | 16.9 ± 1.2 (ns) | 15.8 ± 0.9 (ns) | 16.6 ± 0.9 (ns) | 17.6 ± 0.8 (ns) |
| Total minor cannabinoids | 1.6 ± 0.2 | 1.6 ± 0.2 (ns) | 1.5 ± 0.1 (ns) | 1.6 ± 0.1 (ns) | 2.1 ± 0.2 (**) |
| Total THC | 4.0 ± 0.4 | 4.2 ± 0.3 (ns) | 3.9 ± 0. 2 (ns) | 4.2 ± 0.2 (ns) | 4.4 ± 0.2 (ns) |
| Total CBD | 8.9 ± 0.9 | 9.4 ± 0.7 (ns) | 8.9 ± 0.5 (ns) | 9.2 ± 0.5 (ns) | 10.0 ± 0.4 (ns) |
| Terpene | Absolute Concentration ± SE at t0 | Absolute Concentration ± SE after 6 Days of Controlled Atmospheric Drying | Absolute Concentration ± SE after 15 Days of Drying and Curing (Traditional Drying) | ||
|---|---|---|---|---|---|
| 240 | |||||
| Atm | N2 | C5O5 | Open-air | ||
| α-pinene | 0.0047 ± 0.0003 | 0.0046 ± 0.0006 a | 0.0047 ± 0.0003 | 0.0052 ± 0.0003 | 0.003 ± 0.002 |
| Camphene | 0.0020 ± 0.0001 | 0.001 ± 0.001 | 0.0018 ± 0.0009 | 0.0024 ± 0.0001 | 0.0004 ± 0.0004 |
| (−)-β-pinene | 0.0116 ± 0.0007 | 0.012 ± 0.001 | 0.0124 ± 0.0006 | 0.0137 ± 0.0005 | 0.0110 ± 0.0008 |
| β-myrcene | 0.054 ± 0.004 | 0.044 ± 0.005 (**) b | 0.047 ± 0.003 (ns) c | 0.052 ± 0.003 (ns) | 0.043 ± 0.004 (***) |
| δ-3-carene | <LOD | ||||
| d-limonene | 0.074 ± 0.004 | 0.075 ± 0.007 (ns) | 0.075 ± 0.004 (ns) | 0.083 ± 0.004 (ns) | 0.069 ± 0.005 (ns) |
| Linalool | <LOD | ||||
| Fenchol | 0.003 ± 0.002 | 0.004 ± 0.002 | 0.0048 ± 0.0007 | 0.0057 ± 0.0005 | 0.0050 ± 0.0006 |
| Pinalol | <LOD | ||||
| β-caryophyllene | 0.31 ± 0.01 | 0.41 ± 0.04 (***) | 0.40 ± 0.03 (***) | 0.408 ± 0.006 (***) | 0.40 ± 0.04 (***) |
| α-humulene | 0.132 ± 0.006 | 0.18 ± 0.03 (*) | 0.18 ± 0.03 (*) | 0.173 ± 0.001 (ns) | 0.18 ± 0.03 (*) |
| (−)-guaiol | 0.056 ± 0.002 | 0.074 ± 0.009 (*) | 0.08 ± 0.01 (**) | 0.0747 ± 0.0009 (**) | 0.071 ± 0.008 (*) |
| (−)-α-bisabolol | <LOD | ||||
| Nerolidol | 0.063 ± 0.002 | 0.09 ± 0.02 (*) | 0.09 ± 0.02 (*) | 0.0804 ± 0.0008 (ns) | 0.08 ± 0.01 (ns) |
| γ-elemene | 0.039 ± 0.002 | 0.055 ± 0.009 (**) | 0.055 ± 0.009 (**) | 0.053 ± 0.001 (*) | 0.048 ± 0.008 (ns) |
| α-bergomotene | 0.0152 ± 0.0006 | 0.020 ± 0.006 | 0.022 ± 0.006 | 0.0183 ± 0.0002 | 0.021 ± 0.005 |
| α-guaiene | 0.028 ± 0.001 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.0350 ± 0.0005 | 0.04 ± 0.01 |
| β-farensene | 0.023 ± 0.001 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.024 ± 0.001 | 0.029 ± 0.009 |
| β-eudesmene | 0.0124 ± 0.0006 | 0.015 ± 0.001 | 0.0166 ± 0.0005 | 0.0162 ± 0.0006 | 0.016 ± 0.001 |
| α-selinene | 0.0164 ± 0.0008 | 0.024 ± 0.007 | 0.026 ± 0.007 | 0.0220 ± 0.0005 | 0.024 ± 0.0006 |
| α-bulnesene | 0.054 ± 0.003 | 0.07 ± 0.01 (ns) | 0.08 ± 0.01 (*) | 0.070 ± 0.001 (ns) | 0.07 ± 0.01 (ns) |
| β-bisabolene | <LOD | ||||
| cis-α-bisabolene | <LOD | ||||
| Eudesma-3,7(11)-diene | 0.042 ± 0.002 | 0.06 ± 0.01 (ns) | 0.06 ± 0.01 (*) | 0.057 ± 0.001 (ns) | 0.06 ± 0.01 (*) |
| γ-eudesmol | 0.055 ± 0.002 | 0.073 ± 0.009 (***) | 0.073 ± 0.005 (***) | 0.0734 ± 0.0009 (***) | 0.068 ± 0.006 (**) |
| β-eudesmol | 0.053 ± 0.002 | 0.072 ± 0.007 (****) | 0.075 ± 0.006 (****) | 0.075 ± 0.001 (****) | 0.072 ± 0.007 (****) |
| Bulnesol | 0.051 ± 0.003 | 0.071 ± 0.007 (***) | 0.074 ± 0.007 (****) | 0.071 ± 0.001 (***) | 0.067 ± 0.007 (**) |
| α-gurjunene | 0.0136 ± 0.0005 | 0.02 ± 0.01 | 0.022 ± 0.009 | 0.0175 ± 0.0002 | 0.021 ± 0.007 |
| γ-gurjunene | 0.031 ± 0.001 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.0403 ± 0.0005 | 0.039 ± 0.008 |
| Elemol | 0.0162 ± 0.0006 | 0.020 ± 0.006 | 0.020 ± 0.006 | 0.0172 ± 0.0001 | 0.012 ± 0.003 |
| Total terpenes | 1.17 ± 0.05 | 1.55 ± 0.22 (*) | 1.55 ± 0.21 (*) | 1.49 ± 0.03 (ns) | 1.43 ± 0.21 (ns) |
| Total monoterpenes | 0.15 ± 0.01 | 0.15 ± 0.02 (ns) | 0.146 ± 0.009 (ns) | 0.162 ± 0.008 (ns) | 0.13 ± 0.01 (ns) |
| Total sesquiterpenes | 1.02 ± 0.04 | 1.4 ± 0.2 (*) | 1.4 ± 0.2 (*) | 1.33 ± 0.02 (ns) | 1.3 ± 0.2 (ns) |
| Gen12 | |||||
| Atm | N2 | C5O5 | Open-air | ||
| α-pinene | 0.26 ± 0.02 | 0.30 ± 0.02 (ns) | 0.28 ± 0.03 (ns) | 0.36 ± 0.03 (****) | 0.29 ± 0.01 (ns) |
| Camphene | 0.005 ± 0.001 | 0.0057 ± 0.0005 | 0.0054 ± 0.0006 | 0.0069 ± 0.0004 | 0.0053 ± 0.0003 |
| (−)-β-pinene | 0.14 ± 0.01 | 0.16 ± 0.01 (ns) | 0.15 ± 0.02 (ns) | 0.20 ± 0.03 (****) | 0.151 ± 0.008 (ns) |
| β-myrcene | 0.41 ± 0.03 | 0.35 ± 0.03 (ns) | 0.33 ± 0.04 (**) | 0.48 ± 0.06 (ns) | 0.29 ± 0.02 (***) |
| δ-3-carene | <LOD | ||||
| d-limonene | 0.093 ± 0.005 | 0.06 ± 0.01 (****) | 0.06 ± 0.01 (***) | 0.079 ± 0.007 (ns) | 0.039 ± 0.002 (****) |
| Linalool | 0.025 ± 0.005 | 0.032 ± 0.002 (ns) | 0.028 ± 0.005 (ns) | 0.04 ± 0.01 (**) | 0.023 ± 0.002 (ns) |
| Fenchol | 0.011 ± 0.001 | 0.012 ± 0.001 | 0.013 ± 0.002 | 0.016 ± 0.004 | 0.010 ± 0.002 |
| Pinalol | 0.018 ± 0.001 | 0.0192 ± 0.0008 | 0.020 ± 0.002 | 0.024 ± 0.006 | 0.017 ± 0.001 |
| β-caryophyllene | 0.60 ± 0.03 | 0.64 ± 0.04 (ns) | 0.69 ± 0.07 (ns) | 0.70 ± 0.04 (*) | 0.73 ± 0.05 (**) |
| α-humulene | 0.22 ± 0.01 | 0.24 ± 0.01 (ns) | 0.25 ± 0.03 (*) | 0.25 ± 0.02 (*) | 0.26 ± 0.02 (**) |
| (−)-guaiol | 0.092 ± 0.005 | 0.116 ± 0.005 (*) | 0.12 ± 0.01 (**) | 0.12 ± 0.01 (**) | 0.13 ± 0.01 (****) |
| (−)-α-bisabolol | 0.24 ± 0.01 | 0.29 ± 0.01 (*) | 0.30 ± 0.03 (**) | 0.30 ± 0.03 (**) | 0.34 ± 0.03 (****) |
| Nerolidol | <LOD | ||||
| γ-elemene | 0.094 ± 0.006 | 0.085 ± 0.003 (ns) | 0.086 ± 0.006 (ns) | 0.084 ± 0.003 (ns) | 0.08 ± 0.01 (*) |
| α-bergomotene | 0.032 ± 0.002 | 0.035 ± 0.002 | 0.036 ± 0.005 | 0.038 ± 0.002 | 0.036 ± 0.002 |
| α-guaiene | <LOD | ||||
| β-farensene | 0.026 ± 0.002 | 0.030 ± 0.003 | 0.032 ± 0.004 | 0.031 ± 0.002 | 0.028 ± 0.003 |
| β-eudesmene | 0.088 ± 0.006 | 0.092 ± 0.006 (ns) | 0.11 ± 0.01 (*) | 0.100 ± 0.008 (ns) | 0.105 ± 0.009 (*) |
| α-selinene | 0.091 ± 0.006 | 0.102 ± 0.006 (ns) | 0.11 ± 0.01 (*) | 0.108 ± 0.007 (ns) | 0.107 ± 0.009 (ns) |
| α-bulnesene | <LOD | ||||
| β-bisabolene | 0.070 ± 0.004 | 0.081 ± 0.005 (ns) | 0.08 ± 0.01 (*) | 0.082 ± 0.006 (*) | 0.074 ± 0.006 (ns) |
| cis-α-bisabolene | 0.120 ± 0.009 | 0.15 ± 0.01 (*) | 0.16 ± 0.02 (**) | 0.15 ± 0.01 (*) | 0.14 ± 0.01 (ns) |
| Eudesma-3,7(11)-diene | 0.24 ± 0.01 | 0.31 ± 0.02 (**) | 0.32 ± 0.04 (***) | 0.32 ± 0.02 (***) | 0.34 ± 0.02 (****) |
| γ-eudesmol | 0.087 ± 0.005 | 0.109 ± 0.005 (*) | 0.11 ± 0.01 (**) | 0.11 ± 0.01 (**) | 0.13 ± 0.01 (****) |
| β-eudesmol | 0.110 ± 0.007 | 0.138 ± 0.006 (*) | 0.14 ± 0.01 (**) | 0.14 ± 0.02 (**) | 0.17 ± 0.02 (****) |
| Bulnesol | 0.081 ± 0.004 | 0.110 ± 0.006 (****) | 0.11 ± 0.01 (****) | 0.104 ± 0.009 (***) | 0.079 ± 0.007 (ns) |
| α-gurjunene | <LOD | ||||
| γ-gurjunene | 0.26 ± 0.01 | 0.29 ± 0.02 (ns) | 0.31 ± 0.04 (*) | 0.31 ± 0.02 (ns) | 0.25 ± 0.02 (ns) |
| Elemol | <LOD | ||||
| Total terpenes | 3.41 ± 0.22 | 3.75 ± 0.23 (ns) | 3.85 ± 0.4 (ns) | 4.15 ± 0.35 (**) | 3.8 ± 0.24 (ns) |
| Total monoterpenes | 0.96 ± 0.08 | 0.94 ± 0.08 (ns) | 0.85 ± 0.10 (ns) | 1.2 ± 0.15 (**) | 0.80 ± 0.04 (ns) |
| Total sesquiterpenes | 2.45 ± 0.14 | 2.81 ± 0.15 (ns) | 3.0 ± 0.3 (*) | 3.0 ± 0.2 (*) | 3.0 ± 0.2 (**) |
| Drying Conditions | 240 | Gen12 |
|---|---|---|
| Weight loss in controlled atmosphere drying chambers after 6 days | ||
| Weight loss in open-air drying conditions after 14 days (before curing) | (**) a | (*) a |
| Weight loss in open-air drying conditions after 15 days (including curing) | (ns) a | (ns) a |
| Drying Conditions | CFU/g Dry × 106 | Alternaria alternata Extent of Infestation [2−ΔΔCt] | Botrytis cinerea Extent of Infestation [2−ΔΔCt] |
|---|---|---|---|
| Fresh inflorescences at t0 | Undetermined | ||
| Inflorescences from controlled atmosphere chambers at day 6 | (ns) a | (ns) a | Undetermined |
| Open-air inflorescences at day 12 | (*) a | (****) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birenboim, M.; Brikenstein, N.; Duanis-Assaf, D.; Maurer, D.; Chalupowicz, D.; Kenigsbuch, D.; Shimshoni, J.A. In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis. Plants 2024, 13, 1049. https://doi.org/10.3390/plants13071049
Birenboim M, Brikenstein N, Duanis-Assaf D, Maurer D, Chalupowicz D, Kenigsbuch D, Shimshoni JA. In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis. Plants. 2024; 13(7):1049. https://doi.org/10.3390/plants13071049
Chicago/Turabian StyleBirenboim, Matan, Nimrod Brikenstein, Danielle Duanis-Assaf, Dalia Maurer, Daniel Chalupowicz, David Kenigsbuch, and Jakob A. Shimshoni. 2024. "In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis" Plants 13, no. 7: 1049. https://doi.org/10.3390/plants13071049
APA StyleBirenboim, M., Brikenstein, N., Duanis-Assaf, D., Maurer, D., Chalupowicz, D., Kenigsbuch, D., & Shimshoni, J. A. (2024). In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis. Plants, 13(7), 1049. https://doi.org/10.3390/plants13071049







