Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress
Abstract
1. Introduction
2. Results
2.1. Effects of Salt Stress on AMF Colonization
2.2. Effects of Salt Stress and AMF on Plant Growth and Root System Architecture
2.3. Effects of Salt Stress and AMF on Osmotic Regulating Substances
2.4. Effects of Salt Stress and AMF on H2O2 and Antioxidant Enzyme Activity
2.5. Effects of Salt Stress and AMF on Photosynthesis Parameters in Leaves
2.6. Effects of Salt Stress and AMF on Leaf Fluorescence Parameters
2.7. Effects of Salt Stress and AMF on Root Endogenous Hormone Levels
2.8. Effects of Salt Stress and AMF on Water Content, Chlorophyll Levels, and Nitrogen Balance of Leaves
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Plant Culture
4.3. Variable Determinations
4.4. Analysis of Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saud, S.; Chen, Y.; Baowen, L.; Fahad, S.; Arooj, S. The different impact on the growth of cool season turf grass under the various conditions on salinity and drought stress. Int. J. Agric. Nat. Res. 2013, 3, 77–84. [Google Scholar]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Abdul, K.; Saud, S.; Shah, H.; Darakh, S.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, Y.J.; Liu, G.Y.; Xu, S.Z.; Dai, J.L.; Li, W.J.; Li, Z.H.; Zhang, D.M.; Li, C.D.; Dong, H.Z. Nitric oxide increases the biomass and lint yield of field-grown cotton under temporary waterlogging through physiological and molecular regulation. Field Crops Res. 2021, 261, 107989. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Xu, C.; Wu, J.J.; Huang, J.S. Sunflower seed yield estimation under the interaction of soil salinity and nitrogen application. Field Crops Res. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Rakesh, K.S.; Shiv, S.V. Chapter 21—Proteomics Approach in Horticultural Crops for Abiotic-Stress Tolerance. In Stress Tolerance in Horticultural Crops; Avinash, C.R., Ashutosh, R., Krishna, K.R., Ved, P.R., Ajay, K., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 371–385. [Google Scholar]
- Dong, F.; Wang, Y.; Tao, J.; Xu, T.; Tang, M. Arbuscular mycorrhizal fungi affect the expression of PxNHX gene family, improve photosynthesis and promote Populus simonii × P. nigra growth under saline-alkali stress. Front. Plant Sci. 2023, 14, 1104095. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M. Sodium (Na+) homeostasis and salt tolerance of plants. Environ. Exp. Bot. 2013, 92, 19–31. [Google Scholar] [CrossRef]
- Wei, J.; Cai, Q.A.; Li, Y.; Shang, L.X.; Bu, X.F.; Yu, Z.J.; Ma, R. Research progress of plant response mechanism to saline-alkali stress. Agri. Sci. 2022, 54, 156–164. (In Chinese) [Google Scholar]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Liao, Z.F.; Liu, H.; Shen, Y.Y.; Xie, G.Y.; Wei, G.L.; Qin, S.S.; Miao, J.H.; Wei, K.H. Research progress on antioxidant enzyme system response of medicinal plants under stress. Mol. Plant Breed. 2023, 6, 1–18. (In Chinese) [Google Scholar]
- Wei, L.X.; Lv, B.S.; Li, X.W.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Yang, R.F.; Piao, Z.Z.; Wang, Z.H.; Lou, J.H.; et al. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crops Res. 2017, 203, 86–93. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhou, Y.L.; Liang, J.S. Characterization of organellar-specific aba responses during environmental stresses in tobacco cells and Arabidopsis plants. Cells 2022, 11, 2039. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhu, J.J. Research progress on response, adaptation and regulatory mechanisms of plant hormones to salt stress. Mol. Plant Breed. 2023, 1, 22. (In Chinese) [Google Scholar]
- Liang, J.P.; Shi, W.J. Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crops Res. 2021, 262, 108027. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Song, X.Z.; Yang, G.Z.; Li, Z.H.; Lu, H.Q.; Kong, X.Q.; Eneji, A.E.; Dong, H.Z. Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crops Res. 2015, 179, 164–172. [Google Scholar] [CrossRef]
- Li, X. Trends in international Food and Agriculture: The Ambassador to the UN Food and Agriculture Agency attended the celebration of FAO’s “World Cotton Day” and delivered a speech. World Agric. 2022, 11, 120. (In Chinese) [Google Scholar]
- Huang, L.; Li, G.; Wang, Q.; Meng, Q.; Xu, F.; Chen, Q.; Liu, F.; Hu, Y.; Luo, M. GhCYP710A1 participates in Cotton resistance to verticillium wilt by regulating stigmasterol synthesis and plasma membrane stability. Int. J. Mol. Sci. 2022, 23, 8437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Li, H.J.; Huang, X.L.; Hu, W.; Wang, S.S.; Zhou, Z.G. Phosphorus application affected cottonseed kernel yield and nutritional quality by improving oil yield and quality of two cotton (Gossypium hirsutum L.) cultivars differing in phosphorus sensitivity. Field Crops Res. 2023, 291, 108778. [Google Scholar] [CrossRef]
- Meng, C.M.; Geng, F.F.; Qing, G.X.; Zhang, F.H.; Li, X.L.; Liu, F.J. Cloning and expression analysis of low phosphorus stress response gene GhGDPD1 in Gossypium hirsutum. J. Zhejiang Univ. 2023, 40, 723–730. (In Chinese) [Google Scholar]
- Amangul, M. Research on the Difference of the Influence of Salt Stress on Growth and Physiological Characteristics of Cotton Seedlings of Different Cultispecies; Xinjiang Agricultural University: Urumqi, China, 2016. (In Chinese) [Google Scholar]
- Reyimaiayi, A.; Chen, J.; Chen, Y.; Ma, L.F. Transcriptome analysis and salt tolerance gene screening of cotton root under salt stress. J. South China Norm. Univ. 2020, 52, 85–92. [Google Scholar]
- Zhang, L.; Zhang, G.; Wang, Y.; Zhou, Z.; Meng, Y.; Chen, B. Effect of soil salinity on physiological characteristics of functional leaves of cotton plants. J. Plant Res. 2013, 126, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Sharif, I.; Aleem, S.; Farooq, J.; Rizwan, M.; Younas, A.; Sarwar, G.; Chohan, S.M. Salinity stress in cotton: Effects, mechanism of tolerance and its management strategies. Physiol. Mol. Biol. Plants 2019, 25, 807–820. [Google Scholar] [CrossRef]
- Min, W.; Guo, H.J.; Zhou, G.G.; Zhang, W.; Ma, L.J.; Ye, J.; Hou, Z.N. Root distribution and growth of cotton as affected by drip irrigation with saline water. Field Crops Res. 2014, 169, 1–10. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Chen, L.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. PeerJ 2020, 11, 10486. [Google Scholar] [CrossRef]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- Ju, F.Y.; Pang, J.L.; Huo, Y.Y.; Zhu, J.J.; Yu, K.; Sun, L.Y.; Loka, D.A.; Hu, W.; Zhou, Z.G.; Wang, S.S.; et al. Potassium application alleviates the negative effects of salt stress on cotton (Gossypium hirsutum L.) yield by improving the ionic homeostasis, photosynthetic capacity and carbohydrate metabolism of the leaf subtending the cotton boll. Field Crops Res. 2021, 272, 108288. [Google Scholar] [CrossRef]
- Feng, Y.J.; Chen, Z.N.; Wang, J.W.; Yang, W.T.; Tan, F.X. Change in abuscular mycorrhizal fungi colonization rate and nutrient content in Bt corn. Chin. J. Eco-Agri. 2010, 18, 486–491. (In Chinese) [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R. Influence of arbuscular mycorrhiza fungi and drought stress on fatty acids profile of sesame (Sesamum indicum L.). Field Crops Res. 2021, 262, 108035. [Google Scholar] [CrossRef]
- Liang, B.B.; Wang, W.J.; Fan, X.X.; Kurakov, A.V.; Liu, Y.F.; Song, F.Q.; Chang, W. Arbuscular mycorrhizal fungi can ameliorate salt stress in Elaeagnus angustifolia by improving leaf photosynthetic function and ultrastructure. Plant Biol. 2021, 1, 232–241. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Meng, S.; Wu, F. Effects of arbuscular mycorrhizal inoculation on the growth, photosynthesis and antioxidant enzymatic activity of Euonymus maackii Rupr. under gradient water deficit levels. PLoS ONE. 2021, 16, e0259959. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Mao, Z.; Li, Z. The roles of phosphorus and nitrogen nutrient transporters in the arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2022, 23, 11027. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Hao, H.; Hu, Z.H.; Leng, P.S.; Lu, C.F. Ectomycorrhizal Quercus mongolica seedlings improve their photosynthesis and antioxidant capacity under salt stress. Chin. J. Appl. Environ. Biol. 2023, 1, 15. (In Chinese) [Google Scholar]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Siani, N.G.; Fallah, S.; Pokhrel, L.R.; Rostamnejadi, A. Natural amelioration of Zinc oxide nanoparticle toxicity in fenugreek (Trigonella foenum-gracum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant Physiol. Biochem. 2017, 112, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, L.; Liu, B.; Huang, D.; Liu, S.; Liu, R.; Siddique, K.H.M.; Chen, Y. Arbuscular mycorrhizas regulate photosynthetic capacity and antioxidant defense systems to mediate salt tolerance in Maize. Plants 2020, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Zhang, Z.; Huang, P.; Yang, Y. Arbuscular mycorrhizal fungi alleviates salt stress in Xanthoceras sorbifolium through improved osmotic tolerance, antioxidant activity, and photosynthesis. Front. Microbiol. 2023, 14, 1138771. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-um, S. Alleviation of salt stress in upland Rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 2020, 11, 348. [Google Scholar] [CrossRef]
- Abbaspour, H.; Pour, F.S.N.; Abdel-Wahhab, M.A. Arbuscular mycorrhizal symbiosis regulates the physiological responses, ion distribution and relevant gene expression to trigger salt stress tolerance in pistachio. Physiol. Mol. Biol. Plants 2021, 27, 1765–1778. [Google Scholar] [CrossRef]
- Adnan, A.; Bei, H.; Aamir, H.K.; Cheng, F.; Abid, U.; Abdul, S.K.; Liangro, H. A transcriptomic study reveals salt stress alleviation in cotton plants upon salt tolerant PGPR inoculation. Environ. Exp. Bot. 2022, 200, 104928. [Google Scholar]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Ping, Y.; Mu, L.; Yang, T. The diversity of arbuscular mycorrhizal fungi of Rosa acicularis ‘Luhe’ in saline areas. J. For. Res. 2018, 30, 1507–1512. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, L.; Wang, J.; Liu, X.; Jia, Z.; Li, C. Arbuscular mycorrhizal fungi promote Gleditsia sinensis root growth under salt stress by regulating nutrient uptake and physiology. Forests 2022, 13, 688. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Savvides, A.; Pesando, M.; Lumini, E.; Volpe, M.G.; Ozudogru, E.A.; Faccio, A.; De, F.; Michelozzi, M.; Lambardi, M.; et al. Impact of two arbuscular mycorrhizal fungi on Arundo donax L. response to salt stress. Planta 2018, 247, 573–585. [Google Scholar] [CrossRef]
- Debatosh, D.; Salina, T.; Prema, M.; Dhruv, A.S.; Heike, B. Development and Resource Exchange Processes in Root Symbioses of Legumes. In Symbiosis in Nature; Intechopen: London, UK, 2023; p. 111540. [Google Scholar]
- Gupta, A.; Singh, A.N.; Tiwari, R.K.; Sahu, P.K.; Yadav, J.; Srivastava, A.K.; Kumar, S. Salinity alleviation and reduction in oxidative stress by endophytic and rhizospheric microbes in two rice cultivars. Plants 2023, 12, 976. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhao, Y.; Guo, S.; Cheng, S.; Guan, Y.J.; Cai, H.G.; Mi, G.H.; Yuan, L.X.; Chen, F.J. Enhanced crown root number and length confers potential for yield improvement and fertilizer reduction in nitrogen-efficient maize cultivars. Field Crops Res. 2019, 241, 107562. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular mechanisms of root development in rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Zhang, H.Q.; Liu, Z.K.; Chen, H.; Tang, M. Symbiosis of arbuscular mycorrhizal fungi and Robinia pseudoacacia L. improves root tensile strength and soil aggregate stability. PLoS ONE 2016, 11, 0153378. [Google Scholar] [CrossRef]
- Saleem, S.; Iqbal, A.; Ahmed, F.; Ahmad, M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021, 28, 5317–5324. [Google Scholar] [CrossRef]
- Wha, B.; Ky, A.; Yue, Z.C.; Lba, D.; Hm, E.; Gh, A. Contrasting photosynthesis, photoinhibition and oxidative damage in honeysuckle (Lonicera japonica Thunb.) under iso-osmotic salt and drought stresses. Environ. Exp. Bot. 2020, 182, 104313. [Google Scholar]
- Yooyongwech, S.; Phaukinsang, N.; Cha-Um, S.; Supaibulwatana, K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2013, 69, 285–293. [Google Scholar] [CrossRef]
- Forlani, G.; Bertazzini, M.; Cagnano, G. Stress-driven increase in proline levels, and not proline levels themselves, correlates with the ability to withstand excess salt in a group of 17 Italian rice genotypes. Plant Biol. 2019, 21, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Aldubise, A.; Egamberdieva, D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015, 10, 230–242. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, J.; Ren, Q.; Zhang, B.; Zhang, J.; Huang, R.; Wang, G.G. Arbuscular mycorrhizal fungi enhanced salt tolerance of Gleditsia sinensis by modulating antioxidant activity, ion balance and P/N ratio. Plant Growth Regul. 2022, 97, 33–49. [Google Scholar] [CrossRef]
- Sharma, I. Arsenic-induced oxidative stress and antioxidant defense system of Pisum sativum and Pennisetum typhoides: A comparative study. Res. J. Biotechnol. 2013, 18, 48–56. [Google Scholar]
- Santos, A.A.; Silveira, J.A.G.D.; Bonifacio, A.; Rodrigues, A.C.; Figueiredo, M.D.V.B. Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Braz. J. Microbiol. 2018, 49, 513–521. [Google Scholar] [CrossRef]
- Khan, Y.; Shah, S.; Hui, T. The roles of arbuscular mycorrhizal fungi in influencing plant nutrients, photosynthesis, and metabolites of cereal crops—A review. Agronomy 2022, 12, 2191. [Google Scholar] [CrossRef]
- Athar, H.; Zafar, Z.U.; Ashraf, M. Glycine betaine improved photosynthesis in canola under salt stress: Evaluation of chlorophyll fluorescence parameters as potential indicators. J. Agron. Crop Sci. 2015, 201, 428–442. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef]
- Diao, F.; Dang, Z.; Cui, X.; Xu, J.; Jia, B.; Ding, S. Transcriptomic analysis revealed distinctive modulations of arbuscular mycorrhizal fungi inoculation in halophyte Suaeda salsa under moderate salt conditions. Environ. Exp. Bot. 2021, 183, 104337. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Liang, S.M.; Zhang, F.; Zou, Y.N.; Kuča, K.; Wu, Q.S. Metabolomics analysis reveals drought responses of trifoliate orange by arbuscular mycorrhizal fungi with a focus on terpenoid profile. Front. Plant Sci. 2021, 12, 740524. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; García, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci. Rep. 2016, 6, 37663. [Google Scholar] [CrossRef]
- Jia, X.; Zhu, H.; Wang, H.; Wu, Y.; Zhao, T.; Cheng, L. Study on physiological response of Malus halliana to saline-alkali stress. Acta Ecol. Sin. 2019, 39, 6349–6361. [Google Scholar]
- Kaya, C.; Ashraf, M.; Dikilitas, M.; Tuna, A.L. Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients—A field trial. Aust. J. Crop Sci. 2013, 7, 249–254. [Google Scholar]
- Zhang, D.J.; Yang, Y.J.; Liu, C.Y.; Zhang, F.; Hu, W.; Wu, Q.S. Auxin modulates root-hair growth through its signaling pathway in citrus. Sci. Hortic. 2018, 236, 73–78. [Google Scholar] [CrossRef]
- Hu, C.H.; Zheng, Y.; Tong, C. Effects of exogenous melatonin on plant growth, root hormones and photosynthetic characteristics of trifoliate orange subjected to salt stress. Plant Growth Regul. 2022, 97, 551–558. [Google Scholar] [CrossRef]
- López-Gómez, M.; Hidalgo-Castellanos, J.; Lluch, C.; Herrera-Cervera, J.A. 24-Epibrassinolide ameliorates salt stress effects in the symbiosis Medicago truncatula-Sinorhizobium meliloti and regulates the nodulation in cross-talk with polyamines. Plant Physiol. Biochem. 2016, 108, 212–221. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C.; et al. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Jiang, X.H.; Fu, Y.Y. Effects of salt stress on chlorophyll fluorescence parameters of cotton seedlings. J. Irrig. Drain. 2021, 40, 4023–4028. (In Chinese) [Google Scholar]
- Chen, L.; Liu, L.T.; Ma, T.T. Effects of melatonin on antioxidant oxidase activity and germination of cotton seed under salt stress. J. Cotton Sci. 2019, 31, 438–447. (In Chinese) [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 7, 357–359. [Google Scholar]
- Liu, R.C.; Xiao, Z.Y.; Hashem, A.; Abdallah, E.F.; Wu, Q.S. Mycorrhizal fungal diversity and its relationship with soil properties in Camellia oleifera. Agriculture 2021, 11, 470. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma 2016, 3, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, S.; Padmarajaiah, N.; Al-tayar, N.G.S. Ninhydrin-sodium molybdate chromogenic analytical probe for the assay of amino acids and proteins. Spectrochim. Acta A 2017, 3, 897–903. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Zou, H. Experimental Supervision of Plant Physiology; China Agriculture Press: Beijing, China, 2018. [Google Scholar]
- Fan, W.; Li, W.; Zhang, X. Photosynthetic physiological characteristics of Tetracentron sinense Oliv in different DBH classes and the factors restricting regeneration. J. Plant Growth Regul. 2021, 41, 1943–1952. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Sakakibara, H. Highly sensitive high-throughput profiling of six phytohormones using MS-probe modification and liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 2012, 918, 151–164. [Google Scholar]
- Chen, J. Arbuscular Mycorrhizal Fungi (AMF) Alleviates Salt Stress in Black Locust. Ph.D. Dissertation, Northwest Agriculture & Forestry University, Xianyang, China, 2018. [Google Scholar]
- Fan, K.; Li, F.; Chen, X.; Li, Z.; Mulla, D.J. Nitrogen balance index prediction of winter wheat by canopy hyperspectral transformation and machine learning. Remote Sens. 2022, 14, 3504. [Google Scholar] [CrossRef]
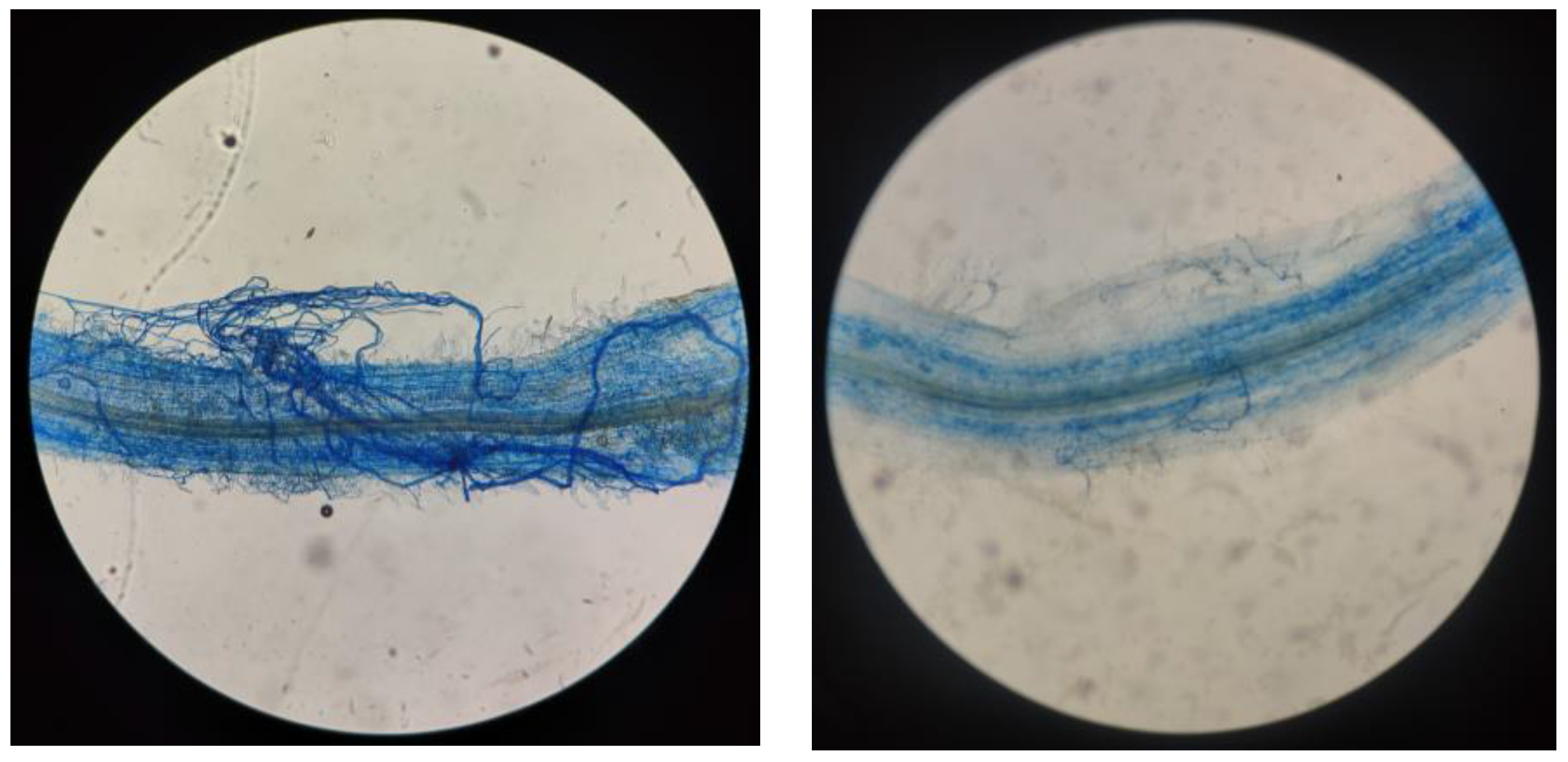

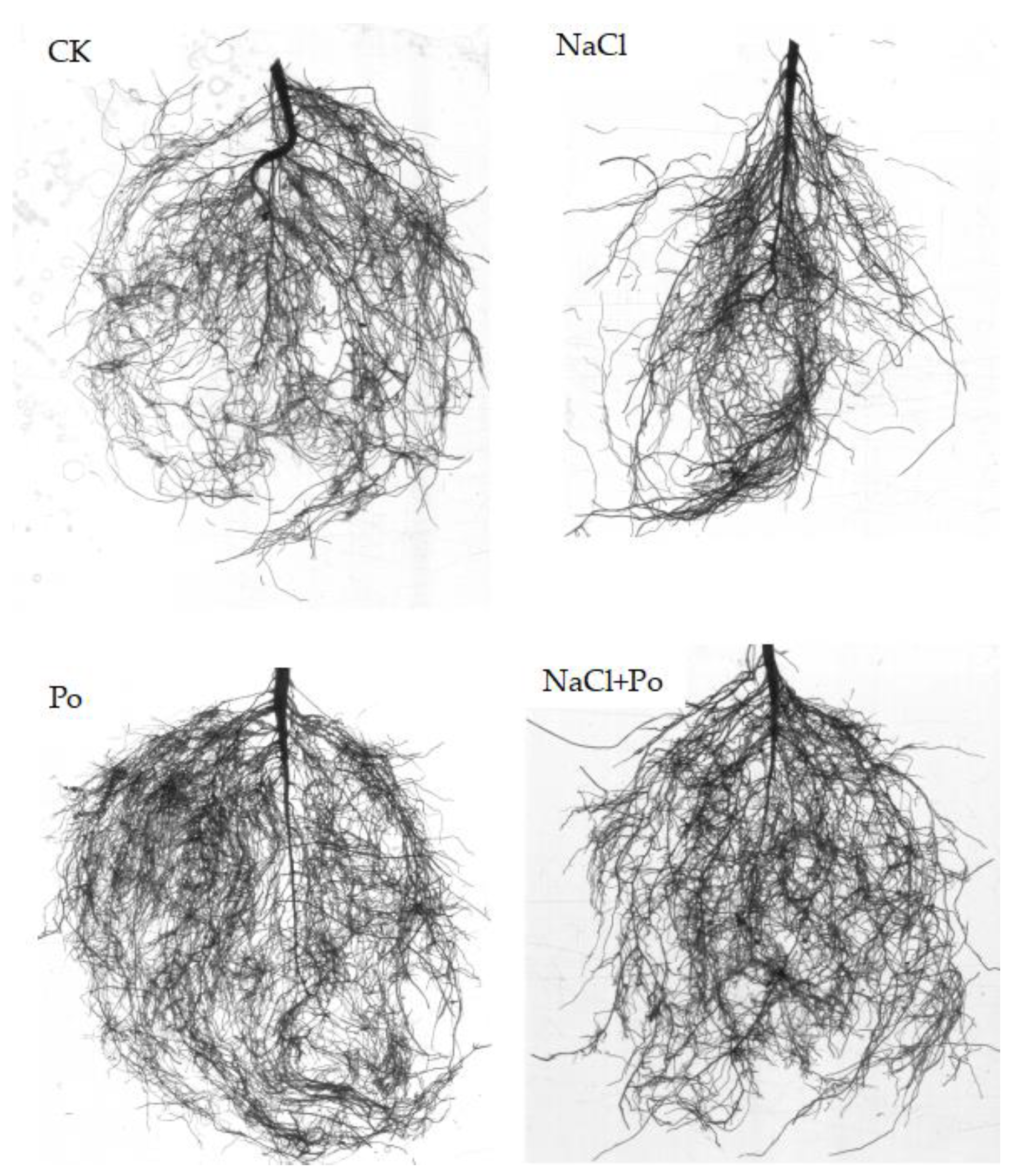
| Treatments | Plant Height (cm/Plant) | Stem Diameter (mm) | Leaf Number (#/Plant) | Shoot Dry Weight (g FW/Plant) | Root Dry Weight (g FW/Plant) | Mycorrhizal Colonization (%) | Soil Hyphal Length (cm/g Soil) |
|---|---|---|---|---|---|---|---|
| CK | 32.04 ± 0.73 b | 2.79 ± 0.06 b | 6.75 ± 0.29 b | 4.59 ± 0.35 c | 2.89 ± 0.25 b | - | - |
| Po | 39.25 ± 0.55 a | 3.17 ± 0.08 a | 7.43 ± 0.21 a | 7.83 ± 0.51 a | 3.33 ± 0.20 a | 77.41% ± 6.20 a | 67.85 ± 5.20 a |
| NaCl | 27.06 ± 0.81 c | 2.45 ± 0.06 c | 4.81 ± 0.25 c | 2.98 ± 0.19 d | 2.45 ± 0.18 c | - | - |
| NaCl + Po | 34.11 ± 0.45 b | 2.82 ± 0.05 b | 6.35 ± 0.19 b | 5.19 ± 0.32 b | 2.73 ± 0.21 b | 52.32% ± 4.20 b | 48.56 ± 4.20 b |
| Treatments | Total Root Length (cm/Plant) | Root Projected Area (cm2/Plant) | Total Root Surface Area (cm2/Plant) | Root Average Diameter (mm/Plant) | Root Volume (cm3/Plant) |
|---|---|---|---|---|---|
| CK | 221.79 ± 7.47 b | 13.07 ± 0.30 ab | 16.98 ± 1.45 ab | 0.48 ± 0.02 a | 2.22 ± 0.20 ab |
| Po | 288.12 ± 9.21 a | 13.21 ± 0.27 a | 17.50 ± 1.35 a | 0.49 ± 0.01 a | 2.34 ± 0.19 a |
| NaCl | 204.09 ± 8.12 c | 12.84 ± 0.37 b | 16.68 ± 1.27 b | 0.41 ± 0.01 a | 2.16 ± 0.11 b |
| NaCl + Po | 231.81 ± 8.91 b | 13.09 ± 0.21 ab | 17.11 ± 1.43 a | 0.47 ± 0.01 a | 2.25 ± 0.10 ab |
| Treatments | Pro (ug/g) | MDA (nmol/Prot) | Soluble Protein (mg/g) | Soluble Sugar (mg/g) |
|---|---|---|---|---|
| CK | 34.89 ± 1.09 c | 502.53 ± 39.44 c | 0.85 ± 0.07 a | 2.21 ± 0.17 a |
| Po | 33.59 ± 2.35 c | 488.54 ± 30.32 c | 0.91 ± 0.04 a | 2.42 ± 0.14 a |
| NaCl | 68.46 ± 5.50 a | 1000.35 ± 60.98 a | 0.81 ± 0.05 a | 2.03 ± 0.19 a |
| NaCl + Po | 48.37 ± 2.39 b | 801.44 ± 51.42 b | 0.83 ± 0.05 a | 2.26 ± 0.15 a |
| Treatments | H2O2 (U/Prot) | SOD (U/Prot) | POD (U/Prot) | CAT (U/Prot) |
|---|---|---|---|---|
| CK | 23.95 ± 2.03 c | 100.04 ± 9.71 d | 30.04 ± 2.45 d | 10.71 ± 1.02 d |
| Po | 23.01 ± 2.15 c | 130.03 ± 8.79 c | 41.73 ± 3.71 c | 21.47 ± 1.12 c |
| NaCl | 33.07 ± 2.62 a | 201.83 ± 18.21 b | 80.03 ± 7.12 b | 30.24 ± 2.02 b |
| NaCl + Po | 28.09 ± 1.87 b | 229.03 ± 20.46 a | 98.41 ± 9.04 a | 38.72 ± 3.07 a |
| Treatments | Pn (μmol/m2·s) | Gs (μmol/m2·s) | Ci (μmol/mol) | Tr (mmol/m2·s) |
|---|---|---|---|---|
| CK | 23.22 ± 0.25 b | 1.71 ± 0.08 b | 183.17 ± 7.95 b | 4.56 ± 0.18 ab |
| Po | 26.20 ± 0.42 a | 1.94 ± 0.16 a | 206.56 ± 8.19 a | 4.87 ± 0.19 a |
| NaCl | 16.81 ± 0.38 d | 1.51 ± 0.13 c | 125.16 ± 11.35 d | 3.74 ± 0.24 c |
| NaCl + Po | 20.58 ± 0.17 c | 1.72 ± 0.07 b | 140.49 ± 6.51 c | 4.26 ± 0.11 b |
| Treatments | φPSII | Fv′/Fm′ | qP | NPQ |
|---|---|---|---|---|
| CK | 0.63 ± 0.04 b | 0.86 ± 0.03 b | 0.97 ± 0.08 b | 0.67 ± 0.03 b |
| Po | 0.83 ± 0.07 a | 0.98 ± 0.06 a | 1.22 ± 0.12 a | 0.44 ± 0.01 c |
| NaCl | 0.54 ± 0.03 c | 0.77 ± 0.04 c | 0.79 ± 0.04 c | 0.88 ± 0.02 a |
| NaCl + Po | 0.62 ± 0.05 b | 0.84 ± 0.05 b | 0.96 ± 0.07 b | 0.68 ± 0.04 b |
| Treatments | IAA (ng/g FW) | BR (ng/g FW) | GA (ng/g FW) | ABA (ng/g FW) |
|---|---|---|---|---|
| CK | 61.13 ± 5.28 bc | 2.41 ± 0.22 b | 6.21 ± 0.36 a | 5.84 ± 0.46 b |
| Po | 72.22 ± 3.79 a | 2.83 ± 0.12 a | 6.15 ± 0.42 a | 5.22 ± 0.22 b |
| NaCl | 54.52 ± 4.21 c | 1.67 ± 0.12 d | 6.11 ± 0.51 a | 6.98 ± 0.42 a |
| NaCl + Po | 65.01 ± 3.44 b | 2.09 ± 0.19 c | 6.22 ± 0.51 a | 5.88 ± 0.35 b |
| Treatments | Relative Water Content (RWC, %) | Water Saturation Deficit (WSD, %) | Chlorophyll (ug/cm2) | Flavonoid (ug/g) | Nitrogen Balance (g/cm2) |
|---|---|---|---|---|---|
| CK | 62.96 ± 2.53 b | 37.04 ± 2.98 a | 31.43 ± 1.91 a | 0.043 ± 0.003 a | 732.13 ± 35.73 a |
| Po | 65.87 ± 2.82 a | 37.13 ± 2.82 a | 32.04 ± 1.29 a | 0.044 ± 0.004 a | 755.51 ± 29.06 a |
| NaCl | 60.10 ± 3.17 c | 29.90 ± 1.98 c | 26.47 ± 2.08 b | 0.048 ± 0.002 a | 549.76 ± 24.29 b |
| NaCl + Po | 64.98 ± 3.23 a | 36.01 ± 2.11 ab | 32.03 ± 2.49 a | 0.045 ± 0.003 a | 748.83 ± 54.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.-J.; Tong, C.-L.; Wang, Q.-S.; Bie, S. Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress. Plants 2024, 13, 805. https://doi.org/10.3390/plants13060805
Zhang D-J, Tong C-L, Wang Q-S, Bie S. Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress. Plants. 2024; 13(6):805. https://doi.org/10.3390/plants13060805
Chicago/Turabian StyleZhang, De-Jian, Cui-Ling Tong, Qiong-Shan Wang, and Shu Bie. 2024. "Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress" Plants 13, no. 6: 805. https://doi.org/10.3390/plants13060805
APA StyleZhang, D.-J., Tong, C.-L., Wang, Q.-S., & Bie, S. (2024). Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress. Plants, 13(6), 805. https://doi.org/10.3390/plants13060805






